Gel Electrophoresis Methods for Protein Sorting
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Protein Sorting Fundamentals and Objectives
Gel electrophoresis has been a cornerstone technique in protein sorting and analysis since its inception in the mid-20th century. This method leverages the fundamental principle that proteins, as charged molecules, migrate through a gel matrix when subjected to an electric field. The rate of migration depends on factors such as protein size, shape, and net charge, allowing for effective separation and characterization.
The primary objective of gel electrophoresis in protein sorting is to achieve high-resolution separation of complex protein mixtures, enabling researchers to isolate, identify, and quantify specific proteins of interest. This technique has evolved significantly over the years, with advancements aimed at improving resolution, sensitivity, and throughput.
One of the key developments in this field has been the introduction of different gel materials and compositions. Polyacrylamide gels, for instance, offer superior resolution for smaller proteins, while agarose gels are better suited for larger proteins and nucleic acids. The advent of gradient gels has further enhanced the ability to separate proteins across a wide range of molecular weights in a single run.
Another critical advancement has been the development of various staining and detection methods. From traditional Coomassie blue staining to more sensitive silver staining, and now to fluorescent dyes and immunoblotting techniques, these innovations have dramatically improved the detection limits and specificity of protein analysis.
The integration of gel electrophoresis with other analytical techniques has opened new avenues for protein research. Two-dimensional gel electrophoresis, which combines isoelectric focusing with SDS-PAGE, has become a powerful tool for proteomics studies, allowing for the separation of thousands of proteins in a single experiment.
Recent trends in gel electrophoresis for protein sorting include the miniaturization of systems for high-throughput analysis, the development of automated platforms, and the incorporation of advanced imaging and data analysis software. These innovations aim to increase efficiency, reduce sample requirements, and enhance data interpretation.
As we look to the future, the goals of gel electrophoresis in protein sorting continue to evolve. Researchers are striving for even higher resolution, increased sensitivity, and the ability to analyze ever-smaller sample volumes. There is also a growing emphasis on developing more environmentally friendly and cost-effective electrophoresis methods, as well as techniques that can better preserve protein structure and function during the separation process.
The primary objective of gel electrophoresis in protein sorting is to achieve high-resolution separation of complex protein mixtures, enabling researchers to isolate, identify, and quantify specific proteins of interest. This technique has evolved significantly over the years, with advancements aimed at improving resolution, sensitivity, and throughput.
One of the key developments in this field has been the introduction of different gel materials and compositions. Polyacrylamide gels, for instance, offer superior resolution for smaller proteins, while agarose gels are better suited for larger proteins and nucleic acids. The advent of gradient gels has further enhanced the ability to separate proteins across a wide range of molecular weights in a single run.
Another critical advancement has been the development of various staining and detection methods. From traditional Coomassie blue staining to more sensitive silver staining, and now to fluorescent dyes and immunoblotting techniques, these innovations have dramatically improved the detection limits and specificity of protein analysis.
The integration of gel electrophoresis with other analytical techniques has opened new avenues for protein research. Two-dimensional gel electrophoresis, which combines isoelectric focusing with SDS-PAGE, has become a powerful tool for proteomics studies, allowing for the separation of thousands of proteins in a single experiment.
Recent trends in gel electrophoresis for protein sorting include the miniaturization of systems for high-throughput analysis, the development of automated platforms, and the incorporation of advanced imaging and data analysis software. These innovations aim to increase efficiency, reduce sample requirements, and enhance data interpretation.
As we look to the future, the goals of gel electrophoresis in protein sorting continue to evolve. Researchers are striving for even higher resolution, increased sensitivity, and the ability to analyze ever-smaller sample volumes. There is also a growing emphasis on developing more environmentally friendly and cost-effective electrophoresis methods, as well as techniques that can better preserve protein structure and function during the separation process.
Market Analysis for Protein Separation Technologies
The protein separation technologies market has experienced significant growth in recent years, driven by advancements in proteomics research, drug discovery, and personalized medicine. Gel electrophoresis methods, particularly for protein sorting, play a crucial role in this expanding market. The global market for protein separation and analysis is projected to reach substantial value in the coming years, with gel electrophoresis techniques contributing significantly to this growth.
