Gel Electrophoresis for Lateral Flow Assays: Innovative Methods
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Gel Electrophoresis LFA Background and Objectives
Gel electrophoresis has been a cornerstone technique in molecular biology for decades, primarily used for separating and analyzing DNA, RNA, and proteins. In recent years, there has been a growing interest in applying this powerful technique to lateral flow assays (LFAs), a widely used point-of-care diagnostic platform. This innovative combination aims to enhance the sensitivity, specificity, and multiplexing capabilities of traditional LFAs.
The evolution of gel electrophoresis in the context of LFAs can be traced back to the early 2000s when researchers began exploring ways to integrate electrophoretic separation into rapid diagnostic tests. The primary goal was to overcome the limitations of conventional LFAs, such as their inability to distinguish between closely related analytes and their limited quantitative capabilities.
As the field progressed, several key technological advancements emerged. One significant development was the miniaturization of electrophoretic systems, allowing for their integration into compact, portable devices suitable for point-of-care testing. This miniaturization was facilitated by advancements in microfluidics and lab-on-a-chip technologies, which enabled the creation of microscale electrophoretic channels within LFA devices.
Another crucial advancement was the development of novel gel materials specifically designed for use in LFAs. These materials needed to combine the separation capabilities of traditional electrophoresis gels with the rapid flow characteristics required for lateral flow devices. Researchers explored various polymer compositions and nanostructured materials to achieve this balance.
The integration of gel electrophoresis into LFAs aims to achieve several objectives. Firstly, it seeks to improve the analytical resolution of LFAs, allowing for the separation and identification of multiple analytes within a single test. This enhanced multiplexing capability is particularly valuable in scenarios where multiple biomarkers need to be detected simultaneously, such as in the diagnosis of complex diseases or in environmental monitoring.
Secondly, the incorporation of gel electrophoresis aims to increase the sensitivity of LFAs. By pre-concentrating analytes through electrophoretic focusing, even low abundance molecules can be detected, potentially rivaling the sensitivity of more complex laboratory-based techniques.
Lastly, this innovative approach seeks to expand the range of analytes that can be detected using LFAs. While traditional LFAs are primarily used for protein and small molecule detection, the integration of gel electrophoresis opens up possibilities for nucleic acid-based tests and other complex biomolecules.
As research in this field continues, the ultimate goal is to develop a new generation of LFAs that combine the simplicity and rapid results of traditional lateral flow tests with the analytical power of gel electrophoresis. This fusion of technologies has the potential to revolutionize point-of-care diagnostics, offering high-performance, multi-analyte testing in resource-limited settings.
The evolution of gel electrophoresis in the context of LFAs can be traced back to the early 2000s when researchers began exploring ways to integrate electrophoretic separation into rapid diagnostic tests. The primary goal was to overcome the limitations of conventional LFAs, such as their inability to distinguish between closely related analytes and their limited quantitative capabilities.
As the field progressed, several key technological advancements emerged. One significant development was the miniaturization of electrophoretic systems, allowing for their integration into compact, portable devices suitable for point-of-care testing. This miniaturization was facilitated by advancements in microfluidics and lab-on-a-chip technologies, which enabled the creation of microscale electrophoretic channels within LFA devices.
Another crucial advancement was the development of novel gel materials specifically designed for use in LFAs. These materials needed to combine the separation capabilities of traditional electrophoresis gels with the rapid flow characteristics required for lateral flow devices. Researchers explored various polymer compositions and nanostructured materials to achieve this balance.
The integration of gel electrophoresis into LFAs aims to achieve several objectives. Firstly, it seeks to improve the analytical resolution of LFAs, allowing for the separation and identification of multiple analytes within a single test. This enhanced multiplexing capability is particularly valuable in scenarios where multiple biomarkers need to be detected simultaneously, such as in the diagnosis of complex diseases or in environmental monitoring.
Secondly, the incorporation of gel electrophoresis aims to increase the sensitivity of LFAs. By pre-concentrating analytes through electrophoretic focusing, even low abundance molecules can be detected, potentially rivaling the sensitivity of more complex laboratory-based techniques.
