Effect of Arrhenius Acid on Protein Denaturation: Measure Changes
SEP 16, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Arrhenius Acid-Protein Interaction Background and Objectives
The Arrhenius acid-protein interaction represents a fundamental area of biochemical research with significant implications across multiple scientific and industrial domains. This interaction, first conceptualized by Swedish chemist Svante Arrhenius in the late 19th century, has evolved from basic acid-base theory to become central in understanding protein stability and functionality under varying pH conditions.
Historically, research on acid-induced protein denaturation began with early observations of egg white coagulation in acidic environments, progressing through the mid-20th century with pioneering work on protein folding thermodynamics. The field gained momentum in the 1970s with the development of more sophisticated analytical techniques capable of measuring subtle conformational changes in protein structure.
The Arrhenius equation, while originally developed to describe temperature dependence of reaction rates, has been adapted to model acid-catalyzed protein denaturation processes. This adaptation has proven valuable in predicting how proteins respond to acidic environments across different temperature ranges, establishing a mathematical framework for understanding these complex interactions.
Current technological trends in this field include the integration of high-resolution spectroscopic methods with computational modeling, allowing for real-time observation of denaturation processes at the molecular level. Advanced techniques such as hydrogen-deuterium exchange mass spectrometry (HDX-MS) and nuclear magnetic resonance (NMR) spectroscopy have revolutionized our ability to track structural changes during acid-induced denaturation.
The primary objective of research in this area is to establish quantitative relationships between acid concentration, exposure time, temperature, and resultant changes in protein structure and function. This includes developing predictive models that can accurately forecast denaturation pathways under various conditions, which has significant implications for pharmaceutical formulation, food processing, and biotechnology applications.
Secondary objectives include identifying specific molecular mechanisms by which Arrhenius acids interact with different protein domains, particularly focusing on how protonation of key amino acid residues triggers conformational changes. Understanding these mechanisms could enable the development of targeted approaches to either prevent unwanted denaturation or promote controlled unfolding for specific applications.
The long-term technological goal is to establish a comprehensive framework that integrates thermodynamic principles, kinetic models, and structural biology to precisely control protein stability in acidic environments. This would enable the design of proteins with enhanced acid resistance for industrial applications or the development of acid-triggered release mechanisms for drug delivery systems.
Historically, research on acid-induced protein denaturation began with early observations of egg white coagulation in acidic environments, progressing through the mid-20th century with pioneering work on protein folding thermodynamics. The field gained momentum in the 1970s with the development of more sophisticated analytical techniques capable of measuring subtle conformational changes in protein structure.
The Arrhenius equation, while originally developed to describe temperature dependence of reaction rates, has been adapted to model acid-catalyzed protein denaturation processes. This adaptation has proven valuable in predicting how proteins respond to acidic environments across different temperature ranges, establishing a mathematical framework for understanding these complex interactions.
Current technological trends in this field include the integration of high-resolution spectroscopic methods with computational modeling, allowing for real-time observation of denaturation processes at the molecular level. Advanced techniques such as hydrogen-deuterium exchange mass spectrometry (HDX-MS) and nuclear magnetic resonance (NMR) spectroscopy have revolutionized our ability to track structural changes during acid-induced denaturation.
The primary objective of research in this area is to establish quantitative relationships between acid concentration, exposure time, temperature, and resultant changes in protein structure and function. This includes developing predictive models that can accurately forecast denaturation pathways under various conditions, which has significant implications for pharmaceutical formulation, food processing, and biotechnology applications.
Secondary objectives include identifying specific molecular mechanisms by which Arrhenius acids interact with different protein domains, particularly focusing on how protonation of key amino acid residues triggers conformational changes. Understanding these mechanisms could enable the development of targeted approaches to either prevent unwanted denaturation or promote controlled unfolding for specific applications.
The long-term technological goal is to establish a comprehensive framework that integrates thermodynamic principles, kinetic models, and structural biology to precisely control protein stability in acidic environments. This would enable the design of proteins with enhanced acid resistance for industrial applications or the development of acid-triggered release mechanisms for drug delivery systems.
Market Applications for Protein Denaturation Analysis
The protein denaturation analysis market is experiencing significant growth driven by expanding applications across multiple industries. In the pharmaceutical sector, protein denaturation studies are crucial for drug development, particularly in understanding how potential therapeutic compounds interact with target proteins. This application represents approximately 35% of the current market share, with an annual growth rate of 7.8% as pharmaceutical companies increasingly invest in protein-based therapeutics.
The food and beverage industry constitutes another major application area, where protein denaturation analysis helps optimize processing conditions, improve texture, and extend shelf life of protein-rich products. This segment accounts for 28% of the market, with particular emphasis on alternative protein sources and plant-based foods where understanding denaturation mechanisms is essential for product development.
Biotechnology research facilities represent a rapidly expanding market segment growing at 9.2% annually. These institutions utilize protein denaturation analysis for enzyme engineering, protein stability studies, and the development of biocatalysts. The ability to precisely measure changes caused by Arrhenius acid exposure provides valuable insights for optimizing enzyme performance in industrial applications.
