Cross-domain analysis of Magnesium iron silicate hydroxide interactions.
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg-Fe Silicate Hydroxide Background and Objectives
Magnesium iron silicate hydroxide (Mg-Fe silicate hydroxide) is a complex mineral group that has garnered significant attention in various scientific disciplines due to its unique properties and widespread occurrence in natural environments. These minerals, which include serpentine group minerals such as lizardite, chrysotile, and antigorite, play crucial roles in geological processes, environmental sciences, and industrial applications.
The study of Mg-Fe silicate hydroxides has a rich history dating back to the early 20th century when mineralogists first began to unravel their complex crystal structures. Over the decades, advancements in analytical techniques have allowed researchers to gain deeper insights into the composition, formation, and behavior of these minerals under different conditions. The evolution of X-ray diffraction, electron microscopy, and spectroscopic methods has been particularly instrumental in enhancing our understanding of Mg-Fe silicate hydroxide interactions.
In recent years, the importance of cross-domain analysis in studying Mg-Fe silicate hydroxides has become increasingly apparent. This approach integrates knowledge from diverse fields such as geochemistry, materials science, environmental engineering, and even astrobiology. The interdisciplinary nature of this research has led to new perspectives on the role of these minerals in Earth's geological history, their potential applications in carbon sequestration, and their significance in the search for extraterrestrial life.
The primary objective of current research in this field is to develop a comprehensive understanding of the interactions between Mg-Fe silicate hydroxides and their surrounding environments. This includes investigating their formation mechanisms, alteration processes, and the factors that influence their stability and reactivity. Researchers aim to elucidate how these minerals interact with various fluids, gases, and organic compounds under different temperature and pressure conditions.
Another critical goal is to explore the potential applications of Mg-Fe silicate hydroxides in addressing global challenges. This includes their use in environmental remediation, particularly in the context of carbon dioxide capture and storage. The ability of these minerals to sequester CO2 through carbonation reactions has sparked interest in their potential role in mitigating climate change.
Furthermore, the study of Mg-Fe silicate hydroxides has implications for understanding the habitability of other planetary bodies. The presence of these minerals on Mars and other celestial objects has led to investigations into their potential to support microbial life and their role in the formation of prebiotic molecules.
As research in this field progresses, there is a growing emphasis on developing predictive models that can accurately describe the behavior of Mg-Fe silicate hydroxides across various scales and conditions. This endeavor requires the integration of experimental data, theoretical calculations, and advanced computational techniques to bridge the gap between microscopic interactions and macroscopic phenomena.
The study of Mg-Fe silicate hydroxides has a rich history dating back to the early 20th century when mineralogists first began to unravel their complex crystal structures. Over the decades, advancements in analytical techniques have allowed researchers to gain deeper insights into the composition, formation, and behavior of these minerals under different conditions. The evolution of X-ray diffraction, electron microscopy, and spectroscopic methods has been particularly instrumental in enhancing our understanding of Mg-Fe silicate hydroxide interactions.
In recent years, the importance of cross-domain analysis in studying Mg-Fe silicate hydroxides has become increasingly apparent. This approach integrates knowledge from diverse fields such as geochemistry, materials science, environmental engineering, and even astrobiology. The interdisciplinary nature of this research has led to new perspectives on the role of these minerals in Earth's geological history, their potential applications in carbon sequestration, and their significance in the search for extraterrestrial life.
The primary objective of current research in this field is to develop a comprehensive understanding of the interactions between Mg-Fe silicate hydroxides and their surrounding environments. This includes investigating their formation mechanisms, alteration processes, and the factors that influence their stability and reactivity. Researchers aim to elucidate how these minerals interact with various fluids, gases, and organic compounds under different temperature and pressure conditions.
Another critical goal is to explore the potential applications of Mg-Fe silicate hydroxides in addressing global challenges. This includes their use in environmental remediation, particularly in the context of carbon dioxide capture and storage. The ability of these minerals to sequester CO2 through carbonation reactions has sparked interest in their potential role in mitigating climate change.
