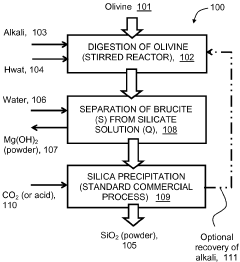
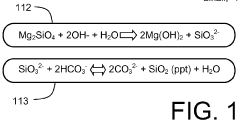
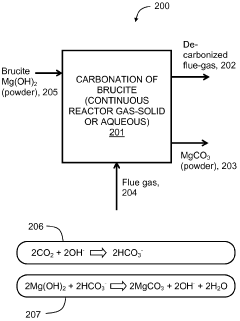
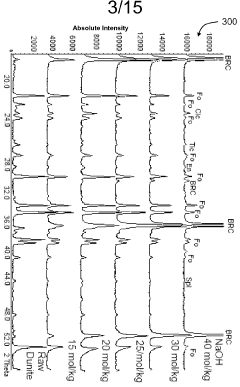
Exploring carbon cycling interactions with Magnesium iron silicate hydroxide.
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Carbon Cycling Background and Objectives
Carbon cycling is a fundamental process in Earth's biogeochemical systems, involving the exchange of carbon between various reservoirs, including the atmosphere, oceans, terrestrial biosphere, and lithosphere. This complex cycle plays a crucial role in regulating global climate and supporting life on our planet. In recent years, the interaction between carbon cycling and magnesium iron silicate hydroxide (MISH) has emerged as a significant area of research, offering potential insights into carbon sequestration and climate change mitigation strategies.
The primary objective of exploring carbon cycling interactions with MISH is to understand the mechanisms by which these minerals can influence carbon dioxide (CO2) uptake and storage. MISH, particularly in the form of serpentine minerals, has shown promising capabilities for carbon mineralization, a process where CO2 is converted into stable carbonate minerals. This natural process occurs over geological timescales, but researchers aim to accelerate and optimize it for practical applications in carbon capture and storage (CCS) technologies.
One key aspect of this research is investigating the weathering processes of MISH-rich rocks and their impact on atmospheric CO2 levels. As these rocks weather, they consume CO2 and release cations such as magnesium and iron, which can then form carbonate minerals. Understanding the rates and factors influencing these reactions is crucial for assessing the potential of MISH in natural and engineered carbon sequestration systems.
Another important objective is to explore the role of MISH in oceanic carbon cycling. The weathering of MISH-containing rocks on land can lead to increased alkalinity in rivers and, ultimately, the oceans. This enhanced alkalinity can boost the ocean's capacity to absorb atmospheric CO2, potentially serving as a natural negative feedback mechanism against rising CO2 levels.
Furthermore, researchers aim to investigate the potential of MISH in developing novel CCS technologies. By harnessing the CO2-binding properties of these minerals, it may be possible to create efficient and environmentally friendly carbon capture systems. This could involve the use of MISH in direct air capture technologies or as a component in enhanced weathering strategies.
The study of carbon cycling interactions with MISH also extends to understanding the long-term stability of sequestered carbon. Assessing the permanence of carbon storage in MISH-derived carbonate minerals is crucial for evaluating the effectiveness of these approaches in mitigating climate change over extended periods.
In conclusion, the exploration of carbon cycling interactions with magnesium iron silicate hydroxide encompasses a wide range of objectives, from enhancing our understanding of natural carbon sequestration processes to developing innovative technologies for climate change mitigation. This research has the potential to contribute significantly to global efforts in reducing atmospheric CO2 levels and addressing the challenges posed by climate change.
The primary objective of exploring carbon cycling interactions with MISH is to understand the mechanisms by which these minerals can influence carbon dioxide (CO2) uptake and storage. MISH, particularly in the form of serpentine minerals, has shown promising capabilities for carbon mineralization, a process where CO2 is converted into stable carbonate minerals. This natural process occurs over geological timescales, but researchers aim to accelerate and optimize it for practical applications in carbon capture and storage (CCS) technologies.
One key aspect of this research is investigating the weathering processes of MISH-rich rocks and their impact on atmospheric CO2 levels. As these rocks weather, they consume CO2 and release cations such as magnesium and iron, which can then form carbonate minerals. Understanding the rates and factors influencing these reactions is crucial for assessing the potential of MISH in natural and engineered carbon sequestration systems.
