Sodium Percarbonate vs. Calcium Hypochlorite in Pool Maintenance
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Background and Objectives
Pool maintenance is a critical aspect of ensuring safe and enjoyable swimming experiences. Over the years, various chemical treatments have been developed to maintain water quality and hygiene in swimming pools. Two prominent contenders in this field are sodium percarbonate and calcium hypochlorite. This technical research report aims to provide a comprehensive comparison of these two chemicals in the context of pool maintenance.
The evolution of pool maintenance technologies has been driven by the need for more effective, safer, and environmentally friendly solutions. Sodium percarbonate, also known as sodium carbonate peroxyhydrate, has gained attention as an alternative to traditional chlorine-based treatments. On the other hand, calcium hypochlorite has long been a staple in pool maintenance due to its powerful disinfecting properties.
Understanding the differences between these two chemicals is crucial for pool owners, maintenance professionals, and manufacturers of pool care products. This comparison will explore various aspects, including their chemical properties, effectiveness in water treatment, safety considerations, environmental impact, and cost-effectiveness.
The primary objective of this research is to evaluate the strengths and weaknesses of sodium percarbonate and calcium hypochlorite in pool maintenance applications. By analyzing their performance across different parameters, we aim to provide insights that can guide decision-making processes for both residential and commercial pool maintenance.
This study will delve into the chemical reactions that occur when these substances are introduced into pool water, examining their efficacy in controlling algae growth, eliminating harmful bacteria, and maintaining proper pH levels. Additionally, we will investigate the long-term effects of using these chemicals on pool equipment and surfaces.
Another key aspect of this research is to assess the safety profiles of sodium percarbonate and calcium hypochlorite. This includes examining potential health risks for swimmers, handling precautions for maintenance personnel, and storage requirements. Environmental considerations, such as the impact on aquatic ecosystems when pool water is discharged, will also be addressed.
Furthermore, this report will explore the market trends and consumer preferences regarding these two pool maintenance solutions. By analyzing adoption rates, user feedback, and industry expert opinions, we aim to provide a comprehensive view of the current landscape and future prospects for both chemicals in the pool maintenance sector.
The evolution of pool maintenance technologies has been driven by the need for more effective, safer, and environmentally friendly solutions. Sodium percarbonate, also known as sodium carbonate peroxyhydrate, has gained attention as an alternative to traditional chlorine-based treatments. On the other hand, calcium hypochlorite has long been a staple in pool maintenance due to its powerful disinfecting properties.
Understanding the differences between these two chemicals is crucial for pool owners, maintenance professionals, and manufacturers of pool care products. This comparison will explore various aspects, including their chemical properties, effectiveness in water treatment, safety considerations, environmental impact, and cost-effectiveness.
The primary objective of this research is to evaluate the strengths and weaknesses of sodium percarbonate and calcium hypochlorite in pool maintenance applications. By analyzing their performance across different parameters, we aim to provide insights that can guide decision-making processes for both residential and commercial pool maintenance.
This study will delve into the chemical reactions that occur when these substances are introduced into pool water, examining their efficacy in controlling algae growth, eliminating harmful bacteria, and maintaining proper pH levels. Additionally, we will investigate the long-term effects of using these chemicals on pool equipment and surfaces.
Another key aspect of this research is to assess the safety profiles of sodium percarbonate and calcium hypochlorite. This includes examining potential health risks for swimmers, handling precautions for maintenance personnel, and storage requirements. Environmental considerations, such as the impact on aquatic ecosystems when pool water is discharged, will also be addressed.
Furthermore, this report will explore the market trends and consumer preferences regarding these two pool maintenance solutions. By analyzing adoption rates, user feedback, and industry expert opinions, we aim to provide a comprehensive view of the current landscape and future prospects for both chemicals in the pool maintenance sector.
Market Analysis
The pool maintenance market has witnessed significant growth in recent years, driven by the increasing number of residential and commercial swimming pools worldwide. The global pool chemicals market, which includes sodium percarbonate and calcium hypochlorite, was valued at approximately $1.2 billion in 2020 and is projected to reach $1.5 billion by 2025, growing at a CAGR of 4.5%.