Lastly, this innovative approach seeks to expand the range of analytes that can be detected using LFAs. While traditional LFAs are primarily used for protein and small molecule detection, the integration of gel electrophoresis opens up possibilities for nucleic acid-based tests and other complex biomolecules.
As research in this field continues, the ultimate goal is to develop a new generation of LFAs that combine the simplicity and rapid results of traditional lateral flow tests with the analytical power of gel electrophoresis. This fusion of technologies has the potential to revolutionize point-of-care diagnostics, offering high-performance, multi-analyte testing in resource-limited settings.
Market Analysis for Gel Electrophoresis LFA
The market for gel electrophoresis in lateral flow assays (LFAs) is experiencing significant growth, driven by the increasing demand for rapid and accurate diagnostic tools. This innovative combination of technologies addresses the limitations of traditional LFAs, offering enhanced sensitivity and quantitative capabilities.
The global lateral flow assay market was valued at approximately $8.7 billion in 2021 and is projected to reach $13.3 billion by 2028, growing at a CAGR of 6.2% during the forecast period. Within this broader market, the segment incorporating gel electrophoresis techniques is gaining traction, particularly in applications requiring higher sensitivity and more precise quantification.
Key factors driving market growth include the rising prevalence of infectious diseases, the need for point-of-care testing, and the increasing adoption of rapid diagnostic tests in both developed and developing countries. The COVID-19 pandemic has further accelerated the demand for rapid, reliable diagnostic solutions, creating new opportunities for innovative LFA technologies.
The healthcare sector remains the primary end-user of gel electrophoresis LFAs, with applications spanning infectious disease diagnosis, pregnancy testing, drug abuse screening, and biomarker detection. The veterinary and environmental testing sectors are also emerging as significant markets, driven by the need for on-site, rapid testing solutions.
Geographically, North America and Europe currently dominate the market due to advanced healthcare infrastructure and higher adoption rates of new technologies. However, the Asia-Pacific region is expected to witness the fastest growth, fueled by improving healthcare systems, increasing awareness, and rising investments in diagnostic technologies.
Key players in the gel electrophoresis LFA market include established diagnostic companies like Abbott Laboratories, Roche Diagnostics, and Quidel Corporation, as well as innovative startups focusing on novel LFA technologies. These companies are investing heavily in R&D to develop more sensitive, specific, and user-friendly assays that incorporate gel electrophoresis principles.
Challenges in the market include the need for standardization of gel electrophoresis LFA protocols, regulatory hurdles for novel diagnostic technologies, and competition from other emerging diagnostic platforms. However, the potential for improved diagnostic accuracy and expanded applications continues to drive interest and investment in this field.
In conclusion, the market for gel electrophoresis in lateral flow assays presents significant opportunities for growth and innovation. As the technology matures and finds wider acceptance, it is poised to play a crucial role in advancing point-of-care diagnostics and addressing global healthcare challenges.
The global lateral flow assay market was valued at approximately $8.7 billion in 2021 and is projected to reach $13.3 billion by 2028, growing at a CAGR of 6.2% during the forecast period. Within this broader market, the segment incorporating gel electrophoresis techniques is gaining traction, particularly in applications requiring higher sensitivity and more precise quantification.
Key factors driving market growth include the rising prevalence of infectious diseases, the need for point-of-care testing, and the increasing adoption of rapid diagnostic tests in both developed and developing countries. The COVID-19 pandemic has further accelerated the demand for rapid, reliable diagnostic solutions, creating new opportunities for innovative LFA technologies.
The healthcare sector remains the primary end-user of gel electrophoresis LFAs, with applications spanning infectious disease diagnosis, pregnancy testing, drug abuse screening, and biomarker detection. The veterinary and environmental testing sectors are also emerging as significant markets, driven by the need for on-site, rapid testing solutions.
Geographically, North America and Europe currently dominate the market due to advanced healthcare infrastructure and higher adoption rates of new technologies. However, the Asia-Pacific region is expected to witness the fastest growth, fueled by improving healthcare systems, increasing awareness, and rising investments in diagnostic technologies.