The demand for protein separation technologies is primarily fueled by the pharmaceutical and biotechnology industries, academic research institutions, and clinical diagnostics laboratories. These sectors require efficient and reliable methods for protein analysis, characterization, and purification. Gel electrophoresis, especially techniques like SDS-PAGE (Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis) and 2D-PAGE (Two-Dimensional Polyacrylamide Gel Electrophoresis), remains a cornerstone in protein separation and analysis workflows.
In the pharmaceutical industry, gel electrophoresis methods are extensively used in drug development processes, including target identification, lead optimization, and quality control of biopharmaceuticals. The growing emphasis on biologics and biosimilars has further boosted the demand for protein separation technologies, as these complex molecules require sophisticated analytical techniques for characterization and comparison.
Academic research institutions continue to be major consumers of gel electrophoresis products and services. The ongoing research in proteomics, structural biology, and molecular biology relies heavily on these techniques for protein analysis and identification. Additionally, the increasing focus on personalized medicine and biomarker discovery has created new opportunities for protein separation technologies in clinical diagnostics.
The market for gel electrophoresis methods in protein sorting is witnessing several trends. There is a growing demand for high-resolution and high-throughput techniques, driving innovations in gel formulations and electrophoresis equipment. Automated systems that integrate sample preparation, separation, and analysis are gaining popularity, especially in large-scale research and clinical settings. Furthermore, the development of specialized gels for specific protein classes or applications is expanding the market potential.
Geographically, North America and Europe dominate the protein separation technologies market, owing to their well-established research infrastructure and significant investments in life sciences. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing research activities, rising healthcare expenditure, and government initiatives to promote biotechnology and pharmaceutical industries.
Despite the advent of newer technologies like mass spectrometry and chromatography, gel electrophoresis methods for protein sorting continue to maintain their relevance due to their cost-effectiveness, reliability, and widespread familiarity among researchers. The market is expected to evolve with technological advancements, focusing on improved resolution, sensitivity, and integration with other analytical techniques.
The demand for protein separation technologies is primarily fueled by the pharmaceutical and biotechnology industries, academic research institutions, and clinical diagnostics laboratories. These sectors require efficient and reliable methods for protein analysis, characterization, and purification. Gel electrophoresis, especially techniques like SDS-PAGE (Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis) and 2D-PAGE (Two-Dimensional Polyacrylamide Gel Electrophoresis), remains a cornerstone in protein separation and analysis workflows.
In the pharmaceutical industry, gel electrophoresis methods are extensively used in drug development processes, including target identification, lead optimization, and quality control of biopharmaceuticals. The growing emphasis on biologics and biosimilars has further boosted the demand for protein separation technologies, as these complex molecules require sophisticated analytical techniques for characterization and comparison.
Academic research institutions continue to be major consumers of gel electrophoresis products and services. The ongoing research in proteomics, structural biology, and molecular biology relies heavily on these techniques for protein analysis and identification. Additionally, the increasing focus on personalized medicine and biomarker discovery has created new opportunities for protein separation technologies in clinical diagnostics.
The market for gel electrophoresis methods in protein sorting is witnessing several trends. There is a growing demand for high-resolution and high-throughput techniques, driving innovations in gel formulations and electrophoresis equipment. Automated systems that integrate sample preparation, separation, and analysis are gaining popularity, especially in large-scale research and clinical settings. Furthermore, the development of specialized gels for specific protein classes or applications is expanding the market potential.
Geographically, North America and Europe dominate the protein separation technologies market, owing to their well-established research infrastructure and significant investments in life sciences. However, the Asia-Pacific region is emerging as a rapidly growing market, driven by increasing research activities, rising healthcare expenditure, and government initiatives to promote biotechnology and pharmaceutical industries.
Despite the advent of newer technologies like mass spectrometry and chromatography, gel electrophoresis methods for protein sorting continue to maintain their relevance due to their cost-effectiveness, reliability, and widespread familiarity among researchers. The market is expected to evolve with technological advancements, focusing on improved resolution, sensitivity, and integration with other analytical techniques.