Another important objective is to explore the role of MISH in oceanic carbon cycling. The weathering of MISH-containing rocks on land can lead to increased alkalinity in rivers and, ultimately, the oceans. This enhanced alkalinity can boost the ocean's capacity to absorb atmospheric CO2, potentially serving as a natural negative feedback mechanism against rising CO2 levels.
Furthermore, researchers aim to investigate the potential of MISH in developing novel CCS technologies. By harnessing the CO2-binding properties of these minerals, it may be possible to create efficient and environmentally friendly carbon capture systems. This could involve the use of MISH in direct air capture technologies or as a component in enhanced weathering strategies.
The study of carbon cycling interactions with MISH also extends to understanding the long-term stability of sequestered carbon. Assessing the permanence of carbon storage in MISH-derived carbonate minerals is crucial for evaluating the effectiveness of these approaches in mitigating climate change over extended periods.
In conclusion, the exploration of carbon cycling interactions with magnesium iron silicate hydroxide encompasses a wide range of objectives, from enhancing our understanding of natural carbon sequestration processes to developing innovative technologies for climate change mitigation. This research has the potential to contribute significantly to global efforts in reducing atmospheric CO2 levels and addressing the challenges posed by climate change.
Market Analysis for Carbon Sequestration Technologies
The market for carbon sequestration technologies has been experiencing significant growth in recent years, driven by increasing global concerns over climate change and the urgent need to reduce greenhouse gas emissions. The exploration of carbon cycling interactions with Magnesium iron silicate hydroxide (MISH) represents a promising avenue within this expanding market.
Carbon sequestration technologies encompass a wide range of approaches, including geological storage, ocean storage, and mineral carbonation. Among these, mineral carbonation, particularly using MISH, has gained attention due to its potential for long-term, stable carbon storage. The global carbon capture and storage (CCS) market was valued at approximately $6.13 billion in 2020 and is projected to reach $11.85 billion by 2028, growing at a CAGR of 7.6% during the forecast period.
The market for MISH-based carbon sequestration is still in its nascent stages but shows promising growth potential. This technology leverages the natural weathering process of silicate minerals, which can be accelerated to capture and store atmospheric CO2. The abundance of magnesium and iron-rich silicate minerals in the Earth's crust makes this approach particularly attractive for large-scale implementation.
Key market drivers include stringent government regulations on carbon emissions, increasing investments in clean energy technologies, and growing corporate commitments to achieve net-zero emissions. The European Union's ambitious target to reduce greenhouse gas emissions by at least 55% by 2030 has created a favorable market environment for carbon sequestration technologies, including MISH-based solutions.
However, the market faces several challenges. The high initial capital costs associated with implementing MISH-based carbon sequestration technologies remain a significant barrier to widespread adoption. Additionally, the energy-intensive nature of the mineral carbonation process and the need for large-scale mining operations raise environmental concerns that need to be addressed.
Despite these challenges, the market outlook for MISH-based carbon sequestration remains positive. Ongoing research and development efforts are focused on improving the efficiency and cost-effectiveness of the process. Collaborations between academic institutions, industry players, and government agencies are driving innovation in this field, potentially leading to breakthroughs that could accelerate market growth.
The market is characterized by a mix of established players in the broader CCS industry and emerging startups specializing in mineral carbonation technologies. As the technology matures and demonstrates its effectiveness at scale, it is expected to attract increased investment and potentially disrupt the existing carbon sequestration market landscape.
Carbon sequestration technologies encompass a wide range of approaches, including geological storage, ocean storage, and mineral carbonation. Among these, mineral carbonation, particularly using MISH, has gained attention due to its potential for long-term, stable carbon storage. The global carbon capture and storage (CCS) market was valued at approximately $6.13 billion in 2020 and is projected to reach $11.85 billion by 2028, growing at a CAGR of 7.6% during the forecast period.
The market for MISH-based carbon sequestration is still in its nascent stages but shows promising growth potential. This technology leverages the natural weathering process of silicate minerals, which can be accelerated to capture and store atmospheric CO2. The abundance of magnesium and iron-rich silicate minerals in the Earth's crust makes this approach particularly attractive for large-scale implementation.
Key market drivers include stringent government regulations on carbon emissions, increasing investments in clean energy technologies, and growing corporate commitments to achieve net-zero emissions. The European Union's ambitious target to reduce greenhouse gas emissions by at least 55% by 2030 has created a favorable market environment for carbon sequestration technologies, including MISH-based solutions.