Key players in the gel electrophoresis LFA market include established diagnostic companies like Abbott Laboratories, Roche Diagnostics, and Quidel Corporation, as well as innovative startups focusing on novel LFA technologies. These companies are investing heavily in R&D to develop more sensitive, specific, and user-friendly assays that incorporate gel electrophoresis principles.
Challenges in the market include the need for standardization of gel electrophoresis LFA protocols, regulatory hurdles for novel diagnostic technologies, and competition from other emerging diagnostic platforms. However, the potential for improved diagnostic accuracy and expanded applications continues to drive interest and investment in this field.
In conclusion, the market for gel electrophoresis in lateral flow assays presents significant opportunities for growth and innovation. As the technology matures and finds wider acceptance, it is poised to play a crucial role in advancing point-of-care diagnostics and addressing global healthcare challenges.
Current Challenges in Gel Electrophoresis LFA
Gel electrophoresis has been a cornerstone technique in lateral flow assays (LFAs) for decades, offering rapid and cost-effective separation of biomolecules. However, as the demand for more sensitive and specific diagnostic tools grows, several challenges have emerged in the application of gel electrophoresis to LFAs.
One of the primary challenges is the limited resolution of traditional gel electrophoresis methods when applied to LFAs. The compact nature of lateral flow devices often restricts the gel length, resulting in reduced separation efficiency for complex biological samples. This limitation becomes particularly problematic when dealing with closely related biomolecules or low-abundance targets, where precise separation is crucial for accurate diagnosis.
Another significant hurdle is the integration of gel electrophoresis into the compact format of LFAs without compromising the simplicity and rapidity that make these assays attractive. The need for external power sources and buffer systems in conventional gel electrophoresis setups conflicts with the self-contained nature of LFAs, posing design and manufacturing challenges.
The stability and shelf-life of gel-based LFAs present additional concerns. Gel materials can degrade over time, affecting the consistency and reliability of test results. This issue is particularly relevant in resource-limited settings or when long-term storage is required, necessitating the development of more robust gel formulations or alternative separation methods.
Sensitivity and detection limits remain critical challenges in gel electrophoresis LFAs. As diagnostic requirements become more stringent, there is a growing need for techniques that can detect and quantify extremely low concentrations of analytes. Current gel-based methods often struggle to meet these sensitivity demands, especially when compared to more advanced laboratory techniques.
The speed of analysis is another area where improvements are needed. While LFAs are generally rapid, the incorporation of gel electrophoresis can introduce time delays that may be unacceptable in certain diagnostic scenarios, such as point-of-care testing or emergency situations.
Lastly, the reproducibility and standardization of gel electrophoresis in LFAs pose significant challenges. Variations in gel composition, electrophoresis conditions, and sample preparation can lead to inconsistent results across different batches or testing sites. Addressing these variability issues is crucial for the widespread adoption and reliability of gel-based LFAs in clinical and field settings.
One of the primary challenges is the limited resolution of traditional gel electrophoresis methods when applied to LFAs. The compact nature of lateral flow devices often restricts the gel length, resulting in reduced separation efficiency for complex biological samples. This limitation becomes particularly problematic when dealing with closely related biomolecules or low-abundance targets, where precise separation is crucial for accurate diagnosis.
Another significant hurdle is the integration of gel electrophoresis into the compact format of LFAs without compromising the simplicity and rapidity that make these assays attractive. The need for external power sources and buffer systems in conventional gel electrophoresis setups conflicts with the self-contained nature of LFAs, posing design and manufacturing challenges.
The stability and shelf-life of gel-based LFAs present additional concerns. Gel materials can degrade over time, affecting the consistency and reliability of test results. This issue is particularly relevant in resource-limited settings or when long-term storage is required, necessitating the development of more robust gel formulations or alternative separation methods.
Sensitivity and detection limits remain critical challenges in gel electrophoresis LFAs. As diagnostic requirements become more stringent, there is a growing need for techniques that can detect and quantify extremely low concentrations of analytes. Current gel-based methods often struggle to meet these sensitivity demands, especially when compared to more advanced laboratory techniques.
The speed of analysis is another area where improvements are needed. While LFAs are generally rapid, the incorporation of gel electrophoresis can introduce time delays that may be unacceptable in certain diagnostic scenarios, such as point-of-care testing or emergency situations.