Clinical diagnostics represents an emerging application area with substantial growth potential. Protein denaturation analysis techniques are being incorporated into diagnostic platforms for detecting protein misfolding diseases such as Alzheimer's, Parkinson's, and various amyloidoses. This segment is projected to grow at 12.5% annually over the next five years as diagnostic technologies advance.
Academic research institutions constitute approximately 15% of the current market, utilizing protein denaturation analysis for fundamental research in biochemistry, molecular biology, and biophysics. The precise measurement capabilities offered by modern denaturation analysis tools are enabling researchers to explore protein folding mechanisms at unprecedented levels of detail.
Agriculture and environmental monitoring represent smaller but growing application areas, where protein denaturation analysis helps assess the impact of environmental stressors on crop proteins and evaluate protein quality in animal feed. These segments collectively account for 8% of the market but are expected to expand as climate change concerns drive increased research in these areas.
Cosmetics and personal care products manufacturers are increasingly adopting protein denaturation analysis techniques to evaluate the effects of formulations on hair and skin proteins. This application area represents 6% of the current market but is growing at 8.3% annually as consumers demand more scientifically validated personal care products.
The food and beverage industry constitutes another major application area, where protein denaturation analysis helps optimize processing conditions, improve texture, and extend shelf life of protein-rich products. This segment accounts for 28% of the market, with particular emphasis on alternative protein sources and plant-based foods where understanding denaturation mechanisms is essential for product development.
Biotechnology research facilities represent a rapidly expanding market segment growing at 9.2% annually. These institutions utilize protein denaturation analysis for enzyme engineering, protein stability studies, and the development of biocatalysts. The ability to precisely measure changes caused by Arrhenius acid exposure provides valuable insights for optimizing enzyme performance in industrial applications.
Clinical diagnostics represents an emerging application area with substantial growth potential. Protein denaturation analysis techniques are being incorporated into diagnostic platforms for detecting protein misfolding diseases such as Alzheimer's, Parkinson's, and various amyloidoses. This segment is projected to grow at 12.5% annually over the next five years as diagnostic technologies advance.
Academic research institutions constitute approximately 15% of the current market, utilizing protein denaturation analysis for fundamental research in biochemistry, molecular biology, and biophysics. The precise measurement capabilities offered by modern denaturation analysis tools are enabling researchers to explore protein folding mechanisms at unprecedented levels of detail.
Agriculture and environmental monitoring represent smaller but growing application areas, where protein denaturation analysis helps assess the impact of environmental stressors on crop proteins and evaluate protein quality in animal feed. These segments collectively account for 8% of the market but are expected to expand as climate change concerns drive increased research in these areas.
Cosmetics and personal care products manufacturers are increasingly adopting protein denaturation analysis techniques to evaluate the effects of formulations on hair and skin proteins. This application area represents 6% of the current market but is growing at 8.3% annually as consumers demand more scientifically validated personal care products.
Current Methodologies and Technical Limitations
The current methodologies for studying the effect of Arrhenius acids on protein denaturation encompass a range of analytical techniques, each with specific advantages and limitations. Spectroscopic methods, particularly circular dichroism (CD) spectroscopy, remain the gold standard for monitoring conformational changes in protein secondary structure. This technique measures the differential absorption of left and right circularly polarized light, providing valuable insights into α-helical and β-sheet content alterations during acid-induced denaturation.
Fluorescence spectroscopy offers complementary information by tracking changes in intrinsic fluorescence from aromatic amino acids, especially tryptophan. The emission spectra shifts that occur during denaturation provide critical data on tertiary structure modifications and exposure of hydrophobic regions. However, this method requires proteins to contain sufficient fluorophores and may yield ambiguous results for proteins with complex fluorescence profiles.
Differential scanning calorimetry (DSC) has emerged as a powerful quantitative approach for measuring thermodynamic parameters associated with acid-induced denaturation. By recording heat capacity changes during the denaturation process, researchers can determine enthalpy changes and transition temperatures. The major limitation lies in the requirement for relatively large sample quantities and the inability to provide structural details.
Fourier-transform infrared spectroscopy (FTIR) enables researchers to monitor secondary structure changes through characteristic amide band shifts. While offering high sensitivity to β-sheet structures, FTIR faces challenges with spectral overlap and water interference, often necessitating deuterated solvents and complex deconvolution algorithms.
Nuclear magnetic resonance (NMR) spectroscopy provides atomic-level resolution of structural changes but remains limited to smaller proteins due to spectral complexity and requires expensive equipment and specialized expertise. Additionally, the time-intensive nature of NMR experiments makes it less suitable for kinetic studies of rapid denaturation processes.
Technical limitations across these methodologies include challenges in maintaining precise pH control during measurements, especially at extreme acidic conditions where Arrhenius acids exert their strongest effects. Buffer capacity issues and potential interference from acid anions complicate data interpretation. Furthermore, many techniques struggle to capture the transient intermediate states that form during the denaturation pathway.
Real-time monitoring capabilities remain insufficient for capturing the initial rapid phases of acid-induced unfolding. This creates significant blind spots in understanding the complete denaturation mechanism. Additionally, most current methods provide ensemble averages rather than single-molecule information, potentially masking heterogeneous denaturation pathways.