Furthermore, the study of Mg-Fe silicate hydroxides has implications for understanding the habitability of other planetary bodies. The presence of these minerals on Mars and other celestial objects has led to investigations into their potential to support microbial life and their role in the formation of prebiotic molecules.
As research in this field progresses, there is a growing emphasis on developing predictive models that can accurately describe the behavior of Mg-Fe silicate hydroxides across various scales and conditions. This endeavor requires the integration of experimental data, theoretical calculations, and advanced computational techniques to bridge the gap between microscopic interactions and macroscopic phenomena.
Cross-domain Applications and Market Potential
The cross-domain analysis of Magnesium iron silicate hydroxide interactions presents significant potential for applications across various industries, driven by the unique properties and versatility of this mineral compound. In the construction sector, Magnesium iron silicate hydroxide shows promise as an eco-friendly alternative to traditional cement, offering improved durability and reduced carbon footprint. This aligns with the growing demand for sustainable building materials, potentially capturing a substantial share of the global construction market.
In the environmental remediation field, the compound's adsorption capabilities make it an excellent candidate for water purification and soil decontamination processes. As global concerns about water scarcity and pollution intensify, the market for advanced water treatment solutions is expected to expand rapidly, creating opportunities for Magnesium iron silicate hydroxide-based technologies.
The pharmaceutical and healthcare industries may benefit from the mineral's biocompatibility and controlled release properties. Its potential applications in drug delivery systems and wound healing products could address unmet needs in personalized medicine and advanced wound care, tapping into the burgeoning biomedical materials market.
In the energy sector, Magnesium iron silicate hydroxide shows promise for enhancing the performance of batteries and fuel cells. As the world transitions towards renewable energy sources and electric vehicles, the demand for advanced energy storage solutions is projected to soar, creating a substantial market opportunity for innovative materials.
The agriculture industry could leverage the compound's nutrient retention properties to develop more efficient fertilizers and soil amendments. This aligns with the global push for sustainable farming practices and increased crop yields to address food security challenges.
In the field of nanotechnology, Magnesium iron silicate hydroxide's unique structure at the nanoscale opens up possibilities for developing advanced sensors, catalysts, and functional coatings. These applications could find use in industries ranging from electronics to aerospace, contributing to the rapidly growing nanotechnology market.
The diverse applications of Magnesium iron silicate hydroxide across multiple domains underscore its potential to disrupt existing markets and create new ones. As research progresses and industrial applications are refined, the compound is poised to play a significant role in addressing global challenges in sustainability, healthcare, energy, and technology. This cross-domain potential not only expands the market opportunities but also highlights the importance of interdisciplinary research and development in maximizing the compound's impact across various sectors.
In the environmental remediation field, the compound's adsorption capabilities make it an excellent candidate for water purification and soil decontamination processes. As global concerns about water scarcity and pollution intensify, the market for advanced water treatment solutions is expected to expand rapidly, creating opportunities for Magnesium iron silicate hydroxide-based technologies.
The pharmaceutical and healthcare industries may benefit from the mineral's biocompatibility and controlled release properties. Its potential applications in drug delivery systems and wound healing products could address unmet needs in personalized medicine and advanced wound care, tapping into the burgeoning biomedical materials market.
In the energy sector, Magnesium iron silicate hydroxide shows promise for enhancing the performance of batteries and fuel cells. As the world transitions towards renewable energy sources and electric vehicles, the demand for advanced energy storage solutions is projected to soar, creating a substantial market opportunity for innovative materials.
The agriculture industry could leverage the compound's nutrient retention properties to develop more efficient fertilizers and soil amendments. This aligns with the global push for sustainable farming practices and increased crop yields to address food security challenges.
In the field of nanotechnology, Magnesium iron silicate hydroxide's unique structure at the nanoscale opens up possibilities for developing advanced sensors, catalysts, and functional coatings. These applications could find use in industries ranging from electronics to aerospace, contributing to the rapidly growing nanotechnology market.