Lastly, the reproducibility and standardization of gel electrophoresis in LFAs pose significant challenges. Variations in gel composition, electrophoresis conditions, and sample preparation can lead to inconsistent results across different batches or testing sites. Addressing these variability issues is crucial for the widespread adoption and reliability of gel-based LFAs in clinical and field settings.
Existing Gel Electrophoresis LFA Methods
01 Microfluidic gel electrophoresis systems
Innovative methods in gel electrophoresis involve the development of microfluidic systems. These systems miniaturize the electrophoresis process, allowing for faster analysis, reduced sample and reagent consumption, and improved resolution. Microfluidic devices can integrate multiple steps of the electrophoresis process, including sample preparation, separation, and detection, into a single chip.- Microfluidic gel electrophoresis systems: Innovative methods in gel electrophoresis involve the development of microfluidic systems. These systems miniaturize the electrophoresis process, allowing for faster analysis, reduced sample and reagent consumption, and improved resolution. Microfluidic devices can integrate multiple steps of the electrophoresis process, including sample preparation, separation, and detection, into a single chip.
- Novel gel materials and compositions: Advancements in gel electrophoresis include the development of new gel materials and compositions. These innovative gels offer improved separation efficiency, increased resolution, and enhanced compatibility with various biomolecules. Some novel gel formulations incorporate nanoparticles or other additives to modify the gel's properties and optimize separation performance.
- Multi-dimensional gel electrophoresis techniques: Innovative methods in gel electrophoresis include the development of multi-dimensional separation techniques. These approaches combine different separation principles or conditions to achieve higher resolution and better separation of complex samples. Two-dimensional and three-dimensional gel electrophoresis systems allow for the analysis of proteins based on multiple properties, such as size, charge, and isoelectric point.
- Advanced detection and imaging methods: Innovative gel electrophoresis methods incorporate advanced detection and imaging techniques. These include the use of fluorescent labels, chemiluminescent markers, and high-sensitivity cameras to visualize and quantify separated biomolecules. Some systems integrate real-time monitoring capabilities, allowing researchers to observe the separation process as it occurs and make adjustments on the fly.
- Automated and high-throughput gel electrophoresis systems: Innovative methods in gel electrophoresis focus on developing automated and high-throughput systems. These systems can handle multiple samples simultaneously, reducing analysis time and increasing laboratory efficiency. Automated systems often integrate sample loading, electrophoresis, staining, and imaging steps, minimizing human intervention and improving reproducibility.
02 Novel gel materials and compositions
Advancements in gel materials and compositions have led to improved separation efficiency and resolution in gel electrophoresis. These innovations include the development of new polymer formulations, incorporation of nanomaterials, and creation of stimuli-responsive gels. Such materials can enhance the separation of biomolecules, increase the range of molecular weights that can be analyzed, and allow for better control over the electrophoresis process.Expand Specific Solutions03 Multi-dimensional gel electrophoresis techniques
Innovative methods in multi-dimensional gel electrophoresis have been developed to improve the separation and analysis of complex mixtures. These techniques involve combining different separation principles, such as isoelectric focusing and size-based separation, in sequential steps. This approach allows for better resolution and identification of proteins or other biomolecules in complex samples.Expand Specific Solutions04 Integration of detection and imaging technologies
Advancements in gel electrophoresis methods include the integration of novel detection and imaging technologies. These innovations involve the use of fluorescent labels, chemiluminescent markers, and high-resolution imaging systems to improve the sensitivity and accuracy of biomolecule detection. Some methods also incorporate real-time monitoring of the separation process, allowing for dynamic adjustments and optimizations.Expand Specific Solutions05 Automated and high-throughput gel electrophoresis systems
Innovative methods in gel electrophoresis focus on developing automated and high-throughput systems. These systems incorporate robotics, advanced software, and parallel processing capabilities to increase sample throughput and reduce manual intervention. Such advancements allow for faster analysis of large numbers of samples, improved reproducibility, and integration with other analytical techniques.Expand Specific Solutions
Key Players in Gel Electrophoresis LFA Industry
The gel electrophoresis for lateral flow assays market is in a growth phase, driven by increasing demand for rapid diagnostic tests. The global market size is projected to expand significantly due to applications in healthcare, environmental monitoring, and food safety. Technologically, the field is advancing rapidly, with companies like Agilent Technologies, Life Technologies, and Beckman Coulter leading innovation. These firms are developing more sensitive and efficient electrophoresis methods for lateral flow devices. Emerging players such as Nanjing Genscript and ARKRAY are also contributing to technological advancements, particularly in miniaturization and integration with microfluidic systems. The competitive landscape is characterized by a mix of established biotechnology giants and specialized diagnostic companies, all vying to improve assay performance and expand applications.