Sodium percarbonate and calcium hypochlorite are two key players in this market, each with its own strengths and market positioning. Sodium percarbonate, also known as oxygen bleach, has gained popularity due to its eco-friendly nature and effectiveness in pool maintenance. It is particularly favored in regions with stringent environmental regulations, such as parts of Europe and North America.
On the other hand, calcium hypochlorite remains a dominant force in the pool maintenance market, especially in developing countries and regions with less stringent environmental regulations. Its lower cost and high effectiveness in killing bacteria and algae have contributed to its widespread use. The calcium hypochlorite market segment is estimated to hold a larger share, accounting for approximately 60% of the chlorine-based pool chemicals market.
Consumer preferences are shifting towards more environmentally friendly and safer pool maintenance solutions, which has led to increased demand for sodium percarbonate. This trend is particularly evident in high-income countries, where consumers are willing to pay a premium for eco-friendly products. The sodium percarbonate market segment is expected to grow at a higher CAGR of 6-7% compared to the overall pool chemicals market.
Regional differences play a significant role in the market dynamics of these two chemicals. North America and Europe show a stronger inclination towards sodium percarbonate due to stricter regulations and higher environmental awareness. In contrast, Asia-Pacific and Latin America still heavily rely on calcium hypochlorite due to its cost-effectiveness and established supply chains.
The COVID-19 pandemic has had a notable impact on the pool maintenance market. With more people staying at home and investing in home improvements, there has been a surge in residential pool installations and maintenance activities. This trend has benefited both sodium percarbonate and calcium hypochlorite manufacturers, although supply chain disruptions have posed challenges.
Looking ahead, the market for both chemicals is expected to grow, albeit at different rates. Sodium percarbonate is likely to see faster adoption in developed markets, while calcium hypochlorite will continue to dominate in developing regions. Manufacturers are increasingly focusing on product innovations, such as improved formulations and packaging, to gain a competitive edge in this evolving market landscape.
Sodium percarbonate and calcium hypochlorite are two key players in this market, each with its own strengths and market positioning. Sodium percarbonate, also known as oxygen bleach, has gained popularity due to its eco-friendly nature and effectiveness in pool maintenance. It is particularly favored in regions with stringent environmental regulations, such as parts of Europe and North America.
On the other hand, calcium hypochlorite remains a dominant force in the pool maintenance market, especially in developing countries and regions with less stringent environmental regulations. Its lower cost and high effectiveness in killing bacteria and algae have contributed to its widespread use. The calcium hypochlorite market segment is estimated to hold a larger share, accounting for approximately 60% of the chlorine-based pool chemicals market.
Consumer preferences are shifting towards more environmentally friendly and safer pool maintenance solutions, which has led to increased demand for sodium percarbonate. This trend is particularly evident in high-income countries, where consumers are willing to pay a premium for eco-friendly products. The sodium percarbonate market segment is expected to grow at a higher CAGR of 6-7% compared to the overall pool chemicals market.
Regional differences play a significant role in the market dynamics of these two chemicals. North America and Europe show a stronger inclination towards sodium percarbonate due to stricter regulations and higher environmental awareness. In contrast, Asia-Pacific and Latin America still heavily rely on calcium hypochlorite due to its cost-effectiveness and established supply chains.
The COVID-19 pandemic has had a notable impact on the pool maintenance market. With more people staying at home and investing in home improvements, there has been a surge in residential pool installations and maintenance activities. This trend has benefited both sodium percarbonate and calcium hypochlorite manufacturers, although supply chain disruptions have posed challenges.
Looking ahead, the market for both chemicals is expected to grow, albeit at different rates. Sodium percarbonate is likely to see faster adoption in developed markets, while calcium hypochlorite will continue to dominate in developing regions. Manufacturers are increasingly focusing on product innovations, such as improved formulations and packaging, to gain a competitive edge in this evolving market landscape.