Current Gel Electrophoresis Techniques and Challenges
Gel electrophoresis remains a cornerstone technique in protein sorting and analysis, with several established methods currently in use. The most prevalent techniques include sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), native PAGE, and two-dimensional gel electrophoresis (2D-PAGE). Each of these methods offers unique advantages for protein separation based on different molecular properties.
SDS-PAGE is widely employed for its ability to separate proteins primarily by molecular weight. This technique utilizes the detergent SDS to denature proteins and impart a uniform negative charge, allowing for separation based solely on size. While highly effective for molecular weight determination, SDS-PAGE faces challenges in maintaining protein structure and function, limiting its use in certain applications requiring native protein analysis.
Native PAGE, on the other hand, preserves protein structure and allows for separation based on both size and charge. This technique is particularly useful for studying protein-protein interactions and enzyme activity. However, it can be less reproducible than SDS-PAGE due to variations in protein charge and conformation, making standardization more challenging.
2D-PAGE combines isoelectric focusing (IEF) with SDS-PAGE to separate proteins based on both their isoelectric point and molecular weight. This powerful technique allows for high-resolution separation of complex protein mixtures but is labor-intensive and can be difficult to reproduce consistently across laboratories.
Despite these established techniques, several challenges persist in gel electrophoresis for protein sorting. One significant issue is the limited dynamic range, which makes it difficult to detect low-abundance proteins in the presence of highly abundant ones. This is particularly problematic when analyzing complex biological samples such as cell lysates or serum.
Another challenge is the poor resolution of proteins with extreme molecular weights or isoelectric points. Very large or small proteins, as well as those with very acidic or basic pIs, can be difficult to separate effectively using standard gel electrophoresis techniques. This limitation can lead to incomplete protein profiles and missed information in proteomic studies.
Reproducibility remains a persistent challenge across all gel electrophoresis techniques. Variations in gel composition, running conditions, and sample preparation can lead to inconsistencies between experiments and laboratories. This issue is particularly pronounced in 2D-PAGE, where small changes in experimental conditions can significantly affect protein spot patterns.
Furthermore, the time-consuming nature of gel electrophoresis techniques, especially 2D-PAGE, limits their applicability in high-throughput analyses. As proteomics research increasingly demands rapid and large-scale protein profiling, the lengthy process of gel-based methods becomes a significant bottleneck.
Efforts to address these challenges have led to the development of various modifications and alternative techniques. These include the use of gradient gels for improved resolution, specialized staining methods for enhanced sensitivity, and the integration of gel electrophoresis with mass spectrometry for more comprehensive protein identification and characterization.
SDS-PAGE is widely employed for its ability to separate proteins primarily by molecular weight. This technique utilizes the detergent SDS to denature proteins and impart a uniform negative charge, allowing for separation based solely on size. While highly effective for molecular weight determination, SDS-PAGE faces challenges in maintaining protein structure and function, limiting its use in certain applications requiring native protein analysis.
Native PAGE, on the other hand, preserves protein structure and allows for separation based on both size and charge. This technique is particularly useful for studying protein-protein interactions and enzyme activity. However, it can be less reproducible than SDS-PAGE due to variations in protein charge and conformation, making standardization more challenging.
2D-PAGE combines isoelectric focusing (IEF) with SDS-PAGE to separate proteins based on both their isoelectric point and molecular weight. This powerful technique allows for high-resolution separation of complex protein mixtures but is labor-intensive and can be difficult to reproduce consistently across laboratories.
Despite these established techniques, several challenges persist in gel electrophoresis for protein sorting. One significant issue is the limited dynamic range, which makes it difficult to detect low-abundance proteins in the presence of highly abundant ones. This is particularly problematic when analyzing complex biological samples such as cell lysates or serum.
Another challenge is the poor resolution of proteins with extreme molecular weights or isoelectric points. Very large or small proteins, as well as those with very acidic or basic pIs, can be difficult to separate effectively using standard gel electrophoresis techniques. This limitation can lead to incomplete protein profiles and missed information in proteomic studies.