Agilent Technologies, Inc.
Technical Solution: Agilent has pioneered a hybrid approach combining gel electrophoresis and lateral flow assays, focusing on multiplexing capabilities. Their system utilizes a specially designed gel matrix that allows for simultaneous separation of multiple analytes based on both size and affinity[7]. This is achieved through the incorporation of specific binding molecules within distinct regions of the gel. After electrophoresis, the separated analytes are transferred to a lateral flow membrane with multiple detection zones. Agilent's technology also includes a novel buffer system that maintains protein stability during electrophoresis while allowing for efficient transfer to the lateral flow membrane[9]. The company has developed a proprietary detection system that can simultaneously read multiple analytes on the lateral flow strip, enabling high-throughput analysis[11].
Strengths: High multiplexing capability, efficient use of sample by combining separation and detection, potential for high-throughput screening. Weaknesses: Complex gel preparation process, may require specialized detection equipment for multiplexed readout.
Life Technologies Corp.
Technical Solution: Life Technologies has developed an innovative approach to integrating gel electrophoresis with lateral flow assays, focusing on enhancing sensitivity for nucleic acid detection. Their system utilizes a specialized gel matrix that allows for size-based separation of nucleic acids, followed by sequence-specific capture on a lateral flow strip[8]. The company has introduced a proprietary gel composition that enables rapid migration of nucleic acids while maintaining high resolution. Life Technologies' approach also incorporates a unique amplification step within the gel matrix, allowing for on-gel PCR or isothermal amplification prior to lateral flow detection[10]. This integration of amplification and separation significantly enhances the sensitivity of the assay. Additionally, the company has developed a novel lateral flow strip design with multiple capture zones, enabling the simultaneous detection of multiple nucleic acid targets[12].
Strengths: High sensitivity for nucleic acid detection, integration of amplification step, capability for multiplex detection. Weaknesses: May be limited to nucleic acid targets, potentially more complex workflow compared to standard lateral flow assays.
Core Innovations in Gel Electrophoresis LFA
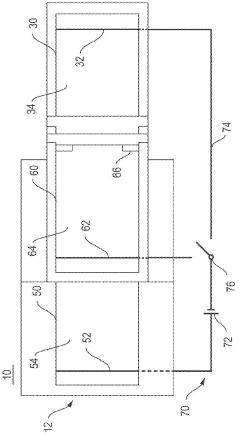
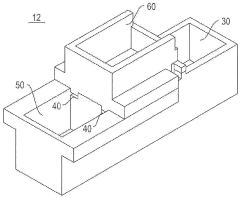
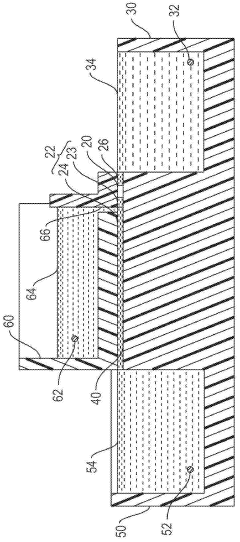
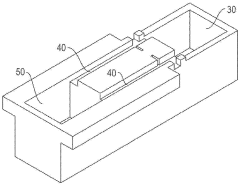
Gel electrophoresis apparatus and gel electrophoresis method
PatentWO2015133190A1
Innovation
- A gel electrophoresis apparatus and method that includes a well for whole blood, with a power supply system to switch voltage application between electrodes, allowing electrophoresis to resume by switching from the first and second electrodes to the second and third electrodes, preventing blood cells from accumulating in the electrophoretic gel and maintaining the energizing function of the second and third electrodes.
