Evaluating Performance of High Pass Filters in Portable Medical Devices
JUL 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
High Pass Filter Background and Objectives
High pass filters have been an integral component of signal processing in electronic devices for decades, with their origins dating back to the early 20th century. These filters are designed to attenuate low-frequency signals while allowing high-frequency signals to pass through, making them crucial in various applications, including portable medical devices. The evolution of high pass filters has been closely tied to advancements in electronic engineering and signal processing technologies.
In the context of portable medical devices, high pass filters play a vital role in improving signal quality and reducing noise. These devices, which include electrocardiograms (ECGs), electroencephalograms (EEGs), and portable ultrasound machines, rely on accurate signal processing to provide reliable diagnostic information. The miniaturization of medical devices has led to increased portability and accessibility, but it has also introduced new challenges in maintaining signal integrity in compact, battery-powered systems.
The primary objective of evaluating the performance of high pass filters in portable medical devices is to optimize their functionality within the constraints of these compact systems. This evaluation aims to address several key aspects, including power efficiency, signal accuracy, and adaptability to various physiological signals. As portable medical devices continue to evolve, there is a growing need for high pass filters that can operate effectively with minimal power consumption while maintaining high precision in signal processing.
Another critical objective is to assess the filters' ability to adapt to different types of physiological signals and environmental conditions. Portable medical devices are often used in diverse settings, from controlled clinical environments to unpredictable field conditions. Therefore, the high pass filters must demonstrate robustness and reliability across a wide range of operating scenarios.
Furthermore, the evaluation seeks to explore innovative filter designs that can overcome the limitations of traditional analog filters. This includes investigating digital filter implementations, adaptive filtering techniques, and hybrid analog-digital solutions. The goal is to identify filter architectures that can provide superior performance in terms of noise reduction, signal preservation, and computational efficiency.
As the healthcare industry moves towards more personalized and remote patient monitoring, the role of high pass filters in portable medical devices becomes increasingly critical. The evaluation of their performance is not only about improving current devices but also about paving the way for future innovations in mobile health technologies. By understanding the strengths and limitations of current high pass filter implementations, researchers and engineers can develop more advanced filtering solutions that will enable the next generation of portable medical devices to deliver more accurate, reliable, and accessible healthcare diagnostics.
In the context of portable medical devices, high pass filters play a vital role in improving signal quality and reducing noise. These devices, which include electrocardiograms (ECGs), electroencephalograms (EEGs), and portable ultrasound machines, rely on accurate signal processing to provide reliable diagnostic information. The miniaturization of medical devices has led to increased portability and accessibility, but it has also introduced new challenges in maintaining signal integrity in compact, battery-powered systems.
The primary objective of evaluating the performance of high pass filters in portable medical devices is to optimize their functionality within the constraints of these compact systems. This evaluation aims to address several key aspects, including power efficiency, signal accuracy, and adaptability to various physiological signals. As portable medical devices continue to evolve, there is a growing need for high pass filters that can operate effectively with minimal power consumption while maintaining high precision in signal processing.
Another critical objective is to assess the filters' ability to adapt to different types of physiological signals and environmental conditions. Portable medical devices are often used in diverse settings, from controlled clinical environments to unpredictable field conditions. Therefore, the high pass filters must demonstrate robustness and reliability across a wide range of operating scenarios.
Furthermore, the evaluation seeks to explore innovative filter designs that can overcome the limitations of traditional analog filters. This includes investigating digital filter implementations, adaptive filtering techniques, and hybrid analog-digital solutions. The goal is to identify filter architectures that can provide superior performance in terms of noise reduction, signal preservation, and computational efficiency.
As the healthcare industry moves towards more personalized and remote patient monitoring, the role of high pass filters in portable medical devices becomes increasingly critical. The evaluation of their performance is not only about improving current devices but also about paving the way for future innovations in mobile health technologies. By understanding the strengths and limitations of current high pass filter implementations, researchers and engineers can develop more advanced filtering solutions that will enable the next generation of portable medical devices to deliver more accurate, reliable, and accessible healthcare diagnostics.
Market Analysis for Portable Medical Devices
The portable medical device market has experienced significant growth in recent years, driven by increasing demand for home healthcare solutions and advancements in technology. This market segment encompasses a wide range of devices, including wearable health monitors, portable diagnostic equipment, and mobile therapy devices. The integration of high pass filters in these devices plays a crucial role in enhancing their performance and reliability.