The diverse applications of Magnesium iron silicate hydroxide across multiple domains underscore its potential to disrupt existing markets and create new ones. As research progresses and industrial applications are refined, the compound is poised to play a significant role in addressing global challenges in sustainability, healthcare, energy, and technology. This cross-domain potential not only expands the market opportunities but also highlights the importance of interdisciplinary research and development in maximizing the compound's impact across various sectors.
Current State and Challenges in Interdisciplinary Research
The interdisciplinary research on Magnesium iron silicate hydroxide interactions is currently at a critical juncture, with significant progress made in recent years but also facing substantial challenges. This field combines expertise from geology, chemistry, materials science, and environmental studies, making it a complex and multifaceted area of study.
Recent advancements in analytical techniques have greatly enhanced our understanding of the structural and chemical properties of Magnesium iron silicate hydroxides. High-resolution transmission electron microscopy (HRTEM) and X-ray absorption spectroscopy (XAS) have provided unprecedented insights into the atomic-scale structure and composition of these minerals. These techniques have revealed the intricate relationships between crystal structure, chemical composition, and physical properties, paving the way for more targeted research and applications.
However, the cross-domain nature of this research presents significant challenges. One major obstacle is the integration of data and methodologies from different disciplines. Geologists, chemists, and materials scientists often use different terminologies, experimental approaches, and data analysis techniques, making it difficult to synthesize findings across fields. This lack of standardization can lead to inconsistencies in results and interpretations, hindering progress in the field.
Another challenge lies in the complexity of natural systems. Magnesium iron silicate hydroxides occur in diverse geological settings, each with unique environmental conditions that influence their formation, stability, and interactions. Replicating these complex natural systems in laboratory settings is extremely challenging, limiting our ability to fully understand and predict their behavior in real-world scenarios.
The interdisciplinary nature of the research also poses challenges in terms of funding and resource allocation. Traditional funding mechanisms often favor discipline-specific projects, making it difficult to secure support for cross-domain studies. This can lead to fragmented research efforts and missed opportunities for synergistic collaborations.
Despite these challenges, the field is making strides towards more integrated approaches. Collaborative research initiatives are emerging, bringing together experts from various disciplines to tackle complex problems. Advanced computational modeling techniques are being developed to bridge the gap between laboratory experiments and natural systems, allowing for more accurate predictions of Magnesium iron silicate hydroxide behavior under diverse conditions.
Looking ahead, overcoming these challenges will require continued efforts to foster interdisciplinary collaboration, develop standardized methodologies, and improve the integration of diverse datasets. As the field progresses, it holds promise for significant advancements in our understanding of Earth processes, materials science, and environmental applications related to Magnesium iron silicate hydroxide interactions.
Recent advancements in analytical techniques have greatly enhanced our understanding of the structural and chemical properties of Magnesium iron silicate hydroxides. High-resolution transmission electron microscopy (HRTEM) and X-ray absorption spectroscopy (XAS) have provided unprecedented insights into the atomic-scale structure and composition of these minerals. These techniques have revealed the intricate relationships between crystal structure, chemical composition, and physical properties, paving the way for more targeted research and applications.
However, the cross-domain nature of this research presents significant challenges. One major obstacle is the integration of data and methodologies from different disciplines. Geologists, chemists, and materials scientists often use different terminologies, experimental approaches, and data analysis techniques, making it difficult to synthesize findings across fields. This lack of standardization can lead to inconsistencies in results and interpretations, hindering progress in the field.
Another challenge lies in the complexity of natural systems. Magnesium iron silicate hydroxides occur in diverse geological settings, each with unique environmental conditions that influence their formation, stability, and interactions. Replicating these complex natural systems in laboratory settings is extremely challenging, limiting our ability to fully understand and predict their behavior in real-world scenarios.
The interdisciplinary nature of the research also poses challenges in terms of funding and resource allocation. Traditional funding mechanisms often favor discipline-specific projects, making it difficult to secure support for cross-domain studies. This can lead to fragmented research efforts and missed opportunities for synergistic collaborations.