Reproducibility remains a persistent challenge across all gel electrophoresis techniques. Variations in gel composition, running conditions, and sample preparation can lead to inconsistencies between experiments and laboratories. This issue is particularly pronounced in 2D-PAGE, where small changes in experimental conditions can significantly affect protein spot patterns.
Furthermore, the time-consuming nature of gel electrophoresis techniques, especially 2D-PAGE, limits their applicability in high-throughput analyses. As proteomics research increasingly demands rapid and large-scale protein profiling, the lengthy process of gel-based methods becomes a significant bottleneck.
Efforts to address these challenges have led to the development of various modifications and alternative techniques. These include the use of gradient gels for improved resolution, specialized staining methods for enhanced sensitivity, and the integration of gel electrophoresis with mass spectrometry for more comprehensive protein identification and characterization.
Existing Gel Electrophoresis Protocols for Protein Sorting
01 Gel composition for protein separation
Various gel compositions are used in electrophoresis for protein sorting. These gels can be made from different materials and concentrations to optimize separation based on protein size, charge, or other properties. Some gels incorporate specific additives or polymers to enhance resolution or target particular protein types.- Gel composition for protein separation: Various gel compositions are used in electrophoresis for efficient protein separation. These gels can be made from different materials and concentrations to optimize the separation of proteins based on their size and charge. Some gels incorporate specific additives or polymers to enhance resolution and reduce background noise.
- Electrophoresis apparatus design: Innovative designs for electrophoresis apparatus improve protein sorting efficiency. These designs may include modifications to electrode configurations, buffer systems, or sample loading mechanisms. Some apparatuses incorporate automated features for precise control of voltage and current during the separation process.
- Sample preparation techniques: Effective sample preparation is crucial for successful protein sorting in gel electrophoresis. Techniques may include protein extraction, purification, and concentration methods. Some approaches involve the use of specific buffers or detergents to maintain protein stability and solubility during the electrophoresis process.
- Detection and analysis methods: Advanced detection and analysis methods enhance the accuracy of protein sorting results. These may include fluorescent labeling techniques, image analysis software, or mass spectrometry integration. Some methods focus on improving the sensitivity and quantification of protein bands in the gel.
- Specialized electrophoresis techniques: Specialized electrophoresis techniques have been developed for specific protein sorting applications. These may include two-dimensional electrophoresis, pulsed-field gel electrophoresis, or capillary electrophoresis. Some techniques focus on separating proteins with similar molecular weights or isoelectric points.
02 Electrophoresis apparatus design
Specialized apparatus designs for gel electrophoresis improve protein sorting efficiency. These may include innovative electrode configurations, buffer circulation systems, or temperature control mechanisms. Some designs focus on miniaturization or integration with other analytical techniques for enhanced performance.Expand Specific Solutions03 Sample preparation techniques
Effective sample preparation is crucial for accurate protein sorting in gel electrophoresis. This includes methods for protein extraction, purification, and loading onto the gel. Some techniques involve pre-treatment of samples to improve resolution or target specific protein fractions.Expand Specific Solutions04 Detection and analysis methods
Various detection and analysis methods are employed to visualize and quantify separated proteins after gel electrophoresis. These may include staining techniques, fluorescent labeling, or integration with mass spectrometry. Some methods focus on improving sensitivity or enabling real-time monitoring of protein migration.Expand Specific Solutions05 Two-dimensional gel electrophoresis
Two-dimensional gel electrophoresis techniques enhance protein sorting capabilities by separating proteins based on two different properties, typically isoelectric point and molecular weight. This approach allows for improved resolution and identification of complex protein mixtures, often coupled with advanced image analysis and database matching.Expand Specific Solutions
Key Players in Protein Separation Industry
The gel electrophoresis methods for protein sorting market is in a mature stage, with established technologies and widespread adoption across research and clinical laboratories. The global market size is estimated to be around $1.5 billion, driven by increasing proteomics research and demand for protein analysis in drug discovery. Technologically, the field has seen incremental improvements in resolution and automation. Key players like Agilent Technologies, Life Technologies, and GE Healthcare dominate with comprehensive product portfolios. Emerging companies such as AmberGen are innovating with novel approaches like mass spectrometry-based protein separation. Academic institutions like Oregon Health & Science University and Carnegie Mellon University contribute cutting-edge research to advance the technology further.