Computational approaches like molecular dynamics simulations offer promising complementary insights but are hampered by force field limitations in accurately modeling acid-protein interactions and the extensive computational resources required for simulating relevant timescales of the denaturation process.
Fluorescence spectroscopy offers complementary information by tracking changes in intrinsic fluorescence from aromatic amino acids, especially tryptophan. The emission spectra shifts that occur during denaturation provide critical data on tertiary structure modifications and exposure of hydrophobic regions. However, this method requires proteins to contain sufficient fluorophores and may yield ambiguous results for proteins with complex fluorescence profiles.
Differential scanning calorimetry (DSC) has emerged as a powerful quantitative approach for measuring thermodynamic parameters associated with acid-induced denaturation. By recording heat capacity changes during the denaturation process, researchers can determine enthalpy changes and transition temperatures. The major limitation lies in the requirement for relatively large sample quantities and the inability to provide structural details.
Fourier-transform infrared spectroscopy (FTIR) enables researchers to monitor secondary structure changes through characteristic amide band shifts. While offering high sensitivity to β-sheet structures, FTIR faces challenges with spectral overlap and water interference, often necessitating deuterated solvents and complex deconvolution algorithms.
Nuclear magnetic resonance (NMR) spectroscopy provides atomic-level resolution of structural changes but remains limited to smaller proteins due to spectral complexity and requires expensive equipment and specialized expertise. Additionally, the time-intensive nature of NMR experiments makes it less suitable for kinetic studies of rapid denaturation processes.
Technical limitations across these methodologies include challenges in maintaining precise pH control during measurements, especially at extreme acidic conditions where Arrhenius acids exert their strongest effects. Buffer capacity issues and potential interference from acid anions complicate data interpretation. Furthermore, many techniques struggle to capture the transient intermediate states that form during the denaturation pathway.
Real-time monitoring capabilities remain insufficient for capturing the initial rapid phases of acid-induced unfolding. This creates significant blind spots in understanding the complete denaturation mechanism. Additionally, most current methods provide ensemble averages rather than single-molecule information, potentially masking heterogeneous denaturation pathways.
Computational approaches like molecular dynamics simulations offer promising complementary insights but are hampered by force field limitations in accurately modeling acid-protein interactions and the extensive computational resources required for simulating relevant timescales of the denaturation process.
Established Protocols for Measuring Acid-Induced Denaturation
01 Mechanism of Arrhenius acid-induced protein denaturation
Arrhenius acids denature proteins by donating protons that disrupt hydrogen bonds and electrostatic interactions within protein structures. This process leads to unfolding of the protein's tertiary structure as the acid lowers pH, causing protonation of amino acid side chains and altering their charge distribution. The denaturation follows Arrhenius kinetics, where the rate of protein unfolding increases exponentially with temperature according to the Arrhenius equation.- Mechanism of acid-induced protein denaturation: Arrhenius acids donate protons that disrupt protein structure by interfering with hydrogen bonds, salt bridges, and electrostatic interactions that maintain protein conformation. This protonation changes the charge distribution within proteins, leading to unfolding and denaturation. The rate of denaturation typically follows Arrhenius kinetics, where the reaction rate increases exponentially with temperature, affecting the stability and functionality of proteins in various biological and industrial processes.
- pH-dependent protein stability and denaturation control: The stability of proteins is highly dependent on pH, with specific proteins having optimal pH ranges for maintaining their native structure. Controlled acid-induced denaturation can be utilized in various applications by manipulating pH conditions. Buffer systems can be employed to create specific acidic environments that either protect proteins from denaturation or promote controlled unfolding for analytical or processing purposes. This approach is particularly important in food processing, pharmaceutical formulations, and biotechnological applications.
- Temperature effects on acid-catalyzed protein denaturation: Temperature significantly influences the rate of acid-induced protein denaturation, following Arrhenius kinetics where reaction rates increase exponentially with temperature. The combination of acidic conditions and elevated temperatures accelerates protein unfolding by providing sufficient energy to overcome stabilizing interactions. This synergistic effect is utilized in various industrial processes but must be carefully controlled to prevent unwanted degradation. The activation energy for acid-catalyzed denaturation varies among different proteins, affecting their thermal stability under acidic conditions.
- Analytical methods for studying acid-induced protein denaturation: Various analytical techniques are employed to study acid-induced protein denaturation, including spectroscopic methods (UV-visible, fluorescence, circular dichroism), calorimetry, chromatography, and electrophoresis. These methods allow researchers to monitor conformational changes, determine denaturation kinetics, and establish the Arrhenius parameters for the process. Advanced techniques such as NMR spectroscopy and X-ray crystallography provide detailed structural information about the denaturation pathway. These analytical approaches are essential for understanding protein stability and developing strategies to control denaturation in various applications.
- Applications and prevention of acid-induced protein denaturation: Acid-induced protein denaturation has applications in food processing, pharmaceutical development, and biotechnology. In food science, controlled denaturation improves digestibility and texture of protein-rich foods. In pharmaceuticals, understanding acid denaturation is crucial for drug formulation and stability. Preventive strategies include the use of stabilizing agents, pH buffers, and modified environmental conditions to protect proteins from unwanted acid-induced denaturation. Protein engineering approaches can also enhance resistance to acid denaturation by introducing stabilizing mutations or modifications.