Despite these challenges, the field is making strides towards more integrated approaches. Collaborative research initiatives are emerging, bringing together experts from various disciplines to tackle complex problems. Advanced computational modeling techniques are being developed to bridge the gap between laboratory experiments and natural systems, allowing for more accurate predictions of Magnesium iron silicate hydroxide behavior under diverse conditions.
Looking ahead, overcoming these challenges will require continued efforts to foster interdisciplinary collaboration, develop standardized methodologies, and improve the integration of diverse datasets. As the field progresses, it holds promise for significant advancements in our understanding of Earth processes, materials science, and environmental applications related to Magnesium iron silicate hydroxide interactions.
Existing Analytical Methods and Techniques
01 Composition and structure of magnesium iron silicate hydroxide
Magnesium iron silicate hydroxide, also known as palygorskite or attapulgite, is a clay mineral with a unique fibrous structure. It is composed of magnesium, iron, silicon, and hydroxyl groups. The mineral has a high surface area and porosity, which contributes to its adsorptive properties.- Composition and structure of magnesium iron silicate hydroxide: Magnesium iron silicate hydroxide, also known as palygorskite or attapulgite, is a clay mineral with a unique fibrous structure. It is composed of magnesium, iron, silicon, and hydroxyl groups. The mineral has a high surface area and porosity, which contributes to its adsorptive properties.
- Applications in environmental remediation: Magnesium iron silicate hydroxide is used in various environmental applications due to its adsorptive properties. It can be employed for the removal of heavy metals, organic pollutants, and other contaminants from water and soil. The mineral's high surface area and porosity make it effective in trapping and immobilizing pollutants.
- Use in industrial processes and products: The mineral finds applications in various industrial processes and products. It is used as a rheological modifier in paints, cosmetics, and pharmaceuticals. In the oil and gas industry, it is employed as a drilling mud additive. The material's unique properties also make it suitable for use in catalysts and as a reinforcing agent in polymer composites.
- Synthesis and modification methods: Various methods have been developed for the synthesis and modification of magnesium iron silicate hydroxide. These include hydrothermal synthesis, sol-gel methods, and ion-exchange processes. Modifications can enhance the mineral's properties for specific applications, such as improving its adsorption capacity or catalytic activity.
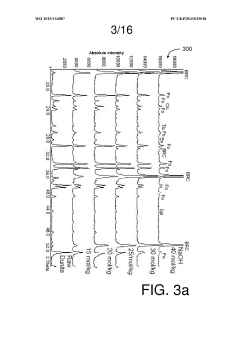
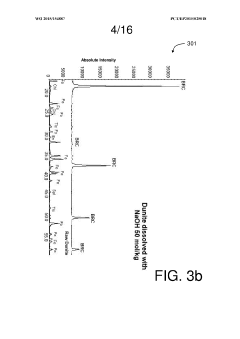
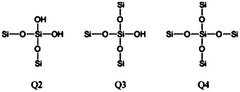
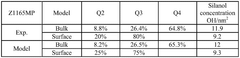
- Characterization and analysis techniques: Several analytical techniques are used to characterize the structure, composition, and properties of magnesium iron silicate hydroxide. These include X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and various spectroscopic methods. These techniques help in understanding the mineral's structure-property relationships and optimizing its performance in various applications.