However, the market faces several challenges. The high initial capital costs associated with implementing MISH-based carbon sequestration technologies remain a significant barrier to widespread adoption. Additionally, the energy-intensive nature of the mineral carbonation process and the need for large-scale mining operations raise environmental concerns that need to be addressed.
Despite these challenges, the market outlook for MISH-based carbon sequestration remains positive. Ongoing research and development efforts are focused on improving the efficiency and cost-effectiveness of the process. Collaborations between academic institutions, industry players, and government agencies are driving innovation in this field, potentially leading to breakthroughs that could accelerate market growth.
The market is characterized by a mix of established players in the broader CCS industry and emerging startups specializing in mineral carbonation technologies. As the technology matures and demonstrates its effectiveness at scale, it is expected to attract increased investment and potentially disrupt the existing carbon sequestration market landscape.
Current State of Magnesium Iron Silicate Hydroxide Research
Magnesium iron silicate hydroxide (MISH) has emerged as a significant focus in carbon cycling research due to its potential role in carbon sequestration and climate change mitigation. Current research on MISH is primarily centered around its unique chemical properties and interactions with carbon dioxide in various environmental settings.
Recent studies have shown that MISH, particularly in its naturally occurring form as serpentine minerals, has a high capacity for carbon dioxide absorption. This characteristic has led to increased interest in its application for carbon capture and storage (CCS) technologies. Researchers are exploring both natural weathering processes and engineered solutions to enhance the carbon sequestration potential of MISH.
One of the key areas of investigation is the kinetics of carbon dioxide reactions with MISH under different environmental conditions. Scientists are working to understand the factors that influence the rate and efficiency of these reactions, including temperature, pressure, and the presence of catalysts. This knowledge is crucial for optimizing carbon sequestration processes and developing more effective CCS strategies.
Another significant aspect of current MISH research is its potential for in-situ carbon mineralization. Studies are being conducted on the feasibility of injecting carbon dioxide into underground formations rich in magnesium and iron silicates, promoting the formation of stable carbonate minerals. This approach offers a promising avenue for long-term carbon storage with minimal environmental impact.
The role of MISH in natural carbon cycling processes is also under intense scrutiny. Researchers are investigating how these minerals interact with atmospheric carbon dioxide in various ecosystems, particularly in ultramafic regions. Understanding these natural processes could provide insights into enhancing carbon sequestration on a global scale.
Furthermore, there is growing interest in the potential of MISH for industrial applications beyond carbon sequestration. Studies are exploring its use in water treatment, as a catalyst in chemical processes, and as a component in advanced materials. These diverse applications highlight the multifaceted nature of current MISH research.
Challenges in MISH research include improving the efficiency of carbon dioxide absorption, reducing the energy requirements for mineral carbonation processes, and addressing potential environmental impacts of large-scale applications. Scientists are working on developing novel techniques and technologies to overcome these challenges, including the use of biotechnology and nanotechnology to enhance MISH reactivity.
Recent studies have shown that MISH, particularly in its naturally occurring form as serpentine minerals, has a high capacity for carbon dioxide absorption. This characteristic has led to increased interest in its application for carbon capture and storage (CCS) technologies. Researchers are exploring both natural weathering processes and engineered solutions to enhance the carbon sequestration potential of MISH.
One of the key areas of investigation is the kinetics of carbon dioxide reactions with MISH under different environmental conditions. Scientists are working to understand the factors that influence the rate and efficiency of these reactions, including temperature, pressure, and the presence of catalysts. This knowledge is crucial for optimizing carbon sequestration processes and developing more effective CCS strategies.
Another significant aspect of current MISH research is its potential for in-situ carbon mineralization. Studies are being conducted on the feasibility of injecting carbon dioxide into underground formations rich in magnesium and iron silicates, promoting the formation of stable carbonate minerals. This approach offers a promising avenue for long-term carbon storage with minimal environmental impact.
The role of MISH in natural carbon cycling processes is also under intense scrutiny. Researchers are investigating how these minerals interact with atmospheric carbon dioxide in various ecosystems, particularly in ultramafic regions. Understanding these natural processes could provide insights into enhancing carbon sequestration on a global scale.