Separation and identification of analytes by GEL electrophoresis
PatentWO2005088292A1
Innovation
- The use of non-polymeric small molecule organogels and hydrogels for analyte separation by applying an electric field, allowing direct transfer of separated analytes to a mass spectrometer for identification and quantification, eliminating the need for complex extraction procedures.
Regulatory Considerations for LFA Devices
Regulatory considerations play a crucial role in the development and commercialization of Lateral Flow Assay (LFA) devices, particularly when incorporating innovative methods such as gel electrophoresis. The regulatory landscape for these devices is complex and varies across different regions, with the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA) being two of the most influential regulatory bodies.
In the United States, LFA devices are typically classified as in vitro diagnostic (IVD) medical devices and are subject to FDA regulations. The specific regulatory pathway depends on the intended use and risk classification of the device. For LFAs incorporating gel electrophoresis, the novel nature of this combination may require a more rigorous regulatory review process, potentially falling under the De Novo classification or Premarket Approval (PMA) pathway.
The FDA's approach to regulating LFA devices focuses on ensuring safety, effectiveness, and quality. Manufacturers must demonstrate that their devices meet these criteria through extensive analytical and clinical performance studies. For LFAs utilizing gel electrophoresis, additional validation may be required to prove the reliability and reproducibility of this innovative method in the context of lateral flow technology.
In the European Union, LFA devices are regulated under the In Vitro Diagnostic Medical Devices Regulation (IVDR), which came into full effect in May 2022. The IVDR introduces a risk-based classification system and more stringent requirements for clinical evidence and post-market surveillance. LFAs incorporating gel electrophoresis may be classified in a higher risk category due to their novel nature, potentially requiring notified body involvement in the conformity assessment process.
Regulatory bodies worldwide are increasingly focusing on the validation of manufacturing processes and quality management systems. For LFAs with gel electrophoresis, this may include specific considerations for the production and integration of gel components, as well as the overall stability and shelf-life of the combined device.
As the field of LFA technology continues to evolve, regulatory agencies are adapting their approaches to keep pace with innovations. Manufacturers developing LFAs with gel electrophoresis should engage in early and frequent communication with regulatory authorities to navigate the complex landscape and ensure compliance throughout the product development lifecycle.
In the United States, LFA devices are typically classified as in vitro diagnostic (IVD) medical devices and are subject to FDA regulations. The specific regulatory pathway depends on the intended use and risk classification of the device. For LFAs incorporating gel electrophoresis, the novel nature of this combination may require a more rigorous regulatory review process, potentially falling under the De Novo classification or Premarket Approval (PMA) pathway.
The FDA's approach to regulating LFA devices focuses on ensuring safety, effectiveness, and quality. Manufacturers must demonstrate that their devices meet these criteria through extensive analytical and clinical performance studies. For LFAs utilizing gel electrophoresis, additional validation may be required to prove the reliability and reproducibility of this innovative method in the context of lateral flow technology.
In the European Union, LFA devices are regulated under the In Vitro Diagnostic Medical Devices Regulation (IVDR), which came into full effect in May 2022. The IVDR introduces a risk-based classification system and more stringent requirements for clinical evidence and post-market surveillance. LFAs incorporating gel electrophoresis may be classified in a higher risk category due to their novel nature, potentially requiring notified body involvement in the conformity assessment process.
Regulatory bodies worldwide are increasingly focusing on the validation of manufacturing processes and quality management systems. For LFAs with gel electrophoresis, this may include specific considerations for the production and integration of gel components, as well as the overall stability and shelf-life of the combined device.
As the field of LFA technology continues to evolve, regulatory agencies are adapting their approaches to keep pace with innovations. Manufacturers developing LFAs with gel electrophoresis should engage in early and frequent communication with regulatory authorities to navigate the complex landscape and ensure compliance throughout the product development lifecycle.
Cost-Effectiveness Analysis of Gel Electrophoresis LFA
The cost-effectiveness analysis of gel electrophoresis for lateral flow assays (LFAs) reveals a complex interplay of factors that influence the overall economic viability of this innovative method. Initial investment in gel electrophoresis equipment and materials may present a higher upfront cost compared to traditional LFA production methods. However, this increased initial expenditure is often offset by improved assay performance and reduced long-term operational costs.