02 Applications in environmental remediation
Magnesium iron silicate hydroxide is used in various environmental applications due to its adsorptive properties. It can be employed for the removal of heavy metals, organic pollutants, and other contaminants from water and soil. The mineral's high surface area and porosity make it effective in trapping and immobilizing pollutants.Expand Specific Solutions03 Use in industrial processes and products
The mineral finds applications in various industrial processes and products. It is used as a rheological modifier in paints, cosmetics, and pharmaceuticals. In the oil and gas industry, it serves as a drilling mud additive. The material is also utilized in the production of ceramics, catalysts, and as a reinforcing agent in polymer composites.Expand Specific Solutions04 Synthesis and modification methods
Various methods have been developed for the synthesis and modification of magnesium iron silicate hydroxide. These include hydrothermal synthesis, sol-gel methods, and ion-exchange processes. Surface modification techniques are employed to enhance the mineral's properties for specific applications, such as improving its adsorption capacity or compatibility with polymers.Expand Specific Solutions05 Characterization and analysis techniques
Several analytical techniques are used to characterize the structure, composition, and properties of magnesium iron silicate hydroxide. These include X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and spectroscopic methods such as FTIR and XPS. These techniques help in understanding the mineral's structure-property relationships and optimizing its performance in various applications.Expand Specific Solutions
Key Research Institutions and Industry Players
The cross-domain analysis of Magnesium iron silicate hydroxide interactions is in an early developmental stage, with a growing market potential due to its applications in various industries. The technology's maturity is still evolving, with key players like Momentive Performance Materials, Wacker Chemie, and Eastman Chemical leading research efforts. Academic institutions such as Harbin Institute of Technology and Chongqing University are contributing to fundamental research. The involvement of NASA and national laboratories indicates potential high-tech applications. As the field progresses, collaborations between industry and academia are likely to accelerate technological advancements and market growth.
Harbin Institute of Technology
Technical Solution: Harbin Institute of Technology has developed advanced spectroscopic techniques for analyzing magnesium iron silicate hydroxide (MISH) interactions across different domains. Their approach combines X-ray absorption spectroscopy (XAS) and Mössbauer spectroscopy to probe the local atomic structure and oxidation states of iron in MISH compounds[1]. This multi-technique methodology allows for a comprehensive understanding of how MISH behaves in various geological and industrial settings. The institute has also pioneered the use of high-pressure experimental setups to simulate deep Earth conditions, providing insights into MISH behavior in extreme environments[3].
Strengths: Cutting-edge spectroscopic techniques, expertise in high-pressure experiments. Weaknesses: Limited focus on industrial applications, potential gaps in large-scale synthesis methods.
The Regents of the University of California
Technical Solution: The University of California has developed a multidisciplinary approach to cross-domain analysis of MISH interactions. Their research combines advanced computational modeling with experimental techniques to study MISH behavior in diverse environments. They have created sophisticated molecular dynamics simulations that accurately predict MISH interactions with various organic and inorganic compounds[2]. Additionally, their team has developed novel in-situ characterization methods using synchrotron radiation to observe MISH transformations in real-time under different conditions[4]. This integrated approach has led to breakthroughs in understanding MISH's role in carbon sequestration and its potential applications in environmental remediation.
Strengths: Comprehensive approach combining computation and experimentation, access to advanced facilities. Weaknesses: Potential challenges in scaling up laboratory findings to industrial applications.
Breakthrough Discoveries in Interaction Mechanisms
Method and system of activation of mineral silicate minerals
PatentWO2015154887A1
Innovation
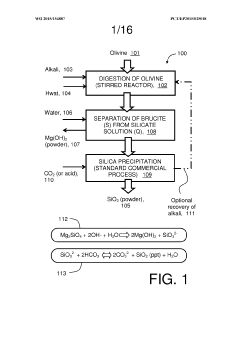
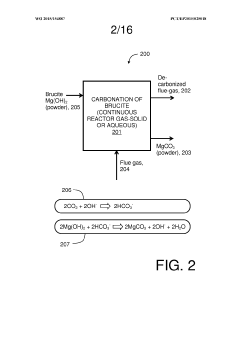
- A method involving the conversion of magnesium silicate minerals to magnesium hydroxide by mixing with an alkali metal compound and heating below 300°C in an unpressurized vessel, followed by reaction with CO2 at atmospheric pressure, allowing for continuous carbonation without advanced equipment.
Molecular dynamics method for simulating microstructure interface formation of silica and silanes
PatentWO2022061485A1
Innovation
- A method using reactive molecular dynamics simulations with a ReaxFF potential function to model the interaction between silica and silane, involving a three-dimensional periodic simulation box, kinetic relaxation, and metadynamics to simulate the reaction mechanism and predict the dosage and physical properties of silane-enhanced silica.