Furthermore, there is growing interest in the potential of MISH for industrial applications beyond carbon sequestration. Studies are exploring its use in water treatment, as a catalyst in chemical processes, and as a component in advanced materials. These diverse applications highlight the multifaceted nature of current MISH research.
Challenges in MISH research include improving the efficiency of carbon dioxide absorption, reducing the energy requirements for mineral carbonation processes, and addressing potential environmental impacts of large-scale applications. Scientists are working on developing novel techniques and technologies to overcome these challenges, including the use of biotechnology and nanotechnology to enhance MISH reactivity.
Existing Carbon-Mineral Interaction Models
01 Magnesium iron silicate hydroxide in carbon sequestration
Magnesium iron silicate hydroxide, also known as serpentine, can be used in carbon sequestration processes. This mineral has the ability to react with carbon dioxide, forming stable carbonate compounds, thus helping to reduce atmospheric CO2 levels. The process involves the weathering of serpentine, which can be enhanced through various methods to increase its carbon capture efficiency.- Magnesium iron silicate hydroxide in carbon sequestration: Magnesium iron silicate hydroxide, also known as serpentine, can be used in carbon sequestration processes. This mineral has the ability to react with carbon dioxide, forming stable carbonate compounds, thus effectively capturing and storing atmospheric CO2. This process can be enhanced through various methods, including mechanical activation and heat treatment, to improve the mineral's reactivity and efficiency in carbon capture.
- Carbon cycling in soil and mineral interactions: The interaction between magnesium iron silicate hydroxide and soil organic matter plays a crucial role in carbon cycling. These minerals can stabilize organic carbon in soils, affecting its turnover rates and long-term storage. Understanding these interactions is essential for developing strategies to enhance soil carbon sequestration and mitigate climate change impacts.
- Mineral weathering and CO2 drawdown: Weathering of magnesium iron silicate hydroxide contributes to natural carbon dioxide drawdown from the atmosphere. This process involves the dissolution of the mineral in slightly acidic rainwater, consuming CO2 and releasing ions that eventually form carbonate minerals. Enhanced weathering techniques aim to accelerate this natural process for more effective carbon sequestration.
- Industrial applications in carbon capture: Magnesium iron silicate hydroxide is being explored for industrial carbon capture applications. This includes its use in direct air capture technologies and as a component in carbon-negative construction materials. The mineral's abundance and relatively low cost make it an attractive option for large-scale carbon dioxide removal strategies.
- Biogeochemical cycling and ecosystem impacts: The role of magnesium iron silicate hydroxide in biogeochemical cycling extends beyond carbon to include other elements such as iron and magnesium. This mineral's interactions with various ecosystem components, including microorganisms and plants, can influence nutrient availability and overall ecosystem functioning. Understanding these complex interactions is crucial for predicting and managing the impacts of climate change on terrestrial and aquatic ecosystems.
02 Carbon cycling in soil and mineral interactions
The interaction between magnesium iron silicate hydroxide and soil organic matter plays a crucial role in carbon cycling. These minerals can stabilize organic carbon in soils, affecting its turnover rates and long-term storage. Understanding these interactions is essential for developing strategies to enhance soil carbon sequestration and mitigate climate change.Expand Specific Solutions03 Synthesis and modification of magnesium iron silicate hydroxide
Various methods for synthesizing and modifying magnesium iron silicate hydroxide have been developed to enhance its properties for carbon cycling applications. These include hydrothermal synthesis, sol-gel methods, and surface modifications to increase reactivity and carbon dioxide absorption capacity.Expand Specific Solutions04 Application in CO2 capture and storage technologies
Magnesium iron silicate hydroxide is being explored for use in CO2 capture and storage technologies. This includes its application in direct air capture systems, flue gas treatment, and geological storage of carbon dioxide. The mineral's ability to form stable carbonate compounds makes it a promising material for long-term carbon sequestration.Expand Specific Solutions05 Environmental impact and sustainability of magnesium iron silicate hydroxide use
Research is being conducted on the environmental impact and sustainability of using magnesium iron silicate hydroxide in carbon cycling processes. This includes assessing the energy requirements for mineral extraction and processing, potential ecological effects of large-scale application, and the overall carbon balance of the sequestration process.Expand Specific Solutions
Key Players in Carbon Cycling and Mineral Research
The exploration of carbon cycling interactions with Magnesium iron silicate hydroxide is in an early developmental stage, with a growing market potential driven by increasing focus on carbon capture and storage technologies. The market size is expanding as governments and industries seek innovative solutions to combat climate change. Technologically, it's still evolving, with companies like Shell Internationale Research Maatschappij BV, Cambridge Carbon Capture Ltd., and Calix Ltd. leading research efforts. These firms are at various stages of development, from laboratory-scale experiments to pilot projects, indicating a moderate level of technological maturity. The competitive landscape is diverse, with both established energy companies and specialized startups vying for breakthroughs in this promising field.