Current Challenges
The current challenges in comparing sodium percarbonate and calcium hypochlorite for pool maintenance stem from various factors, including chemical properties, environmental impact, and user preferences. One significant challenge is the difference in stability between these two compounds. Calcium hypochlorite is generally more stable and has a longer shelf life, while sodium percarbonate can degrade more quickly, especially when exposed to moisture or heat.
Another challenge lies in the varying pH effects of these chemicals on pool water. Calcium hypochlorite tends to raise the pH of the water, potentially requiring additional pH adjustment. In contrast, sodium percarbonate has a more neutral effect on pH, which can be advantageous but may also complicate direct comparisons of effectiveness.
The environmental impact of these chemicals presents another challenge in their comparison. Calcium hypochlorite is known for its strong oxidizing properties, which can be harsh on the environment if not properly managed. Sodium percarbonate, being an oxygen-based bleach, is often perceived as more environmentally friendly. However, quantifying and comparing their long-term environmental effects remains a challenge.
Effectiveness in different water conditions poses another hurdle. Calcium hypochlorite is generally more effective in hard water conditions, while sodium percarbonate may perform better in softer water. This variability makes it difficult to establish a universal comparison, as pool water composition can vary significantly across different regions.
The cost-effectiveness of these chemicals also presents a challenge in comparison. While calcium hypochlorite is often less expensive upfront, sodium percarbonate may require less frequent application, potentially balancing out costs over time. Accurately comparing long-term costs requires consideration of factors such as storage requirements, application frequency, and additional water treatment needs.
User safety and handling requirements differ between these chemicals, adding another layer of complexity to their comparison. Calcium hypochlorite requires more careful handling due to its corrosive nature and potential for harmful fumes. Sodium percarbonate is generally considered safer to handle but may be less effective in certain situations, creating a trade-off between safety and efficacy.
Lastly, the challenge of consumer perception and market trends cannot be overlooked. As eco-friendly options gain popularity, sodium percarbonate may be favored by some pool owners despite potential drawbacks. This shift in consumer preference complicates objective comparisons based solely on technical performance.
Another challenge lies in the varying pH effects of these chemicals on pool water. Calcium hypochlorite tends to raise the pH of the water, potentially requiring additional pH adjustment. In contrast, sodium percarbonate has a more neutral effect on pH, which can be advantageous but may also complicate direct comparisons of effectiveness.
The environmental impact of these chemicals presents another challenge in their comparison. Calcium hypochlorite is known for its strong oxidizing properties, which can be harsh on the environment if not properly managed. Sodium percarbonate, being an oxygen-based bleach, is often perceived as more environmentally friendly. However, quantifying and comparing their long-term environmental effects remains a challenge.
Effectiveness in different water conditions poses another hurdle. Calcium hypochlorite is generally more effective in hard water conditions, while sodium percarbonate may perform better in softer water. This variability makes it difficult to establish a universal comparison, as pool water composition can vary significantly across different regions.
The cost-effectiveness of these chemicals also presents a challenge in comparison. While calcium hypochlorite is often less expensive upfront, sodium percarbonate may require less frequent application, potentially balancing out costs over time. Accurately comparing long-term costs requires consideration of factors such as storage requirements, application frequency, and additional water treatment needs.
User safety and handling requirements differ between these chemicals, adding another layer of complexity to their comparison. Calcium hypochlorite requires more careful handling due to its corrosive nature and potential for harmful fumes. Sodium percarbonate is generally considered safer to handle but may be less effective in certain situations, creating a trade-off between safety and efficacy.
Lastly, the challenge of consumer perception and market trends cannot be overlooked. As eco-friendly options gain popularity, sodium percarbonate may be favored by some pool owners despite potential drawbacks. This shift in consumer preference complicates objective comparisons based solely on technical performance.