Life Technologies Corp.
Technical Solution: Life Technologies has pioneered several innovative gel electrophoresis methods for protein sorting. Their NuPAGE Bis-Tris gel system offers improved protein separation and stability compared to traditional Laemmli gels[4]. The company has also developed the E-Gel system, which utilizes pre-cast agarose gels with integrated electrodes for rapid and convenient protein electrophoresis[5]. Life Technologies' Novex line of pre-cast polyacrylamide gels provides a range of formulations optimized for different protein sizes and applications. Furthermore, they have introduced stain-free gel technology, allowing for immediate visualization of proteins without the need for post-electrophoresis staining steps[6]. The company also offers advanced imaging systems and analysis software to complement their gel electrophoresis products.
Strengths: Wide range of pre-cast gel options, user-friendly systems, and integrated solutions for various protein analysis needs. Weaknesses: Proprietary systems may limit flexibility and increase costs for some users.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has developed advanced gel electrophoresis methods for protein sorting, including their 2100 Bioanalyzer system. This microfluidics-based platform utilizes lab-on-a-chip technology to perform gel electrophoresis for protein analysis[1]. The system employs a miniaturized version of gel electrophoresis, allowing for rapid and automated protein separation and quantification. Agilent's technology incorporates fluorescence detection methods, enabling high sensitivity and accurate protein sizing. The company has also introduced improvements in sample preparation techniques and gel matrix formulations to enhance resolution and reproducibility[2]. Additionally, Agilent has developed software tools for data analysis and interpretation, streamlining the protein characterization process[3].
Strengths: High-throughput capability, automation, and miniaturization reduce sample and reagent consumption. Weaknesses: Limited to smaller proteins and may not be suitable for all types of protein samples.
Innovative Approaches in Gel-Based Protein Separation
Method and devices for forming a plurality of wells on a gel
PatentInactiveUS20100213065A1
Innovation
- A multiwell template apparatus is used to form a plurality of wells on a gel, allowing for the formation of wells without cutting the gel, and an automatic liquid dispensing and eluting device for non-electroelution-based analyte extraction, facilitating easier handling and processing of gel fractions without pre-staining.
Method for separating substances
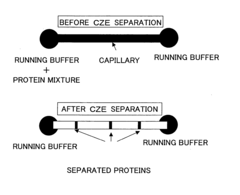
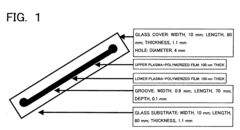
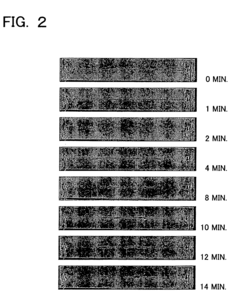
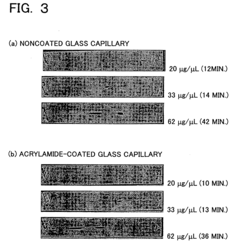
PatentInactiveUS20060113189A1
Innovation
- The use of plasma polymerization, surface polymerization, and polymer immobilization to coat substrate surfaces with polymer membranes that regulate electric potential, hydrophobicity, and hydrophilicity, enabling controlled separation and analysis of proteins on miniaturized substrates.
Regulatory Considerations for Protein Analysis Methods
Regulatory considerations play a crucial role in the development and implementation of protein analysis methods, including gel electrophoresis techniques for protein sorting. These considerations are essential to ensure the safety, efficacy, and reliability of the analytical methods used in various fields, such as pharmaceuticals, biotechnology, and clinical diagnostics.
