Gel Electrophoresis for Proteomics: Latest Developments
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Proteomics Gel Electrophoresis Background and Objectives
Gel electrophoresis has been a cornerstone technique in proteomics for decades, enabling the separation and analysis of complex protein mixtures. This method, which utilizes an electric field to separate proteins based on their size and charge, has undergone significant advancements since its inception in the 1930s. The evolution of gel electrophoresis has been driven by the increasing demand for higher resolution, improved sensitivity, and enhanced reproducibility in protein analysis.
The primary objective of recent developments in gel electrophoresis for proteomics is to overcome the limitations of traditional techniques and to meet the growing needs of modern proteomic research. These advancements aim to provide more comprehensive protein profiling, better quantification, and increased throughput, all while maintaining or improving the reliability of results.
One of the key trends in the field has been the shift towards two-dimensional gel electrophoresis (2-DE), which separates proteins based on two independent properties: isoelectric point and molecular weight. This technique has dramatically improved the resolution of protein separation, allowing for the visualization of thousands of proteins in a single gel. However, 2-DE has its own challenges, including limited dynamic range and difficulties in resolving hydrophobic or very basic proteins.
To address these issues, researchers have been exploring various modifications and enhancements to the 2-DE technique. These include the development of immobilized pH gradient (IPG) strips for improved first-dimension separation, the use of fluorescent dyes for better protein detection and quantification, and the integration of mass spectrometry for protein identification.
Another significant trend is the move towards miniaturization and automation of gel electrophoresis systems. Microfluidic devices and lab-on-a-chip technologies are being developed to perform gel electrophoresis on a much smaller scale, offering advantages such as reduced sample consumption, faster analysis times, and the potential for high-throughput screening.
The integration of gel electrophoresis with other analytical techniques is also a key focus area. Combining gel-based separations with mass spectrometry, for instance, has become increasingly common, allowing for more detailed protein characterization and identification. This approach, often referred to as gel-based proteomics, has become a powerful tool for studying complex protein mixtures and post-translational modifications.
As we look to the future, the field of gel electrophoresis in proteomics continues to evolve. Researchers are exploring novel materials for gel matrices, developing more sensitive detection methods, and working on improved data analysis tools to handle the vast amount of information generated by these techniques. The ultimate goal is to create more robust, sensitive, and high-throughput methods for protein separation and analysis, enabling a deeper understanding of proteomes and their role in biological systems.
The primary objective of recent developments in gel electrophoresis for proteomics is to overcome the limitations of traditional techniques and to meet the growing needs of modern proteomic research. These advancements aim to provide more comprehensive protein profiling, better quantification, and increased throughput, all while maintaining or improving the reliability of results.
One of the key trends in the field has been the shift towards two-dimensional gel electrophoresis (2-DE), which separates proteins based on two independent properties: isoelectric point and molecular weight. This technique has dramatically improved the resolution of protein separation, allowing for the visualization of thousands of proteins in a single gel. However, 2-DE has its own challenges, including limited dynamic range and difficulties in resolving hydrophobic or very basic proteins.
To address these issues, researchers have been exploring various modifications and enhancements to the 2-DE technique. These include the development of immobilized pH gradient (IPG) strips for improved first-dimension separation, the use of fluorescent dyes for better protein detection and quantification, and the integration of mass spectrometry for protein identification.
Another significant trend is the move towards miniaturization and automation of gel electrophoresis systems. Microfluidic devices and lab-on-a-chip technologies are being developed to perform gel electrophoresis on a much smaller scale, offering advantages such as reduced sample consumption, faster analysis times, and the potential for high-throughput screening.
The integration of gel electrophoresis with other analytical techniques is also a key focus area. Combining gel-based separations with mass spectrometry, for instance, has become increasingly common, allowing for more detailed protein characterization and identification. This approach, often referred to as gel-based proteomics, has become a powerful tool for studying complex protein mixtures and post-translational modifications.
As we look to the future, the field of gel electrophoresis in proteomics continues to evolve. Researchers are exploring novel materials for gel matrices, developing more sensitive detection methods, and working on improved data analysis tools to handle the vast amount of information generated by these techniques. The ultimate goal is to create more robust, sensitive, and high-throughput methods for protein separation and analysis, enabling a deeper understanding of proteomes and their role in biological systems.
Market Demand for Advanced Protein Separation Techniques
The market demand for advanced protein separation techniques, particularly in the field of proteomics, has been steadily increasing over the past decade. This growth is primarily driven by the expanding applications of proteomics in various sectors, including pharmaceutical research, diagnostics, and personalized medicine. Gel electrophoresis, especially in its advanced forms, remains a cornerstone technique in this field due to its versatility and effectiveness in protein separation and analysis.