Existing Solutions
01 Effectiveness of sodium percarbonate and calcium hypochlorite as oxidizing agents
Sodium percarbonate and calcium hypochlorite are effective oxidizing agents used in various applications. They are known for their strong disinfecting and bleaching properties, making them suitable for water treatment, cleaning, and sanitization purposes. These compounds release active oxygen or chlorine, respectively, which can effectively eliminate bacteria, viruses, and other microorganisms.- Effectiveness of sodium percarbonate and calcium hypochlorite as oxidizing agents: Sodium percarbonate and calcium hypochlorite are effective oxidizing agents used in various applications. They are known for their strong disinfecting and bleaching properties, making them suitable for water treatment, cleaning, and sanitization purposes. These compounds release active oxygen or chlorine, respectively, which can effectively eliminate bacteria, viruses, and other microorganisms.
- Safety considerations for handling and storage: While effective, both sodium percarbonate and calcium hypochlorite require careful handling and storage due to their reactive nature. Proper safety measures should be implemented to prevent accidental exposure or reactions. This includes storing them in cool, dry places away from incompatible materials, using appropriate personal protective equipment during handling, and following recommended disposal procedures to minimize environmental impact.
- Applications in water treatment and disinfection: Sodium percarbonate and calcium hypochlorite are widely used in water treatment and disinfection processes. They are effective in purifying drinking water, treating swimming pools, and sanitizing industrial water systems. These compounds can remove organic contaminants, control algae growth, and maintain proper pH levels in water, making them valuable in ensuring water safety and quality.
- Formulation and stability enhancements: Research has focused on improving the formulation and stability of products containing sodium percarbonate and calcium hypochlorite. This includes developing coatings or stabilizers to enhance shelf life, improve dissolution rates, and maintain effectiveness over time. Advanced formulations may also incorporate other compounds to enhance their performance or address specific application requirements.
- Comparative effectiveness and environmental impact: Studies have been conducted to compare the effectiveness of sodium percarbonate and calcium hypochlorite in various applications. Factors such as concentration, contact time, and target microorganisms are considered. Additionally, research has examined the environmental impact of these compounds, including their degradation products and potential effects on aquatic ecosystems, to ensure their safe and sustainable use.
02 Safety considerations for handling and storage
While effective, both sodium percarbonate and calcium hypochlorite require careful handling and storage due to their reactive nature. Proper safety measures should be implemented to prevent accidental exposure or reactions. This includes storing them in cool, dry places away from incompatible materials, using appropriate personal protective equipment during handling, and following recommended dilution ratios for various applications.Expand Specific Solutions03 Comparative effectiveness in water treatment applications
Both compounds are widely used in water treatment, but their effectiveness can vary depending on factors such as pH, temperature, and organic matter content. Calcium hypochlorite generally provides longer-lasting residual disinfection, while sodium percarbonate may be preferred in situations where chlorine odor or taste is undesirable. The choice between the two often depends on specific application requirements and environmental considerations.Expand Specific Solutions04 Environmental impact and degradation products
The environmental impact of these compounds is an important consideration. Sodium percarbonate breaks down into oxygen, water, and sodium carbonate, which are generally considered environmentally friendly. Calcium hypochlorite, on the other hand, can form chlorinated byproducts that may have potential environmental concerns. Understanding the degradation pathways and potential effects on aquatic ecosystems is crucial for their responsible use.Expand Specific Solutions05 Formulation and stability enhancements
Research has been conducted to improve the stability and effectiveness of both compounds in various formulations. This includes developing coated or stabilized forms of sodium percarbonate to enhance its shelf life and performance in detergent applications. For calcium hypochlorite, efforts have focused on improving its stability in tablet or granular forms for easier handling and dosing in water treatment applications.Expand Specific Solutions
Key Industry Players
The market for pool maintenance chemicals, specifically comparing sodium percarbonate and calcium hypochlorite, is in a mature stage with established players and technologies. The global pool chemicals market size was valued at approximately $1.2 billion in 2020, with steady growth projected. Technologically, both compounds are well-understood, with ongoing research focusing on improving efficiency and environmental impact. Key players like Innovative Water Care LLC, Evonik Operations GmbH, and Henkel AG & Co. KGaA are investing in R&D to enhance product performance and sustainability. The competition is intense, with companies like Fluidra Group Australia Pty Ltd. and The Clorox Co. offering innovative solutions to differentiate themselves in this saturated market.