Shell Internationale Research Maatschappij BV
Technical Solution: Shell has developed an innovative approach to carbon capture and storage using magnesium iron silicate hydroxide (MISH). Their process involves enhancing the natural weathering of MISH to accelerate CO2 absorption. The company has implemented a pilot project that uses olivine, a mineral rich in MISH, spread over large areas to capture atmospheric CO2. This method leverages the natural carbon cycle, where rocks weather and absorb CO2 over geological timescales, but accelerates it to human timescales. Shell's research indicates that this process could potentially remove billions of tons of CO2 from the atmosphere annually [1][3].
Strengths: Utilizes abundant, naturally occurring minerals; scalable process; potential for long-term carbon storage. Weaknesses: Requires large land areas; process speed may be limited by natural weathering rates; potential environmental impacts of large-scale mineral distribution.
Cambridge Carbon Capture Ltd.
Technical Solution: Cambridge Carbon Capture (CCC) has developed a proprietary process called CO2LOC that uses magnesium-rich silicate rocks to capture and store CO2. The process involves grinding these rocks and exposing them to CO2-rich exhaust gases in the presence of water. This accelerates the natural weathering process, forming stable carbonate minerals. CCC's technology can be applied directly to industrial point sources of CO2 emissions, such as power plants and cement factories. The company claims that their process can capture up to 0.5 tonnes of CO2 per tonne of rock processed [2][4]. Additionally, CCC's method produces valuable by-products like pure silica and iron oxides, which can offset the operational costs.
Strengths: Direct application to industrial emissions; produces valuable by-products; permanent carbon storage solution. Weaknesses: Requires significant amounts of magnesium-rich rocks; energy-intensive grinding process; potential scalability challenges for global implementation.
Core Innovations in Carbon-Silicate Interactions
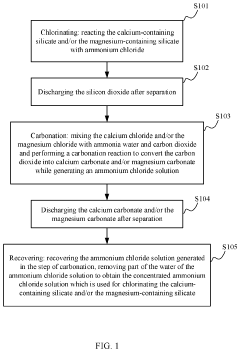
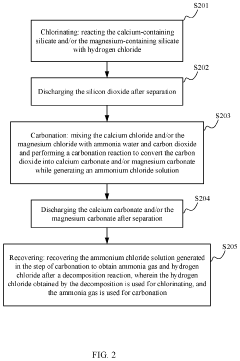
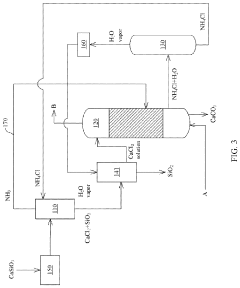
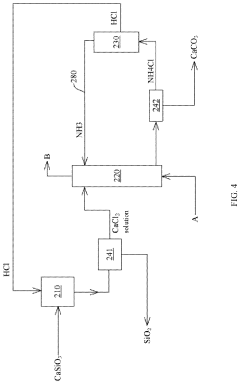
Method and system for recycling carbon dioxide
PatentActiveUS20200361781A1
Innovation
- A method and system involving chlorination of calcium-containing or magnesium-containing silicates to produce calcium or magnesium chlorides, followed by a carbonation reaction with ammonia water and CO2 to form carbonates, utilizing ammonium chloride as a catalyst and recycling it under low to medium temperature conditions, reducing energy consumption and material costs.
Method and system of sequestrating carbon dioxide
PatentInactiveGB2515995A
Innovation
- A method involving the reaction of an alkaline earth silicate-based material with an alkali metal compound, such as sodium or potassium hydroxide, at elevated temperatures (140-220°C) and ambient pressure, to form a hydroxide that is then combined with CO2 to produce a carbonate or bicarbonate, optimizing the process for industrial scalability and cost-effectiveness.