The global portable medical device market was valued at approximately $44.5 billion in 2020 and is projected to reach $85.3 billion by 2027, growing at a CAGR of 9.8% during the forecast period. This growth is attributed to factors such as the rising prevalence of chronic diseases, an aging population, and the increasing adoption of telemedicine and remote patient monitoring solutions.
Within this market, devices incorporating high pass filters are gaining traction due to their ability to improve signal quality and reduce noise in various medical applications. These filters are particularly valuable in portable electrocardiogram (ECG) monitors, blood glucose meters, and portable ultrasound devices, where accurate signal processing is critical for reliable diagnostics.
The demand for portable medical devices with enhanced filtering capabilities is driven by healthcare providers' need for accurate and reliable data in both clinical and home settings. Patients are also increasingly seeking user-friendly, compact devices that can provide accurate measurements and seamless data transmission to healthcare professionals.
Key market players in the portable medical device sector include Medtronic, GE Healthcare, Philips Healthcare, and Abbott Laboratories. These companies are investing heavily in research and development to improve the performance of their devices, with a focus on integrating advanced filtering technologies to enhance signal quality and reduce interference.
Geographically, North America dominates the portable medical device market, followed by Europe and Asia-Pacific. The United States, in particular, holds a significant market share due to its advanced healthcare infrastructure and high adoption rate of new technologies. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness rapid growth in the coming years, driven by improving healthcare access and increasing healthcare expenditure.
The COVID-19 pandemic has further accelerated the adoption of portable medical devices, as healthcare systems worldwide sought to reduce hospital visits and implement remote patient monitoring solutions. This trend is expected to continue post-pandemic, creating new opportunities for devices with advanced filtering capabilities to support telemedicine and home-based care.
The global portable medical device market was valued at approximately $44.5 billion in 2020 and is projected to reach $85.3 billion by 2027, growing at a CAGR of 9.8% during the forecast period. This growth is attributed to factors such as the rising prevalence of chronic diseases, an aging population, and the increasing adoption of telemedicine and remote patient monitoring solutions.
Within this market, devices incorporating high pass filters are gaining traction due to their ability to improve signal quality and reduce noise in various medical applications. These filters are particularly valuable in portable electrocardiogram (ECG) monitors, blood glucose meters, and portable ultrasound devices, where accurate signal processing is critical for reliable diagnostics.
The demand for portable medical devices with enhanced filtering capabilities is driven by healthcare providers' need for accurate and reliable data in both clinical and home settings. Patients are also increasingly seeking user-friendly, compact devices that can provide accurate measurements and seamless data transmission to healthcare professionals.
Key market players in the portable medical device sector include Medtronic, GE Healthcare, Philips Healthcare, and Abbott Laboratories. These companies are investing heavily in research and development to improve the performance of their devices, with a focus on integrating advanced filtering technologies to enhance signal quality and reduce interference.
Geographically, North America dominates the portable medical device market, followed by Europe and Asia-Pacific. The United States, in particular, holds a significant market share due to its advanced healthcare infrastructure and high adoption rate of new technologies. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness rapid growth in the coming years, driven by improving healthcare access and increasing healthcare expenditure.
The COVID-19 pandemic has further accelerated the adoption of portable medical devices, as healthcare systems worldwide sought to reduce hospital visits and implement remote patient monitoring solutions. This trend is expected to continue post-pandemic, creating new opportunities for devices with advanced filtering capabilities to support telemedicine and home-based care.
Current Challenges in High Pass Filter Implementation
The implementation of high pass filters in portable medical devices faces several significant challenges that impact their performance and effectiveness. One of the primary issues is the miniaturization of components while maintaining filter accuracy and efficiency. As portable devices become increasingly compact, designers struggle to integrate high-quality filters within limited space constraints without compromising functionality.
Power consumption is another critical challenge. Portable medical devices rely on battery power, necessitating energy-efficient filter designs. However, achieving optimal filter performance often requires more power, creating a delicate balance between energy conservation and filter effectiveness. This trade-off becomes particularly crucial in devices intended for long-term monitoring or continuous use.
Signal integrity is a paramount concern in medical applications. High pass filters must effectively remove low-frequency noise and DC offset without distorting the desired signal. Achieving this balance is challenging, especially when dealing with weak bioelectric signals commonly encountered in portable medical devices. Environmental interference, such as motion artifacts and electromagnetic noise, further complicates the filtering process.