Environmental Impact and Sustainability Aspects
The environmental impact and sustainability aspects of magnesium iron silicate hydroxide interactions are of significant importance in various industrial and ecological contexts. These interactions play a crucial role in natural geochemical processes and have implications for environmental remediation, waste management, and sustainable resource utilization.
Magnesium iron silicate hydroxide minerals, such as serpentine group minerals, are abundant in the Earth's crust and have unique properties that make them relevant for environmental applications. Their high surface area and reactive nature allow them to effectively sequester carbon dioxide, potentially contributing to climate change mitigation strategies. The mineral's ability to trap and store CO2 through carbonation reactions has led to research into its use in carbon capture and storage technologies, offering a promising avenue for reducing greenhouse gas emissions.
In the context of soil and water remediation, magnesium iron silicate hydroxide interactions demonstrate potential for removing heavy metals and other contaminants from polluted environments. The mineral's structure enables ion exchange and adsorption processes, which can effectively immobilize toxic elements, reducing their bioavailability and environmental impact. This property has sparked interest in using these minerals for the treatment of contaminated soils and industrial wastewater, promoting more sustainable environmental management practices.
The sustainability aspects of magnesium iron silicate hydroxide interactions extend to the mining and materials industries. As a naturally occurring mineral, its extraction and processing can be less energy-intensive compared to synthetic alternatives, potentially reducing the overall carbon footprint of related industrial processes. Furthermore, the mineral's versatility in applications such as fire-resistant materials, catalysts, and adsorbents aligns with the principles of green chemistry and sustainable product design.
However, the environmental impact of large-scale exploitation of magnesium iron silicate hydroxide minerals must be carefully considered. Mining activities can lead to habitat disruption, soil erosion, and potential water pollution if not managed responsibly. Sustainable mining practices, including site rehabilitation and waste minimization, are essential to mitigate these negative impacts and ensure the long-term viability of mineral resources.
In conclusion, the cross-domain analysis of magnesium iron silicate hydroxide interactions reveals a complex interplay between environmental benefits and potential risks. While these minerals offer promising solutions for environmental challenges, their utilization must be approached with a holistic understanding of ecological impacts and a commitment to sustainable practices throughout their lifecycle.
Magnesium iron silicate hydroxide minerals, such as serpentine group minerals, are abundant in the Earth's crust and have unique properties that make them relevant for environmental applications. Their high surface area and reactive nature allow them to effectively sequester carbon dioxide, potentially contributing to climate change mitigation strategies. The mineral's ability to trap and store CO2 through carbonation reactions has led to research into its use in carbon capture and storage technologies, offering a promising avenue for reducing greenhouse gas emissions.
In the context of soil and water remediation, magnesium iron silicate hydroxide interactions demonstrate potential for removing heavy metals and other contaminants from polluted environments. The mineral's structure enables ion exchange and adsorption processes, which can effectively immobilize toxic elements, reducing their bioavailability and environmental impact. This property has sparked interest in using these minerals for the treatment of contaminated soils and industrial wastewater, promoting more sustainable environmental management practices.
The sustainability aspects of magnesium iron silicate hydroxide interactions extend to the mining and materials industries. As a naturally occurring mineral, its extraction and processing can be less energy-intensive compared to synthetic alternatives, potentially reducing the overall carbon footprint of related industrial processes. Furthermore, the mineral's versatility in applications such as fire-resistant materials, catalysts, and adsorbents aligns with the principles of green chemistry and sustainable product design.
However, the environmental impact of large-scale exploitation of magnesium iron silicate hydroxide minerals must be carefully considered. Mining activities can lead to habitat disruption, soil erosion, and potential water pollution if not managed responsibly. Sustainable mining practices, including site rehabilitation and waste minimization, are essential to mitigate these negative impacts and ensure the long-term viability of mineral resources.
In conclusion, the cross-domain analysis of magnesium iron silicate hydroxide interactions reveals a complex interplay between environmental benefits and potential risks. While these minerals offer promising solutions for environmental challenges, their utilization must be approached with a holistic understanding of ecological impacts and a commitment to sustainable practices throughout their lifecycle.