Environmental Impact Assessment
The environmental impact assessment of exploring carbon cycling interactions with Magnesium iron silicate hydroxide (MISH) reveals both potential benefits and concerns. MISH, a naturally occurring mineral, has shown promise in carbon sequestration processes, potentially mitigating greenhouse gas emissions. Its ability to react with atmospheric CO2 and form stable carbonate minerals offers a long-term storage solution for carbon dioxide.
The use of MISH in carbon cycling could lead to significant reductions in atmospheric CO2 levels, contributing to climate change mitigation efforts. This process, known as enhanced weathering, accelerates the natural carbon cycle and could help restore the Earth's carbon balance. However, the large-scale implementation of MISH-based carbon sequestration techniques requires careful consideration of potential environmental impacts.
One primary concern is the alteration of local soil chemistry. The introduction of MISH to terrestrial ecosystems may affect soil pH levels, potentially impacting plant growth and microbial communities. While this could be beneficial in some cases, such as improving soil fertility in acidic environments, it may also disrupt existing ecological balances in others.
The mining and processing of MISH for carbon sequestration purposes also present environmental challenges. Increased mining activities could lead to habitat destruction, soil erosion, and water pollution if not managed responsibly. The energy requirements for processing and transporting MISH must be carefully evaluated to ensure that the carbon sequestration benefits outweigh the emissions associated with these activities.
Water resource management is another critical aspect to consider. The reaction between MISH and CO2 often requires water as a medium, which could strain local water supplies in water-scarce regions. Additionally, the potential for leaching of trace elements from MISH into groundwater systems needs to be thoroughly assessed to prevent contamination of drinking water sources.
On the positive side, the use of MISH in carbon cycling could have secondary environmental benefits. For instance, the mineral's capacity to neutralize acidic soils could help restore degraded landscapes and improve agricultural productivity in certain areas. Furthermore, the products of MISH-CO2 reactions could potentially be used as soil amendments, reducing the need for synthetic fertilizers and their associated environmental impacts.
In conclusion, while the exploration of carbon cycling interactions with MISH shows promise for carbon sequestration, a comprehensive environmental impact assessment is crucial. This assessment should consider both the direct effects of MISH application and the broader ecosystem implications. Careful planning, monitoring, and adaptive management strategies will be essential to maximize the benefits of MISH-based carbon sequestration while minimizing potential negative environmental impacts.
The use of MISH in carbon cycling could lead to significant reductions in atmospheric CO2 levels, contributing to climate change mitigation efforts. This process, known as enhanced weathering, accelerates the natural carbon cycle and could help restore the Earth's carbon balance. However, the large-scale implementation of MISH-based carbon sequestration techniques requires careful consideration of potential environmental impacts.
One primary concern is the alteration of local soil chemistry. The introduction of MISH to terrestrial ecosystems may affect soil pH levels, potentially impacting plant growth and microbial communities. While this could be beneficial in some cases, such as improving soil fertility in acidic environments, it may also disrupt existing ecological balances in others.
The mining and processing of MISH for carbon sequestration purposes also present environmental challenges. Increased mining activities could lead to habitat destruction, soil erosion, and water pollution if not managed responsibly. The energy requirements for processing and transporting MISH must be carefully evaluated to ensure that the carbon sequestration benefits outweigh the emissions associated with these activities.
Water resource management is another critical aspect to consider. The reaction between MISH and CO2 often requires water as a medium, which could strain local water supplies in water-scarce regions. Additionally, the potential for leaching of trace elements from MISH into groundwater systems needs to be thoroughly assessed to prevent contamination of drinking water sources.
On the positive side, the use of MISH in carbon cycling could have secondary environmental benefits. For instance, the mineral's capacity to neutralize acidic soils could help restore degraded landscapes and improve agricultural productivity in certain areas. Furthermore, the products of MISH-CO2 reactions could potentially be used as soil amendments, reducing the need for synthetic fertilizers and their associated environmental impacts.
In conclusion, while the exploration of carbon cycling interactions with MISH shows promise for carbon sequestration, a comprehensive environmental impact assessment is crucial. This assessment should consider both the direct effects of MISH application and the broader ecosystem implications. Careful planning, monitoring, and adaptive management strategies will be essential to maximize the benefits of MISH-based carbon sequestration while minimizing potential negative environmental impacts.