Innovative Water Care LLC
Technical Solution: Innovative Water Care LLC has developed advanced formulations for both sodium percarbonate and calcium hypochlorite pool treatments. Their sodium percarbonate solution, marketed as "OxyShock", utilizes a stabilized form of the compound that releases oxygen more slowly, providing longer-lasting sanitization[1]. For calcium hypochlorite, they've introduced "Cal-Hypo Pro", a proprietary blend that includes scale inhibitors to prevent calcium build-up in pool equipment[2]. The company has also invested in research comparing the two chemicals' effectiveness in various pool conditions, finding that sodium percarbonate is more effective in soft water, while calcium hypochlorite performs better in hard water environments[3].
Strengths: Tailored solutions for different water types, innovative formulations to address common issues like equipment scaling. Weaknesses: May be more expensive than generic alternatives, requires specific application methods for optimal performance.
Fluidra Group Australia Pty Ltd.
Technical Solution: Fluidra Group Australia has developed innovative pool maintenance solutions utilizing both sodium percarbonate and calcium hypochlorite. Their "AstralPool Ox-Active" system uses a stabilized form of sodium percarbonate that provides up to 72 hours of active oxygen release, significantly longer than traditional formulations[1]. For calcium hypochlorite, they've introduced "Hypocal Plus", which incorporates a unique dissolution technology that reduces scaling by up to 50% compared to standard cal-hypo products[2]. Fluidra has also conducted extensive research on the comparative effectiveness of these chemicals in different pool types, finding that sodium percarbonate is particularly effective in saltwater pools, reducing salt system maintenance by up to 30%[3]. Their studies also show that their calcium hypochlorite solution maintains a more stable pH level, requiring up to 40% less pH adjustment chemicals over a swimming season[4].
Strengths: Long-lasting oxygen release for sodium percarbonate, reduced scaling for calcium hypochlorite, effective solutions for different pool types. Weaknesses: May require specific water balance conditions for optimal performance, potentially higher initial cost.
Technical Innovations
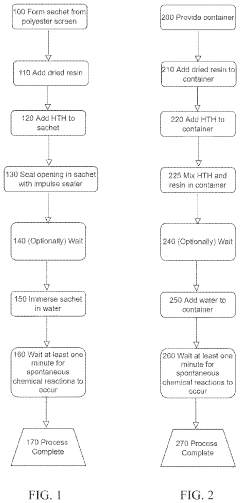
Coated sodium percarbonate particle
PatentActiveEP1889901A1
Innovation
- Sodium percarbonate particles with a shell layer comprising 70 to 99.8% anhydrous sodium sulfate and 0.2 to 20% sodium borate, providing improved stabilization and reducing boron content, along with a core composed of sodium carbonate perhydrate and optional stabilizing additives like alkali metal silicates, enhance storage stability.
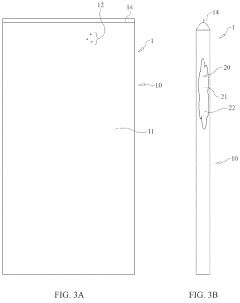
Compositions, Processes and Systems to Produce Hypochlorous Acid
PatentPendingUS20230391620A1
Innovation
- A composition of shelf-stable alkali metal or alkaline earth hypochlorite salts combined with acid form cation exchange resins, which are mixed with water at the point of use to produce hypochlorous acid solutions with controlled pH and reduced dissolved solids, using a modular system that allows for on-demand generation of hypochlorous acid.
Environmental Impact
The environmental impact of pool maintenance chemicals is a critical consideration in the comparison between sodium percarbonate and calcium hypochlorite. Both chemicals are widely used for pool sanitation, but their effects on the environment differ significantly.
Sodium percarbonate, also known as oxygen bleach, is generally considered more environmentally friendly. When dissolved in water, it breaks down into hydrogen peroxide and sodium carbonate, both of which are relatively harmless to aquatic ecosystems. Hydrogen peroxide decomposes into water and oxygen, leaving no harmful residues. Sodium carbonate, while it can temporarily increase water pH, is not toxic to aquatic life in the concentrations typically used for pool maintenance.