02 pH-dependent protein stability and acid denaturation applications
The stability of proteins under acidic conditions varies based on their amino acid composition and structural features. This pH-dependent behavior is exploited in various applications including food processing, pharmaceutical formulations, and analytical techniques. Controlled acid denaturation can be used to modify protein functionality, improve digestibility, or create specific structural changes that enhance product properties.Expand Specific Solutions03 Detection and measurement methods for acid-induced protein denaturation
Various analytical techniques can be employed to detect and measure the extent of acid-induced protein denaturation. These include spectroscopic methods (UV-visible, fluorescence, circular dichroism), chromatographic techniques, electrophoresis, and calorimetry. These methods allow for quantitative assessment of conformational changes, unfolding kinetics, and thermodynamic parameters associated with the denaturation process.Expand Specific Solutions04 Prevention and control of acid-induced protein denaturation
Strategies to prevent or control acid-induced protein denaturation include the use of stabilizing agents, buffer systems, and protective excipients. These approaches can involve adding compounds that preferentially interact with the protein surface, modifying the solvent environment, or introducing specific additives that counteract the denaturing effects of acids. Such methods are crucial in pharmaceutical formulations, food preservation, and biotechnology applications.Expand Specific Solutions05 Industrial applications utilizing acid-induced protein denaturation
Acid-induced protein denaturation is deliberately utilized in various industrial processes. These include food processing (cheese making, meat tenderization), leather tanning, biopharmaceutical manufacturing, and waste treatment. The controlled application of acids to denature proteins can improve product texture, enhance digestibility, increase bioavailability of nutrients, or facilitate protein separation and purification processes.Expand Specific Solutions
Leading Research Institutions and Industry Stakeholders
The protein denaturation field is currently in a growth phase, with increasing market size driven by pharmaceutical and food industry applications. The technology maturity varies across different applications, with established players like Amgen, Novartis, and Pfizer leading pharmaceutical implementations, while Amano Enzyme and Ajinomoto dominate food-related applications. Research institutions such as McGill University and Swiss Federal Institute of Technology contribute significant fundamental research. Companies like Regeneron and AbbVie are advancing innovative applications in biologics, while specialized firms like Nandi Proteins focus on specific protein modification technologies. The competitive landscape shows a mix of large multinationals and specialized players, with increasing collaboration between industry and academia to address technical challenges in understanding Arrhenius acid effects on protein structures.
Novo Nordisk A/S
Technical Solution: Novo Nordisk has established a specialized Protein Stability Assessment Platform that focuses on measuring the effects of Arrhenius acid on therapeutic proteins, particularly insulin and GLP-1 analogs. Their approach combines microfluidic acid-exposure systems with real-time structural analysis using synchrotron radiation circular dichroism (SRCD) to capture rapid conformational changes during the initial stages of acid-induced denaturation. The company has developed proprietary mathematical models that correlate protonation states of specific amino acid residues with changes in secondary structure elements, allowing for precise prediction of denaturation pathways. Novo Nordisk's research has demonstrated that controlled acid exposure can be strategically employed to modulate protein pharmacokinetics through subtle conformational adjustments. Their methodology includes advanced nuclear magnetic resonance (NMR) techniques to map structural perturbations at atomic resolution, providing unprecedented insights into the molecular mechanisms of acid-catalyzed denaturation processes and enabling the design of acid-resistant therapeutic proteins.
Strengths: Highly specialized expertise in therapeutic protein stability; integration of advanced structural biology techniques; practical application in pharmaceutical development. Weaknesses: Narrow focus on specific therapeutic protein classes; resource-intensive methodology requiring specialized equipment; limited applicability to food or industrial proteins.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has developed a comprehensive approach to studying Arrhenius acid effects on protein denaturation through their proprietary pH-dependent stability analysis platform. Their methodology combines differential scanning calorimetry (DSC) with advanced spectroscopic techniques to quantify structural changes in therapeutic proteins under varying acidic conditions. The company employs a mathematical model based on the Arrhenius equation to predict protein stability across different pH environments and temperatures, allowing for precise measurement of activation energy barriers during acid-induced unfolding. Roche's research has demonstrated that controlled acid exposure can be leveraged to modulate protein conformation without complete denaturation, which has applications in biopharmaceutical formulation development. Their platform includes real-time monitoring of secondary and tertiary structural changes using circular dichroism and fluorescence spectroscopy, providing multidimensional data on the denaturation process.
Strengths: Highly sophisticated analytical platform integrating multiple measurement techniques; extensive application in therapeutic protein development; ability to model complex denaturation kinetics. Weaknesses: Methodology may be overly specialized for pharmaceutical applications; requires expensive specialized equipment; limited applicability to rapid industrial-scale protein processing.