Temperature sensitivity poses another significant hurdle. Portable devices are subject to varying environmental conditions, and filter components can be affected by temperature fluctuations. Maintaining consistent filter performance across a wide temperature range is essential for reliable operation but presents design challenges.
Component tolerances and variations in manufacturing processes can lead to inconsistencies in filter characteristics. This variability can result in performance discrepancies between individual devices, potentially affecting diagnostic accuracy. Implementing robust calibration methods and quality control measures becomes crucial to mitigate these issues.
The dynamic range of input signals in medical applications can be extensive, requiring filters to handle both small-amplitude physiological signals and larger interfering signals. Designing filters that can effectively process this wide range of inputs without saturation or distortion is a complex task.
Lastly, the need for adaptability in filter characteristics presents a significant challenge. Different medical applications may require varying filter responses, and some devices may need to adjust filter parameters in real-time based on changing physiological conditions or measurement requirements. Implementing programmable or adaptive filtering solutions while maintaining simplicity and reliability is an ongoing challenge in the field of portable medical devices.
Power consumption is another critical challenge. Portable medical devices rely on battery power, necessitating energy-efficient filter designs. However, achieving optimal filter performance often requires more power, creating a delicate balance between energy conservation and filter effectiveness. This trade-off becomes particularly crucial in devices intended for long-term monitoring or continuous use.
Signal integrity is a paramount concern in medical applications. High pass filters must effectively remove low-frequency noise and DC offset without distorting the desired signal. Achieving this balance is challenging, especially when dealing with weak bioelectric signals commonly encountered in portable medical devices. Environmental interference, such as motion artifacts and electromagnetic noise, further complicates the filtering process.
Temperature sensitivity poses another significant hurdle. Portable devices are subject to varying environmental conditions, and filter components can be affected by temperature fluctuations. Maintaining consistent filter performance across a wide temperature range is essential for reliable operation but presents design challenges.
Component tolerances and variations in manufacturing processes can lead to inconsistencies in filter characteristics. This variability can result in performance discrepancies between individual devices, potentially affecting diagnostic accuracy. Implementing robust calibration methods and quality control measures becomes crucial to mitigate these issues.
The dynamic range of input signals in medical applications can be extensive, requiring filters to handle both small-amplitude physiological signals and larger interfering signals. Designing filters that can effectively process this wide range of inputs without saturation or distortion is a complex task.
Lastly, the need for adaptability in filter characteristics presents a significant challenge. Different medical applications may require varying filter responses, and some devices may need to adjust filter parameters in real-time based on changing physiological conditions or measurement requirements. Implementing programmable or adaptive filtering solutions while maintaining simplicity and reliability is an ongoing challenge in the field of portable medical devices.
Existing High Pass Filter Designs for Portable Devices
01 Circuit design for high pass filters
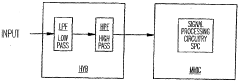
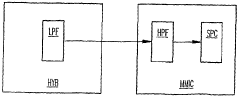
High pass filters can be designed using various circuit configurations to improve performance. These designs may include active and passive components, such as capacitors, inductors, and operational amplifiers, to achieve desired frequency response and signal attenuation characteristics. Advanced circuit topologies can enhance filter selectivity, reduce noise, and improve overall system performance.- Design and optimization of high pass filter circuits: High pass filters are designed and optimized to improve their performance in various applications. This includes the development of novel circuit topologies, component selection, and parameter tuning to achieve desired frequency response, cut-off characteristics, and signal quality.
- Integration of high pass filters in signal processing systems: High pass filters are integrated into various signal processing systems to enhance overall performance. This involves incorporating filters into audio, video, and communication systems to remove low-frequency noise, improve signal clarity, and optimize system efficiency.
- Digital implementation of high pass filters: Digital high pass filters are implemented using advanced algorithms and digital signal processing techniques. This approach offers improved flexibility, precision, and adaptability compared to analog counterparts, enabling enhanced performance in various applications.
- High pass filter applications in specific industries: High pass filters are tailored for specific industry applications, such as automotive, telecommunications, and medical devices. These specialized filters are designed to meet unique performance requirements and regulatory standards within each sector.