In contrast, calcium hypochlorite has a more substantial environmental footprint. It releases chlorine into the water, which can form harmful byproducts such as chloramines and trihalomethanes. These compounds can be toxic to aquatic organisms and may persist in the environment for extended periods. Additionally, the production of calcium hypochlorite often involves energy-intensive processes and the use of hazardous materials, contributing to its overall environmental impact.
The disposal of pool water treated with these chemicals also presents different environmental challenges. Water treated with sodium percarbonate can often be safely used for irrigation or released into the environment with minimal impact. However, water treated with calcium hypochlorite may require dechlorination before disposal to prevent harm to plants and aquatic life.
In terms of long-term environmental effects, sodium percarbonate has a lower potential for accumulation in ecosystems. Its breakdown products are naturally occurring and easily assimilated by the environment. Calcium hypochlorite, on the other hand, can contribute to the buildup of chlorine compounds in water bodies, potentially affecting the balance of aquatic ecosystems over time.
Energy consumption and carbon footprint are also important factors to consider. The production of sodium percarbonate is generally less energy-intensive compared to calcium hypochlorite, resulting in a lower carbon footprint. This aspect becomes particularly significant when considering the large-scale use of these chemicals in commercial and public pools.
While both chemicals have their place in pool maintenance, the growing emphasis on environmental sustainability is shifting preferences towards more eco-friendly options like sodium percarbonate. However, it's important to note that the overall environmental impact also depends on factors such as proper usage, dosage, and water management practices in pool maintenance.
Sodium percarbonate, also known as oxygen bleach, is generally considered more environmentally friendly. When dissolved in water, it breaks down into hydrogen peroxide and sodium carbonate, both of which are relatively harmless to aquatic ecosystems. Hydrogen peroxide decomposes into water and oxygen, leaving no harmful residues. Sodium carbonate, while it can temporarily increase water pH, is not toxic to aquatic life in the concentrations typically used for pool maintenance.
In contrast, calcium hypochlorite has a more substantial environmental footprint. It releases chlorine into the water, which can form harmful byproducts such as chloramines and trihalomethanes. These compounds can be toxic to aquatic organisms and may persist in the environment for extended periods. Additionally, the production of calcium hypochlorite often involves energy-intensive processes and the use of hazardous materials, contributing to its overall environmental impact.
The disposal of pool water treated with these chemicals also presents different environmental challenges. Water treated with sodium percarbonate can often be safely used for irrigation or released into the environment with minimal impact. However, water treated with calcium hypochlorite may require dechlorination before disposal to prevent harm to plants and aquatic life.
In terms of long-term environmental effects, sodium percarbonate has a lower potential for accumulation in ecosystems. Its breakdown products are naturally occurring and easily assimilated by the environment. Calcium hypochlorite, on the other hand, can contribute to the buildup of chlorine compounds in water bodies, potentially affecting the balance of aquatic ecosystems over time.
Energy consumption and carbon footprint are also important factors to consider. The production of sodium percarbonate is generally less energy-intensive compared to calcium hypochlorite, resulting in a lower carbon footprint. This aspect becomes particularly significant when considering the large-scale use of these chemicals in commercial and public pools.
While both chemicals have their place in pool maintenance, the growing emphasis on environmental sustainability is shifting preferences towards more eco-friendly options like sodium percarbonate. However, it's important to note that the overall environmental impact also depends on factors such as proper usage, dosage, and water management practices in pool maintenance.
Safety Regulations
Safety regulations play a crucial role in the use and handling of pool maintenance chemicals, particularly when comparing sodium percarbonate and calcium hypochlorite. These regulations are designed to protect both consumers and professionals from potential hazards associated with these chemicals.
For sodium percarbonate, safety regulations typically focus on its oxidizing properties and potential skin and eye irritation. Storage requirements often include keeping the product in a cool, dry place away from direct sunlight and incompatible materials. Handling guidelines usually emphasize the use of personal protective equipment (PPE) such as gloves, goggles, and dust masks when measuring and applying the product.