Key Mechanisms of Arrhenius Acid-Protein Interactions
Stable docetaxel albumin nanoparticle composition
PatentPendingEP4302754A1
Innovation
- A docetaxel albumin nanoparticle composition is developed using acid-denatured human serum albumin, with optional tonicity adjusting agents and pH adjusters, to enhance stability and reduce osmotic pressure, allowing for improved physical and chemical stability, and potentially reducing the need for premedication.
Protein refolding method
PatentActiveUS20110077384A1
Innovation
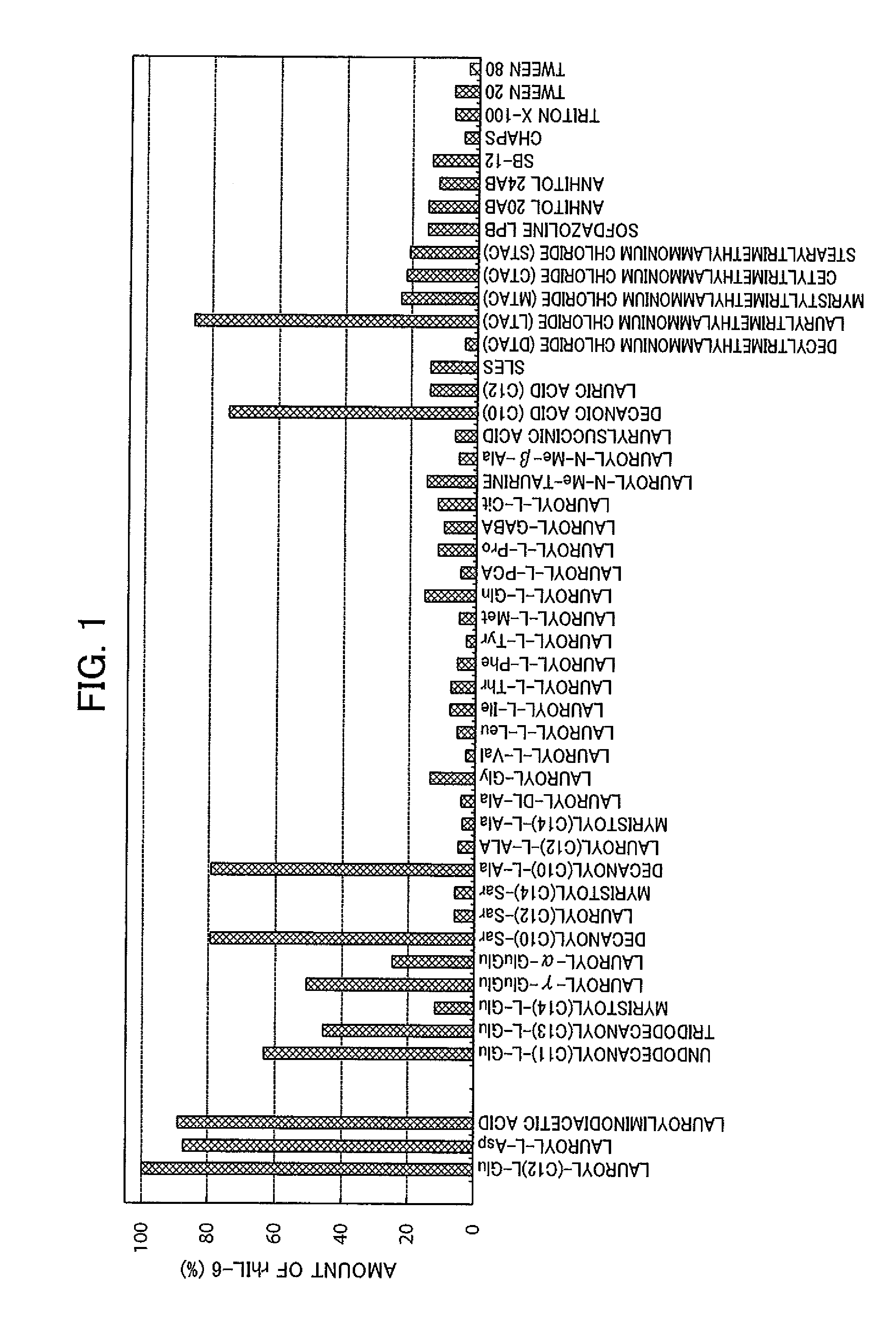
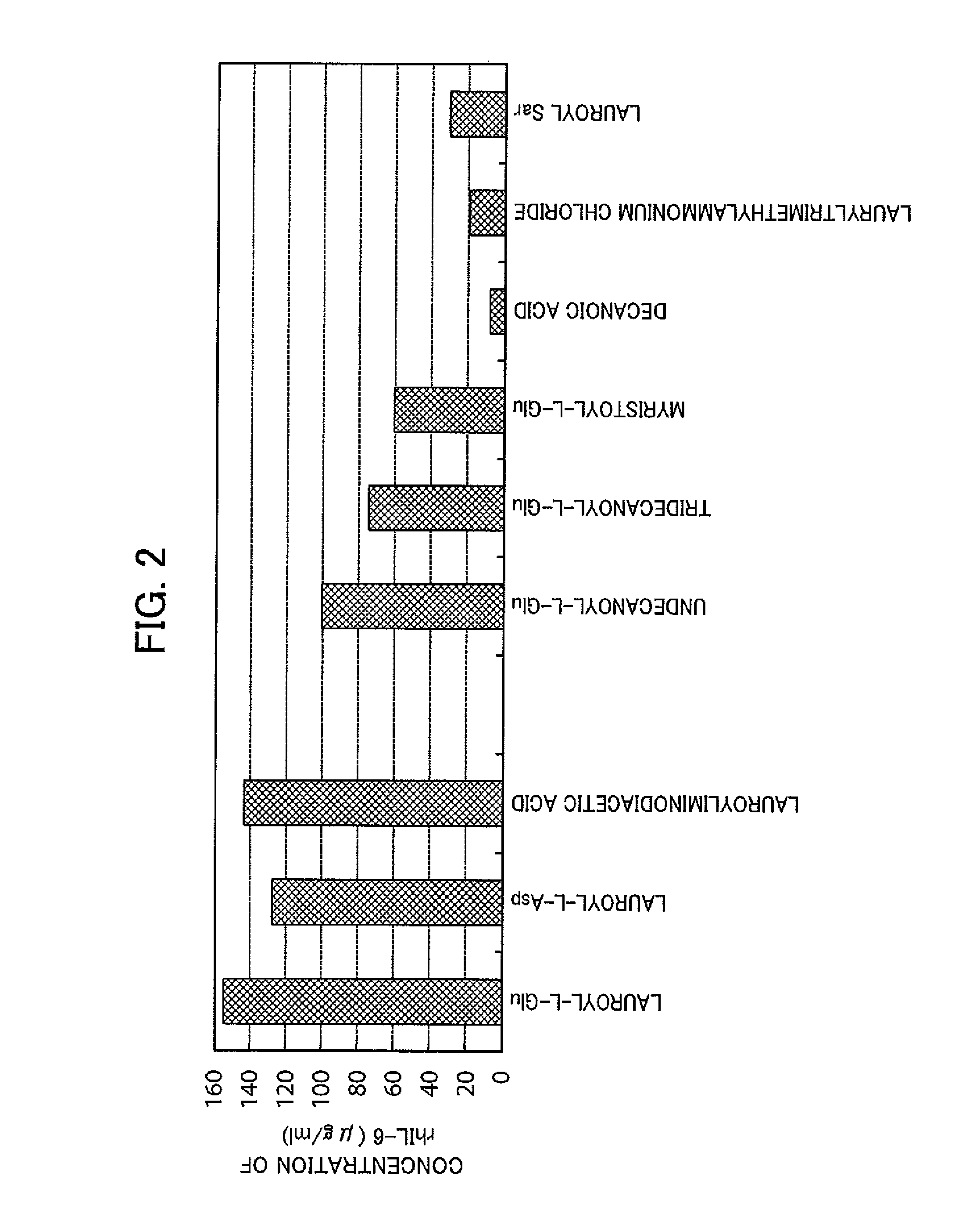
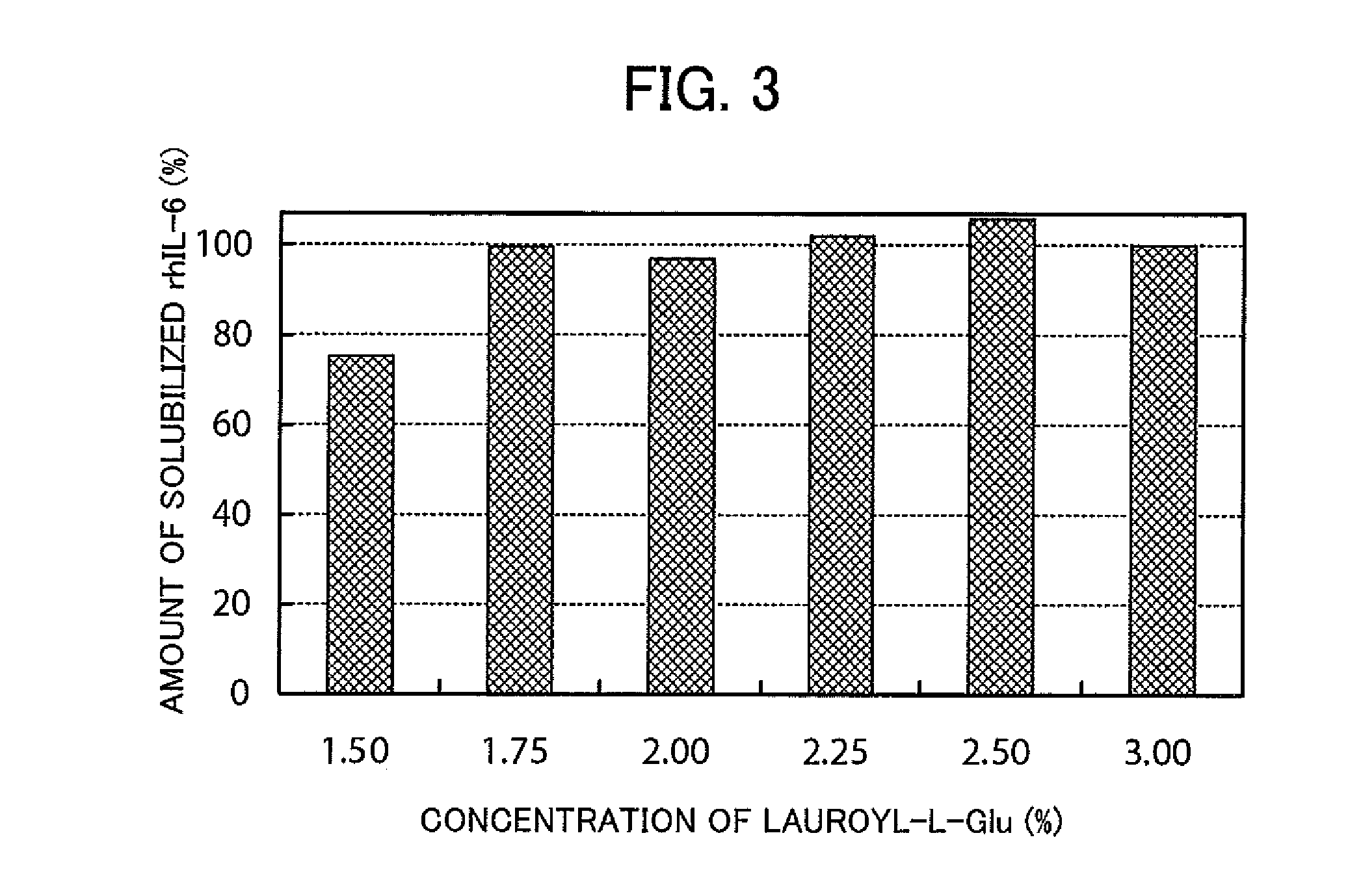
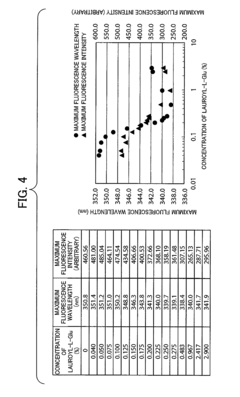
- A method involving a specific surfactant, such as lauroyl-L-Glu, is used to solubilize denatured proteins, followed by dilution with a buffer containing arginine or its derivatives to restore the native higher-order structure while easily stripping the surfactant, preventing aggregation.
Regulatory Considerations for Protein Analysis Methods
Protein analysis methods used in studying the effects of Arrhenius acid on protein denaturation must adhere to stringent regulatory frameworks established by various international and national authorities. The FDA in the United States has specific guidelines under 21 CFR Part 58 (Good Laboratory Practices) that govern analytical procedures for protein characterization, particularly when these methods may inform pharmaceutical development. Similarly, the European Medicines Agency (EMA) provides detailed guidance through ICH Q2(R1) for validation of analytical procedures used in protein analysis, emphasizing parameters such as specificity, accuracy, precision, and robustness.