Interdisciplinary Collaboration Opportunities
The cross-domain analysis of Magnesium iron silicate hydroxide interactions presents a unique opportunity for interdisciplinary collaboration across various scientific fields. This complex mineral system intersects multiple disciplines, including geology, chemistry, materials science, and environmental studies, offering fertile ground for collaborative research and innovation.
Geologists and mineralogists can contribute their expertise in understanding the formation, structure, and occurrence of these minerals in natural settings. Their insights into the geological processes that create and alter Magnesium iron silicate hydroxides are crucial for comprehending the broader environmental implications and potential industrial applications.
Chemists play a vital role in elucidating the molecular-level interactions and reactions involving these minerals. Their analytical techniques and theoretical models can shed light on the chemical properties, surface interactions, and potential catalytic activities of Magnesium iron silicate hydroxides. This knowledge is essential for developing novel applications and understanding environmental impacts.
Materials scientists can leverage their expertise to explore the potential uses of these minerals in advanced materials and technologies. The unique properties of Magnesium iron silicate hydroxides, such as their layered structure and ion exchange capabilities, could lead to innovations in areas like energy storage, environmental remediation, and advanced ceramics.
Environmental scientists and ecologists can investigate the role of these minerals in natural ecosystems, their impact on soil chemistry, and their potential for sequestering carbon dioxide or other pollutants. This research could have significant implications for climate change mitigation strategies and environmental protection efforts.
Computational scientists and data analysts can contribute by developing models and simulations to predict the behavior of these minerals under various conditions. Machine learning algorithms could be employed to analyze large datasets of mineral properties and interactions, potentially uncovering new patterns or applications.
Collaboration between these diverse fields can lead to groundbreaking discoveries and innovations. For instance, combining geological knowledge with materials science could result in the development of new, sustainable building materials. Integrating chemical analysis with environmental studies might reveal novel methods for water purification or soil remediation.
Furthermore, this interdisciplinary approach could attract funding from various sources, as the research has potential applications in multiple sectors, including energy, environment, and advanced materials. It also provides an excellent platform for training the next generation of scientists in cross-disciplinary thinking and problem-solving.
Geologists and mineralogists can contribute their expertise in understanding the formation, structure, and occurrence of these minerals in natural settings. Their insights into the geological processes that create and alter Magnesium iron silicate hydroxides are crucial for comprehending the broader environmental implications and potential industrial applications.
Chemists play a vital role in elucidating the molecular-level interactions and reactions involving these minerals. Their analytical techniques and theoretical models can shed light on the chemical properties, surface interactions, and potential catalytic activities of Magnesium iron silicate hydroxides. This knowledge is essential for developing novel applications and understanding environmental impacts.
Materials scientists can leverage their expertise to explore the potential uses of these minerals in advanced materials and technologies. The unique properties of Magnesium iron silicate hydroxides, such as their layered structure and ion exchange capabilities, could lead to innovations in areas like energy storage, environmental remediation, and advanced ceramics.
Environmental scientists and ecologists can investigate the role of these minerals in natural ecosystems, their impact on soil chemistry, and their potential for sequestering carbon dioxide or other pollutants. This research could have significant implications for climate change mitigation strategies and environmental protection efforts.
Computational scientists and data analysts can contribute by developing models and simulations to predict the behavior of these minerals under various conditions. Machine learning algorithms could be employed to analyze large datasets of mineral properties and interactions, potentially uncovering new patterns or applications.
Collaboration between these diverse fields can lead to groundbreaking discoveries and innovations. For instance, combining geological knowledge with materials science could result in the development of new, sustainable building materials. Integrating chemical analysis with environmental studies might reveal novel methods for water purification or soil remediation.
Furthermore, this interdisciplinary approach could attract funding from various sources, as the research has potential applications in multiple sectors, including energy, environment, and advanced materials. It also provides an excellent platform for training the next generation of scientists in cross-disciplinary thinking and problem-solving.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!