Calcium hypochlorite, being a more potent oxidizer, is subject to stricter safety regulations. It is classified as a hazardous material for transportation purposes, requiring special packaging and labeling. Storage regulations for calcium hypochlorite are more stringent, mandating segregation from other chemicals and flammable materials. Handling procedures often require more extensive PPE, including chemical-resistant clothing and respiratory protection in certain situations.
Both chemicals are subject to regulations regarding proper disposal. Used containers and unused product must be disposed of in accordance with local, state, and federal guidelines to prevent environmental contamination. Many jurisdictions require special handling and disposal procedures for these chemicals, often involving neutralization before disposal.
Safety data sheets (SDS) for both sodium percarbonate and calcium hypochlorite must be readily available at pool maintenance facilities and retail locations. These documents provide detailed information on hazards, handling procedures, and emergency measures. Employers are typically required to train staff on the proper use and handling of these chemicals, as well as emergency response procedures in case of spills or exposure.
Water quality regulations also impact the use of these chemicals in pool maintenance. Many jurisdictions set limits on the concentration of chlorine and other disinfectants in pool water to ensure swimmer safety. Regular testing and documentation of water chemistry are often mandated by local health departments.
In recent years, there has been a trend towards more environmentally friendly pool maintenance practices. This has led to some jurisdictions implementing regulations that favor the use of less aggressive chemicals like sodium percarbonate over traditional chlorine-based products like calcium hypochlorite. However, these regulations vary widely by region and are still evolving.
Overall, while both sodium percarbonate and calcium hypochlorite are subject to safety regulations, the more hazardous nature of calcium hypochlorite generally results in more stringent requirements for its use, storage, and handling in pool maintenance applications. Pool operators and homeowners must stay informed about local regulations and best practices to ensure compliance and maintain a safe swimming environment.
For sodium percarbonate, safety regulations typically focus on its oxidizing properties and potential skin and eye irritation. Storage requirements often include keeping the product in a cool, dry place away from direct sunlight and incompatible materials. Handling guidelines usually emphasize the use of personal protective equipment (PPE) such as gloves, goggles, and dust masks when measuring and applying the product.
Calcium hypochlorite, being a more potent oxidizer, is subject to stricter safety regulations. It is classified as a hazardous material for transportation purposes, requiring special packaging and labeling. Storage regulations for calcium hypochlorite are more stringent, mandating segregation from other chemicals and flammable materials. Handling procedures often require more extensive PPE, including chemical-resistant clothing and respiratory protection in certain situations.
Both chemicals are subject to regulations regarding proper disposal. Used containers and unused product must be disposed of in accordance with local, state, and federal guidelines to prevent environmental contamination. Many jurisdictions require special handling and disposal procedures for these chemicals, often involving neutralization before disposal.
Safety data sheets (SDS) for both sodium percarbonate and calcium hypochlorite must be readily available at pool maintenance facilities and retail locations. These documents provide detailed information on hazards, handling procedures, and emergency measures. Employers are typically required to train staff on the proper use and handling of these chemicals, as well as emergency response procedures in case of spills or exposure.
Water quality regulations also impact the use of these chemicals in pool maintenance. Many jurisdictions set limits on the concentration of chlorine and other disinfectants in pool water to ensure swimmer safety. Regular testing and documentation of water chemistry are often mandated by local health departments.
In recent years, there has been a trend towards more environmentally friendly pool maintenance practices. This has led to some jurisdictions implementing regulations that favor the use of less aggressive chemicals like sodium percarbonate over traditional chlorine-based products like calcium hypochlorite. However, these regulations vary widely by region and are still evolving.
Overall, while both sodium percarbonate and calcium hypochlorite are subject to safety regulations, the more hazardous nature of calcium hypochlorite generally results in more stringent requirements for its use, storage, and handling in pool maintenance applications. Pool operators and homeowners must stay informed about local regulations and best practices to ensure compliance and maintain a safe swimming environment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!