Researchers investigating Arrhenius acid effects on protein denaturation must consider ISO/IEC 17025 standards, which establish general requirements for the competence of testing laboratories. These standards are particularly relevant when measuring structural changes in proteins using techniques such as circular dichroism spectroscopy or differential scanning calorimetry. Compliance with these standards ensures that data generated is reliable and reproducible across different laboratory settings.
For methods involving human or animal-derived proteins, additional ethical considerations come into play. Institutional Review Board (IRB) approval may be necessary when working with human samples, while animal-derived materials must comply with regulations such as the Animal Welfare Act in the US or Directive 2010/63/EU in Europe. Documentation of ethical sourcing becomes a critical regulatory component of the research protocol.
Quality control measures represent another significant regulatory aspect. The implementation of quality management systems that include regular calibration of instruments, validation of analytical methods, and proper documentation of procedures is essential for compliance. For quantitative measurements of protein denaturation, statistical validation approaches must meet criteria outlined in USP <1010> or equivalent international standards.
Data integrity regulations, including 21 CFR Part 11 for electronic records, apply when using computerized systems to collect and analyze protein denaturation data. These regulations require implementation of audit trails, electronic signatures, and data security measures to ensure that experimental results remain trustworthy and tamper-proof throughout the research process.
Researchers must also consider emerging regulatory trends, such as the increasing emphasis on orthogonal analytical approaches. Regulatory bodies increasingly expect multiple, complementary techniques to characterize protein changes, rather than reliance on a single analytical method. This multi-method approach provides more robust evidence of protein denaturation mechanisms when studying Arrhenius acid effects.
Researchers investigating Arrhenius acid effects on protein denaturation must consider ISO/IEC 17025 standards, which establish general requirements for the competence of testing laboratories. These standards are particularly relevant when measuring structural changes in proteins using techniques such as circular dichroism spectroscopy or differential scanning calorimetry. Compliance with these standards ensures that data generated is reliable and reproducible across different laboratory settings.
For methods involving human or animal-derived proteins, additional ethical considerations come into play. Institutional Review Board (IRB) approval may be necessary when working with human samples, while animal-derived materials must comply with regulations such as the Animal Welfare Act in the US or Directive 2010/63/EU in Europe. Documentation of ethical sourcing becomes a critical regulatory component of the research protocol.