One of the primary regulatory bodies overseeing protein analysis methods is the Food and Drug Administration (FDA) in the United States. The FDA has established guidelines for the validation of analytical procedures, including those used in gel electrophoresis. These guidelines outline the requirements for method validation, including specificity, accuracy, precision, linearity, and robustness.
In addition to the FDA, other regulatory agencies worldwide have their own set of requirements for protein analysis methods. The European Medicines Agency (EMA) and the International Conference on Harmonisation (ICH) have also published guidelines that address the validation of analytical procedures used in protein characterization and quantification.
For gel electrophoresis methods specifically, regulatory considerations often focus on the reproducibility and consistency of results. This includes factors such as gel composition, running conditions, and staining techniques. Manufacturers of gel electrophoresis equipment and reagents must adhere to Good Manufacturing Practices (GMP) to ensure the quality and consistency of their products.
Regulatory bodies also emphasize the importance of proper documentation and record-keeping throughout the analytical process. This includes maintaining detailed standard operating procedures (SOPs), instrument calibration records, and raw data from experiments. Such documentation is crucial for demonstrating compliance with regulatory requirements and ensuring the traceability of results.
Another important aspect of regulatory compliance is the validation of software used in gel electrophoresis analysis. As image analysis software becomes increasingly sophisticated, regulatory agencies require that these tools undergo thorough validation to ensure the accuracy and reliability of the results they produce.
Furthermore, regulatory considerations extend to the interpretation and reporting of gel electrophoresis results. Clear guidelines must be established for data analysis, including the identification and quantification of protein bands. This is particularly important in clinical settings, where gel electrophoresis results may be used for diagnostic purposes.
In conclusion, regulatory considerations for protein analysis methods, including gel electrophoresis, are multifaceted and evolving. As new technologies and applications emerge, regulatory bodies continue to adapt their guidelines to ensure the highest standards of safety and efficacy in protein analysis.
One of the primary regulatory bodies overseeing protein analysis methods is the Food and Drug Administration (FDA) in the United States. The FDA has established guidelines for the validation of analytical procedures, including those used in gel electrophoresis. These guidelines outline the requirements for method validation, including specificity, accuracy, precision, linearity, and robustness.
In addition to the FDA, other regulatory agencies worldwide have their own set of requirements for protein analysis methods. The European Medicines Agency (EMA) and the International Conference on Harmonisation (ICH) have also published guidelines that address the validation of analytical procedures used in protein characterization and quantification.
For gel electrophoresis methods specifically, regulatory considerations often focus on the reproducibility and consistency of results. This includes factors such as gel composition, running conditions, and staining techniques. Manufacturers of gel electrophoresis equipment and reagents must adhere to Good Manufacturing Practices (GMP) to ensure the quality and consistency of their products.
Regulatory bodies also emphasize the importance of proper documentation and record-keeping throughout the analytical process. This includes maintaining detailed standard operating procedures (SOPs), instrument calibration records, and raw data from experiments. Such documentation is crucial for demonstrating compliance with regulatory requirements and ensuring the traceability of results.
Another important aspect of regulatory compliance is the validation of software used in gel electrophoresis analysis. As image analysis software becomes increasingly sophisticated, regulatory agencies require that these tools undergo thorough validation to ensure the accuracy and reliability of the results they produce.
Furthermore, regulatory considerations extend to the interpretation and reporting of gel electrophoresis results. Clear guidelines must be established for data analysis, including the identification and quantification of protein bands. This is particularly important in clinical settings, where gel electrophoresis results may be used for diagnostic purposes.
In conclusion, regulatory considerations for protein analysis methods, including gel electrophoresis, are multifaceted and evolving. As new technologies and applications emerge, regulatory bodies continue to adapt their guidelines to ensure the highest standards of safety and efficacy in protein analysis.
Environmental Impact of Gel Electrophoresis Practices
Gel electrophoresis, a widely used technique in protein sorting and analysis, has significant environmental implications that warrant careful consideration. The process involves the use of various chemicals and materials, some of which can have detrimental effects on the environment if not properly managed.