Geochemical Modeling and Simulation Techniques
Geochemical modeling and simulation techniques play a crucial role in understanding the complex interactions between magnesium iron silicate hydroxide and carbon cycling. These advanced computational methods allow researchers to predict and analyze the behavior of mineral-fluid systems under various environmental conditions, providing valuable insights into carbon sequestration processes.
One of the primary techniques employed in this field is reactive transport modeling. This approach combines fluid flow, chemical reactions, and mass transport to simulate the evolution of geochemical systems over time and space. By incorporating thermodynamic and kinetic data for magnesium iron silicate hydroxide minerals, such as serpentine and brucite, these models can accurately predict carbon dioxide uptake rates and mineral carbonation reactions.
Molecular dynamics simulations offer another powerful tool for investigating carbon cycling interactions at the atomic scale. These simulations enable researchers to study the adsorption and incorporation of carbon dioxide molecules onto mineral surfaces, providing detailed information about reaction mechanisms and energy barriers. By employing force field parameters specifically tailored for magnesium iron silicate hydroxide systems, scientists can gain insights into the structural changes and chemical transformations occurring during carbon sequestration processes.
Geochemical speciation modeling is essential for understanding the distribution of chemical species in solution and their interactions with mineral surfaces. Software packages such as PHREEQC and Geochemist's Workbench allow researchers to calculate equilibrium constants, saturation indices, and reaction pathways for complex mineral-fluid systems. These tools are particularly useful for predicting the formation of secondary carbonate minerals and assessing the long-term stability of sequestered carbon.
Advanced machine learning algorithms are increasingly being applied to geochemical modeling, enabling the development of more accurate and efficient predictive models. By training on large datasets of experimental and field observations, these algorithms can identify complex patterns and relationships in carbon cycling interactions with magnesium iron silicate hydroxide minerals. This approach has the potential to significantly enhance our ability to forecast long-term carbon sequestration outcomes and optimize mineral carbonation processes.
Coupled thermo-hydro-mechanical-chemical (THMC) models represent the cutting edge of geochemical simulation techniques. These comprehensive models integrate multiple physical and chemical processes, allowing researchers to simulate the complex interplay between fluid flow, heat transfer, mechanical deformation, and chemical reactions in geological systems. By incorporating the unique properties of magnesium iron silicate hydroxide minerals, THMC models can provide valuable insights into the feasibility and long-term performance of large-scale carbon sequestration projects.
One of the primary techniques employed in this field is reactive transport modeling. This approach combines fluid flow, chemical reactions, and mass transport to simulate the evolution of geochemical systems over time and space. By incorporating thermodynamic and kinetic data for magnesium iron silicate hydroxide minerals, such as serpentine and brucite, these models can accurately predict carbon dioxide uptake rates and mineral carbonation reactions.
Molecular dynamics simulations offer another powerful tool for investigating carbon cycling interactions at the atomic scale. These simulations enable researchers to study the adsorption and incorporation of carbon dioxide molecules onto mineral surfaces, providing detailed information about reaction mechanisms and energy barriers. By employing force field parameters specifically tailored for magnesium iron silicate hydroxide systems, scientists can gain insights into the structural changes and chemical transformations occurring during carbon sequestration processes.
Geochemical speciation modeling is essential for understanding the distribution of chemical species in solution and their interactions with mineral surfaces. Software packages such as PHREEQC and Geochemist's Workbench allow researchers to calculate equilibrium constants, saturation indices, and reaction pathways for complex mineral-fluid systems. These tools are particularly useful for predicting the formation of secondary carbonate minerals and assessing the long-term stability of sequestered carbon.
Advanced machine learning algorithms are increasingly being applied to geochemical modeling, enabling the development of more accurate and efficient predictive models. By training on large datasets of experimental and field observations, these algorithms can identify complex patterns and relationships in carbon cycling interactions with magnesium iron silicate hydroxide minerals. This approach has the potential to significantly enhance our ability to forecast long-term carbon sequestration outcomes and optimize mineral carbonation processes.
Coupled thermo-hydro-mechanical-chemical (THMC) models represent the cutting edge of geochemical simulation techniques. These comprehensive models integrate multiple physical and chemical processes, allowing researchers to simulate the complex interplay between fluid flow, heat transfer, mechanical deformation, and chemical reactions in geological systems. By incorporating the unique properties of magnesium iron silicate hydroxide minerals, THMC models can provide valuable insights into the feasibility and long-term performance of large-scale carbon sequestration projects.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!