Quality control measures represent another significant regulatory aspect. The implementation of quality management systems that include regular calibration of instruments, validation of analytical methods, and proper documentation of procedures is essential for compliance. For quantitative measurements of protein denaturation, statistical validation approaches must meet criteria outlined in USP <1010> or equivalent international standards.
Data integrity regulations, including 21 CFR Part 11 for electronic records, apply when using computerized systems to collect and analyze protein denaturation data. These regulations require implementation of audit trails, electronic signatures, and data security measures to ensure that experimental results remain trustworthy and tamper-proof throughout the research process.
Researchers must also consider emerging regulatory trends, such as the increasing emphasis on orthogonal analytical approaches. Regulatory bodies increasingly expect multiple, complementary techniques to characterize protein changes, rather than reliance on a single analytical method. This multi-method approach provides more robust evidence of protein denaturation mechanisms when studying Arrhenius acid effects.
Computational Modeling of Acid-Protein Denaturation Processes
Computational modeling has emerged as a powerful tool for understanding the complex interactions between Arrhenius acids and protein structures during denaturation processes. These models integrate principles from physical chemistry, molecular dynamics, and quantum mechanics to simulate how proton transfer from acids affects protein stability and conformation.
Current computational approaches primarily utilize molecular dynamics (MD) simulations with specialized force fields that can accurately represent protonation states and their changes under varying pH conditions. These simulations typically employ constant-pH molecular dynamics (CpHMD) methods that allow protonation states to fluctuate during the simulation, providing a more realistic representation of acid-induced denaturation.
Advanced quantum mechanics/molecular mechanics (QM/MM) hybrid methods have shown particular promise in modeling the specific interactions between Arrhenius acids and protein functional groups. These approaches treat the acid-protein interface with quantum mechanical precision while using classical mechanics for the remainder of the system, enabling accurate representation of proton transfer events that initiate denaturation.
Machine learning algorithms have recently been integrated into these computational frameworks, allowing for more efficient exploration of the vast conformational space associated with protein denaturation. Neural network potentials trained on quantum mechanical calculations can now predict structural changes with accuracy approaching ab initio methods but at a fraction of the computational cost.
Time-dependent simulations reveal that acid-induced denaturation typically proceeds through distinct phases: initial protonation of susceptible amino acid residues, followed by disruption of hydrogen bonds, exposure of hydrophobic cores, and finally complete unfolding. These simulations have successfully reproduced experimental measurements of denaturation rates and intermediate states observed in circular dichroism and fluorescence spectroscopy studies.
Coarse-grained models have been developed to extend simulation timescales to biologically relevant ranges, allowing researchers to observe complete denaturation events that would be computationally prohibitive with all-atom models. These approaches sacrifice atomic detail but capture essential thermodynamic and kinetic properties of the denaturation process.
Validation of computational models against experimental data remains crucial, with researchers typically comparing predicted changes in protein secondary structure content, solvent-accessible surface area, and denaturation rates with laboratory measurements. Recent advances in time-resolved experimental techniques have provided unprecedented opportunities to refine and validate computational predictions of intermediate states during acid-induced unfolding.
Current computational approaches primarily utilize molecular dynamics (MD) simulations with specialized force fields that can accurately represent protonation states and their changes under varying pH conditions. These simulations typically employ constant-pH molecular dynamics (CpHMD) methods that allow protonation states to fluctuate during the simulation, providing a more realistic representation of acid-induced denaturation.
Advanced quantum mechanics/molecular mechanics (QM/MM) hybrid methods have shown particular promise in modeling the specific interactions between Arrhenius acids and protein functional groups. These approaches treat the acid-protein interface with quantum mechanical precision while using classical mechanics for the remainder of the system, enabling accurate representation of proton transfer events that initiate denaturation.
Machine learning algorithms have recently been integrated into these computational frameworks, allowing for more efficient exploration of the vast conformational space associated with protein denaturation. Neural network potentials trained on quantum mechanical calculations can now predict structural changes with accuracy approaching ab initio methods but at a fraction of the computational cost.
Time-dependent simulations reveal that acid-induced denaturation typically proceeds through distinct phases: initial protonation of susceptible amino acid residues, followed by disruption of hydrogen bonds, exposure of hydrophobic cores, and finally complete unfolding. These simulations have successfully reproduced experimental measurements of denaturation rates and intermediate states observed in circular dichroism and fluorescence spectroscopy studies.
Coarse-grained models have been developed to extend simulation timescales to biologically relevant ranges, allowing researchers to observe complete denaturation events that would be computationally prohibitive with all-atom models. These approaches sacrifice atomic detail but capture essential thermodynamic and kinetic properties of the denaturation process.
Validation of computational models against experimental data remains crucial, with researchers typically comparing predicted changes in protein secondary structure content, solvent-accessible surface area, and denaturation rates with laboratory measurements. Recent advances in time-resolved experimental techniques have provided unprecedented opportunities to refine and validate computational predictions of intermediate states during acid-induced unfolding.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!