Gel electrophoresis LFAs demonstrate enhanced sensitivity and specificity, leading to more accurate test results. This improvement in diagnostic accuracy can significantly reduce false positives and negatives, thereby minimizing the need for repeat testing and follow-up procedures. The reduction in unnecessary medical interventions and associated costs contributes to the overall cost-effectiveness of the method.
The scalability of gel electrophoresis LFA production is another crucial factor in its cost-effectiveness analysis. While the initial setup may require specialized equipment, the process can be readily automated for high-throughput manufacturing. This automation potential allows for economies of scale, reducing per-unit production costs as volume increases. Additionally, the consistency and quality control afforded by gel electrophoresis methods can lead to fewer product rejections and waste, further improving cost efficiency.
Material costs for gel electrophoresis LFAs may vary depending on the specific reagents and gels used. However, the ability to precisely control sample and reagent volumes through electrophoretic separation often results in more efficient use of materials. This optimization can lead to reduced reagent consumption and lower per-test costs compared to traditional LFA production methods.
The durability and shelf life of gel electrophoresis LFAs also factor into their cost-effectiveness. The improved stability of biomolecules within the gel matrix can extend the shelf life of the final product, reducing inventory turnover and associated costs. This increased longevity is particularly valuable in resource-limited settings or for tests that are not frequently used but must be readily available.
When considering the broader economic impact, gel electrophoresis LFAs may offer advantages in terms of reduced healthcare costs and improved patient outcomes. The higher accuracy and sensitivity of these tests can lead to earlier disease detection and intervention, potentially reducing the overall cost of treatment and improving patient prognosis. This aspect, while challenging to quantify precisely, should be factored into comprehensive cost-effectiveness analyses.
In conclusion, while gel electrophoresis LFAs may present higher initial costs, their improved performance, scalability, and potential for long-term cost savings make them an economically viable option in many scenarios. The cost-effectiveness of this method is likely to improve further as the technology matures and becomes more widely adopted, potentially leading to reduced equipment and material costs in the future.
Gel electrophoresis LFAs demonstrate enhanced sensitivity and specificity, leading to more accurate test results. This improvement in diagnostic accuracy can significantly reduce false positives and negatives, thereby minimizing the need for repeat testing and follow-up procedures. The reduction in unnecessary medical interventions and associated costs contributes to the overall cost-effectiveness of the method.
The scalability of gel electrophoresis LFA production is another crucial factor in its cost-effectiveness analysis. While the initial setup may require specialized equipment, the process can be readily automated for high-throughput manufacturing. This automation potential allows for economies of scale, reducing per-unit production costs as volume increases. Additionally, the consistency and quality control afforded by gel electrophoresis methods can lead to fewer product rejections and waste, further improving cost efficiency.
Material costs for gel electrophoresis LFAs may vary depending on the specific reagents and gels used. However, the ability to precisely control sample and reagent volumes through electrophoretic separation often results in more efficient use of materials. This optimization can lead to reduced reagent consumption and lower per-test costs compared to traditional LFA production methods.
The durability and shelf life of gel electrophoresis LFAs also factor into their cost-effectiveness. The improved stability of biomolecules within the gel matrix can extend the shelf life of the final product, reducing inventory turnover and associated costs. This increased longevity is particularly valuable in resource-limited settings or for tests that are not frequently used but must be readily available.
When considering the broader economic impact, gel electrophoresis LFAs may offer advantages in terms of reduced healthcare costs and improved patient outcomes. The higher accuracy and sensitivity of these tests can lead to earlier disease detection and intervention, potentially reducing the overall cost of treatment and improving patient prognosis. This aspect, while challenging to quantify precisely, should be factored into comprehensive cost-effectiveness analyses.
In conclusion, while gel electrophoresis LFAs may present higher initial costs, their improved performance, scalability, and potential for long-term cost savings make them an economically viable option in many scenarios. The cost-effectiveness of this method is likely to improve further as the technology matures and becomes more widely adopted, potentially leading to reduced equipment and material costs in the future.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!