One of the primary environmental concerns associated with gel electrophoresis is the disposal of acrylamide, a neurotoxin used in polyacrylamide gels. Improper disposal of acrylamide-containing waste can lead to soil and water contamination, potentially affecting ecosystems and human health. To mitigate this risk, many laboratories have implemented strict protocols for the safe handling and disposal of acrylamide waste, including polymerization and treatment before disposal.
The use of staining agents, such as Coomassie Brilliant Blue and silver nitrate, also poses environmental challenges. These dyes can be toxic to aquatic life if released into water systems. Furthermore, the fixatives and destaining solutions used in conjunction with these stains often contain methanol and acetic acid, which are harmful to the environment and require specialized disposal methods.
Electrophoresis buffer systems, typically containing Tris, glycine, and SDS (sodium dodecyl sulfate), contribute to the environmental footprint of gel electrophoresis. While these compounds are generally less toxic than acrylamide or staining agents, their regular use and disposal in large quantities can impact local water treatment systems and aquatic environments.
The energy consumption of gel electrophoresis equipment is another environmental consideration. Power supplies and cooling systems used in electrophoresis contribute to laboratory energy usage, indirectly impacting carbon emissions. Efforts to develop more energy-efficient equipment and optimize run times can help reduce this environmental impact.
Plastic waste generated from disposable gel cassettes, combs, and packaging materials is a growing concern in the scientific community. The increasing use of pre-cast gels, while convenient, has led to an increase in non-biodegradable waste. Some manufacturers are exploring recyclable or biodegradable alternatives to address this issue.
To minimize the environmental impact of gel electrophoresis practices, many research institutions and biotechnology companies are adopting more sustainable approaches. These include implementing recycling programs for plastic consumables, using alternative staining methods with lower toxicity, and exploring gel-free protein separation techniques.
Additionally, there is a growing trend towards miniaturization in gel electrophoresis, which not only improves efficiency but also reduces reagent consumption and waste generation. Microfluidic devices and capillary electrophoresis systems are examples of technologies that offer more environmentally friendly alternatives to traditional slab gel electrophoresis.
One of the primary environmental concerns associated with gel electrophoresis is the disposal of acrylamide, a neurotoxin used in polyacrylamide gels. Improper disposal of acrylamide-containing waste can lead to soil and water contamination, potentially affecting ecosystems and human health. To mitigate this risk, many laboratories have implemented strict protocols for the safe handling and disposal of acrylamide waste, including polymerization and treatment before disposal.
The use of staining agents, such as Coomassie Brilliant Blue and silver nitrate, also poses environmental challenges. These dyes can be toxic to aquatic life if released into water systems. Furthermore, the fixatives and destaining solutions used in conjunction with these stains often contain methanol and acetic acid, which are harmful to the environment and require specialized disposal methods.
Electrophoresis buffer systems, typically containing Tris, glycine, and SDS (sodium dodecyl sulfate), contribute to the environmental footprint of gel electrophoresis. While these compounds are generally less toxic than acrylamide or staining agents, their regular use and disposal in large quantities can impact local water treatment systems and aquatic environments.
The energy consumption of gel electrophoresis equipment is another environmental consideration. Power supplies and cooling systems used in electrophoresis contribute to laboratory energy usage, indirectly impacting carbon emissions. Efforts to develop more energy-efficient equipment and optimize run times can help reduce this environmental impact.
Plastic waste generated from disposable gel cassettes, combs, and packaging materials is a growing concern in the scientific community. The increasing use of pre-cast gels, while convenient, has led to an increase in non-biodegradable waste. Some manufacturers are exploring recyclable or biodegradable alternatives to address this issue.
To minimize the environmental impact of gel electrophoresis practices, many research institutions and biotechnology companies are adopting more sustainable approaches. These include implementing recycling programs for plastic consumables, using alternative staining methods with lower toxicity, and exploring gel-free protein separation techniques.
Additionally, there is a growing trend towards miniaturization in gel electrophoresis, which not only improves efficiency but also reduces reagent consumption and waste generation. Microfluidic devices and capillary electrophoresis systems are examples of technologies that offer more environmentally friendly alternatives to traditional slab gel electrophoresis.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!