- Performance evaluation and testing of high pass filters: Methods and systems are developed for evaluating and testing the performance of high pass filters. This includes the use of advanced measurement techniques, simulation tools, and standardized testing procedures to assess filter characteristics and ensure optimal performance across various operating conditions.
02 Digital implementation of high pass filters
Digital signal processing techniques can be used to implement high pass filters with improved performance. These digital filters can be realized using microprocessors, DSP chips, or FPGAs, allowing for precise control over filter characteristics and adaptability to changing requirements. Digital implementations can offer advantages in terms of flexibility, stability, and ease of integration with other digital systems.Expand Specific Solutions03 High pass filter applications in communication systems
High pass filters play a crucial role in various communication systems, including wireless and optical communications. They are used for noise reduction, signal conditioning, and frequency selection in transmitters and receivers. Optimizing high pass filter performance can lead to improved signal quality, increased data rates, and enhanced overall system efficiency in communication applications.Expand Specific Solutions04 Adaptive and tunable high pass filters
Adaptive and tunable high pass filters can dynamically adjust their characteristics based on input signals or system requirements. These filters use feedback mechanisms or programmable components to optimize performance in real-time. Such adaptability allows for improved filtering in changing environments, enhanced interference rejection, and better overall system performance across various operating conditions.Expand Specific Solutions05 High pass filter performance optimization techniques
Various techniques can be employed to optimize high pass filter performance, including component selection, layout optimization, and advanced materials. These methods aim to minimize parasitic effects, reduce insertion loss, improve stopband attenuation, and enhance overall filter response. Additionally, simulation and modeling tools can be used to predict and fine-tune filter performance before physical implementation.Expand Specific Solutions
Key Players in Medical Device Filtering Solutions
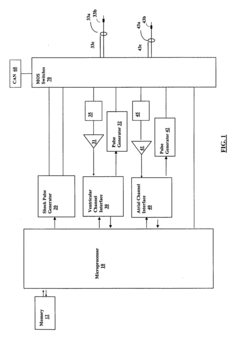
The evaluation of high pass filter performance in portable medical devices is at a mature stage of development, with a significant market size driven by the growing demand for compact and efficient medical equipment. The technology has reached a high level of maturity, with key players like Murata Manufacturing, STMicroelectronics, and Taiyo Yuden leading the field. These companies, along with others such as NXP Semiconductors and Infineon Technologies, are continuously innovating to improve filter performance, miniaturization, and power efficiency. The competitive landscape is characterized by a mix of established electronics giants and specialized component manufacturers, all vying to meet the stringent requirements of the medical device industry.
Murata Manufacturing Co. Ltd.
Technical Solution: Murata has developed advanced high-pass filter solutions for portable medical devices, focusing on miniaturization and high performance. Their EMIFIL® series employs a three-terminal structure with ferrite beads and capacitors integrated into a single chip, achieving superior noise suppression characteristics[1]. The company has also introduced multilayer ceramic capacitors (MLCCs) with ultra-low equivalent series inductance (ESL), which are crucial for high-frequency filtering in medical equipment[2]. Murata's filters demonstrate excellent attenuation in the high-frequency range, typically above 100 MHz, making them ideal for eliminating electromagnetic interference in sensitive medical instruments[3].
Strengths: Compact size, high integration, and excellent high-frequency performance. Weaknesses: Potentially higher cost compared to discrete components and limited flexibility in filter characteristics adjustment.
Stmicroelectronics Srl
Technical Solution: STMicroelectronics has developed a range of high-performance analog filters for portable medical devices, including their STPF series of programmable filters. These filters utilize switched-capacitor technology, allowing for precise cutoff frequency control and filter response shaping[1]. The company's solutions often integrate multiple filtering stages, analog-to-digital converters (ADCs), and digital signal processing (DSP) capabilities on a single chip, optimizing power consumption and reducing overall system size[2]. STMicroelectronics' filters typically achieve a stopband attenuation of over 60 dB and can operate at frequencies up to several MHz, making them suitable for various medical applications such as ECG and EEG signal processing[3].
Strengths: Programmability, integration of multiple functions, and low power consumption. Weaknesses: Potential for clock feedthrough noise and limited maximum operating frequency compared to passive solutions.
Core Innovations in High Pass Filter Technology
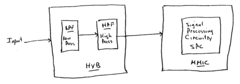
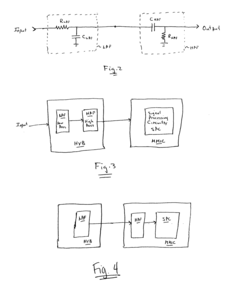
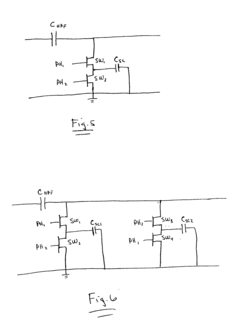
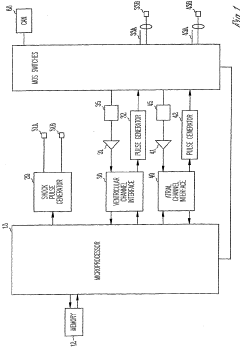
Passive switched capacitor high-pass filter for implantable cardiac device
PatentInactiveUS20050261596A1
Innovation
- Implementing a switched capacitor passive high-pass filter on an integrated circuit chip, which reduces the capacitance and resistance values to acceptable dimensions, allowing for the integration of high-pass filtering functionality within the device.
Passive switched capacitor high-pass filter for implantable medical device
PatentWO2005115538A1
Innovation
- A passive switched capacitor high-pass filter is implemented, using a series capacitor and switched resistances formed by capacitors and switches, allowing for a reduced capacitance value that is feasible for chip fabrication and achieving the desired RC time constant, enabling effective filtering of repolarization components from electrogram signals.
Regulatory Compliance for Medical Device Filters
Regulatory compliance is a critical aspect of developing and implementing high pass filters in portable medical devices. The stringent requirements set by regulatory bodies ensure the safety, efficacy, and reliability of these devices in healthcare settings. In the United States, the Food and Drug Administration (FDA) oversees the regulation of medical devices, including those incorporating high pass filters.
The FDA classifies medical devices into three categories based on their risk level and intended use. Portable medical devices with high pass filters typically fall under Class II, which requires a 510(k) premarket notification submission. This process involves demonstrating that the device is substantially equivalent to a legally marketed predicate device in terms of safety and effectiveness.
For high pass filters in portable medical devices, manufacturers must adhere to specific standards and guidelines. The International Electrotechnical Commission (IEC) 60601-1 standard is particularly relevant, as it outlines safety requirements for medical electrical equipment. Additionally, the IEC 60601-1-2 standard addresses electromagnetic compatibility (EMC) requirements, which is crucial for devices utilizing high pass filters.
Manufacturers must also comply with quality management system regulations, such as the FDA's Quality System Regulation (QSR) or ISO 13485. These systems ensure consistent design, production, and distribution processes that meet regulatory requirements and maintain product quality throughout the device lifecycle.
Documentation plays a vital role in regulatory compliance. Manufacturers must maintain detailed records of design controls, risk management processes, and verification and validation testing. For high pass filters, this includes documenting filter specifications, performance characteristics, and any potential risks associated with their use in the medical device.
Regulatory bodies also require post-market surveillance to monitor the performance and safety of medical devices once they are in use. This involves collecting and analyzing data on device performance, adverse events, and user feedback. For high pass filters, this may include monitoring filter degradation over time or identifying any unexpected interactions with other device components.
As medical device technology evolves, regulatory requirements may also change. Manufacturers must stay informed about updates to standards and regulations that may affect their devices. This includes participating in industry forums, engaging with regulatory bodies, and continuously evaluating their compliance strategies to ensure they meet current and future requirements.
The FDA classifies medical devices into three categories based on their risk level and intended use. Portable medical devices with high pass filters typically fall under Class II, which requires a 510(k) premarket notification submission. This process involves demonstrating that the device is substantially equivalent to a legally marketed predicate device in terms of safety and effectiveness.
For high pass filters in portable medical devices, manufacturers must adhere to specific standards and guidelines. The International Electrotechnical Commission (IEC) 60601-1 standard is particularly relevant, as it outlines safety requirements for medical electrical equipment. Additionally, the IEC 60601-1-2 standard addresses electromagnetic compatibility (EMC) requirements, which is crucial for devices utilizing high pass filters.
Manufacturers must also comply with quality management system regulations, such as the FDA's Quality System Regulation (QSR) or ISO 13485. These systems ensure consistent design, production, and distribution processes that meet regulatory requirements and maintain product quality throughout the device lifecycle.
Documentation plays a vital role in regulatory compliance. Manufacturers must maintain detailed records of design controls, risk management processes, and verification and validation testing. For high pass filters, this includes documenting filter specifications, performance characteristics, and any potential risks associated with their use in the medical device.
Regulatory bodies also require post-market surveillance to monitor the performance and safety of medical devices once they are in use. This involves collecting and analyzing data on device performance, adverse events, and user feedback. For high pass filters, this may include monitoring filter degradation over time or identifying any unexpected interactions with other device components.
As medical device technology evolves, regulatory requirements may also change. Manufacturers must stay informed about updates to standards and regulations that may affect their devices. This includes participating in industry forums, engaging with regulatory bodies, and continuously evaluating their compliance strategies to ensure they meet current and future requirements.
Power Efficiency in Portable Filter Design
Power efficiency is a critical consideration in the design of portable medical devices, particularly when implementing high pass filters. As these devices often rely on battery power, optimizing energy consumption is paramount to ensure extended operational time and improved user experience.
The design of power-efficient high pass filters for portable medical devices requires a multifaceted approach. One key strategy involves the selection of low-power components, such as operational amplifiers and passive elements with minimal current draw. These components should be carefully chosen to balance performance requirements with power consumption, often necessitating trade-offs between filter characteristics and energy efficiency.
Another important aspect of power-efficient filter design is the implementation of adaptive power management techniques. This may include dynamic adjustment of filter parameters based on signal conditions or device operating modes. For instance, the filter's cutoff frequency or order could be dynamically modified to conserve power when high-precision filtering is not required, reverting to more power-intensive configurations only when necessary.
The use of advanced circuit topologies can also contribute significantly to power efficiency. For example, switched-capacitor filters offer the advantage of precise frequency control without the need for large, power-hungry inductors. Similarly, current-mode filters can operate at lower supply voltages, reducing overall power consumption.
In recent years, the integration of digital signal processing (DSP) techniques has opened new avenues for power-efficient filter design. By leveraging the flexibility and programmability of DSP, designers can implement adaptive filtering algorithms that optimize power usage based on real-time signal characteristics. This approach allows for more intelligent power management, potentially extending battery life without compromising filter performance.
Consideration must also be given to the overall system architecture when designing power-efficient filters. This includes optimizing the interaction between the filter stage and other components of the medical device, such as analog-to-digital converters and microcontrollers. Proper interfacing and synchronization between these elements can minimize unnecessary power consumption and data processing.
As portable medical devices continue to evolve, emerging technologies such as energy harvesting and ultra-low-power circuit design techniques are likely to play an increasingly important role in filter power efficiency. These advancements may enable the development of self-powered or extremely long-lasting portable devices, further expanding their utility in various medical applications.
The design of power-efficient high pass filters for portable medical devices requires a multifaceted approach. One key strategy involves the selection of low-power components, such as operational amplifiers and passive elements with minimal current draw. These components should be carefully chosen to balance performance requirements with power consumption, often necessitating trade-offs between filter characteristics and energy efficiency.
Another important aspect of power-efficient filter design is the implementation of adaptive power management techniques. This may include dynamic adjustment of filter parameters based on signal conditions or device operating modes. For instance, the filter's cutoff frequency or order could be dynamically modified to conserve power when high-precision filtering is not required, reverting to more power-intensive configurations only when necessary.
The use of advanced circuit topologies can also contribute significantly to power efficiency. For example, switched-capacitor filters offer the advantage of precise frequency control without the need for large, power-hungry inductors. Similarly, current-mode filters can operate at lower supply voltages, reducing overall power consumption.
In recent years, the integration of digital signal processing (DSP) techniques has opened new avenues for power-efficient filter design. By leveraging the flexibility and programmability of DSP, designers can implement adaptive filtering algorithms that optimize power usage based on real-time signal characteristics. This approach allows for more intelligent power management, potentially extending battery life without compromising filter performance.
Consideration must also be given to the overall system architecture when designing power-efficient filters. This includes optimizing the interaction between the filter stage and other components of the medical device, such as analog-to-digital converters and microcontrollers. Proper interfacing and synchronization between these elements can minimize unnecessary power consumption and data processing.
As portable medical devices continue to evolve, emerging technologies such as energy harvesting and ultra-low-power circuit design techniques are likely to play an increasingly important role in filter power efficiency. These advancements may enable the development of self-powered or extremely long-lasting portable devices, further expanding their utility in various medical applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!