The global proteomics market, which heavily relies on advanced separation techniques, is projected to experience significant growth in the coming years. This expansion is fueled by the rising prevalence of chronic diseases, increased funding for proteomics research, and technological advancements in protein analysis tools. Pharmaceutical and biotechnology companies are major contributors to this demand, as they increasingly utilize proteomic approaches in drug discovery and development processes.
In the academic and research sector, there is a growing need for high-resolution protein separation techniques to support complex proteomic studies. Universities and research institutions are investing in advanced gel electrophoresis systems to enhance their research capabilities in areas such as biomarker discovery, protein-protein interactions, and post-translational modifications.
The healthcare industry is another significant driver of demand for advanced protein separation techniques. As personalized medicine gains traction, there is an increasing need for precise protein analysis in clinical diagnostics and treatment monitoring. Hospitals and diagnostic laboratories are adopting sophisticated gel electrophoresis systems to improve their ability to detect and analyze disease-related proteins.
Environmental and food safety sectors are also contributing to the market demand. These industries require advanced protein separation techniques for detecting contaminants, allergens, and pathogens in food and environmental samples. The ability of gel electrophoresis to provide detailed protein profiles makes it an invaluable tool in these applications.
Geographically, North America and Europe currently dominate the market for advanced protein separation techniques, owing to their well-established research infrastructure and high investment in life sciences. However, the Asia-Pacific region is expected to show the fastest growth in demand, driven by increasing research activities, rising healthcare expenditure, and growing awareness of proteomics applications.
The demand for gel electrophoresis in proteomics is also being shaped by the need for more efficient and high-throughput systems. There is a growing market preference for automated and integrated platforms that can handle complex proteomic workflows, from sample preparation to data analysis. This trend is pushing manufacturers to develop more sophisticated gel electrophoresis systems that offer improved resolution, sensitivity, and reproducibility.
The global proteomics market, which heavily relies on advanced separation techniques, is projected to experience significant growth in the coming years. This expansion is fueled by the rising prevalence of chronic diseases, increased funding for proteomics research, and technological advancements in protein analysis tools. Pharmaceutical and biotechnology companies are major contributors to this demand, as they increasingly utilize proteomic approaches in drug discovery and development processes.
In the academic and research sector, there is a growing need for high-resolution protein separation techniques to support complex proteomic studies. Universities and research institutions are investing in advanced gel electrophoresis systems to enhance their research capabilities in areas such as biomarker discovery, protein-protein interactions, and post-translational modifications.
The healthcare industry is another significant driver of demand for advanced protein separation techniques. As personalized medicine gains traction, there is an increasing need for precise protein analysis in clinical diagnostics and treatment monitoring. Hospitals and diagnostic laboratories are adopting sophisticated gel electrophoresis systems to improve their ability to detect and analyze disease-related proteins.
Environmental and food safety sectors are also contributing to the market demand. These industries require advanced protein separation techniques for detecting contaminants, allergens, and pathogens in food and environmental samples. The ability of gel electrophoresis to provide detailed protein profiles makes it an invaluable tool in these applications.
Geographically, North America and Europe currently dominate the market for advanced protein separation techniques, owing to their well-established research infrastructure and high investment in life sciences. However, the Asia-Pacific region is expected to show the fastest growth in demand, driven by increasing research activities, rising healthcare expenditure, and growing awareness of proteomics applications.
The demand for gel electrophoresis in proteomics is also being shaped by the need for more efficient and high-throughput systems. There is a growing market preference for automated and integrated platforms that can handle complex proteomic workflows, from sample preparation to data analysis. This trend is pushing manufacturers to develop more sophisticated gel electrophoresis systems that offer improved resolution, sensitivity, and reproducibility.
Current Challenges in Gel Electrophoresis for Proteomics
Gel electrophoresis remains a cornerstone technique in proteomics, yet it faces several challenges that limit its effectiveness in modern research applications. One of the primary issues is the limited resolution and sensitivity for low-abundance proteins. As proteomes become increasingly complex, the ability to detect and quantify proteins present in small quantities becomes crucial. Current gel-based methods often struggle to provide the necessary sensitivity, potentially missing important biological signals.
Another significant challenge is the difficulty in separating and visualizing proteins with extreme molecular weights or isoelectric points. Proteins that are very large, very small, highly acidic, or highly basic often fail to resolve properly on standard gels, leading to incomplete proteome coverage. This limitation can result in biased analyses and incomplete understanding of cellular processes.
The time-consuming nature of gel electrophoresis poses a substantial hurdle in high-throughput proteomics studies. The manual steps involved in gel preparation, running, staining, and image analysis can be labor-intensive and prone to variability. This not only reduces sample throughput but also introduces potential sources of error and inconsistency between experiments.
Reproducibility is another critical challenge facing gel electrophoresis in proteomics. Variations in gel composition, running conditions, and staining procedures can lead to significant differences in protein migration patterns and spot intensities between gels. This lack of reproducibility complicates comparative analyses and makes it difficult to establish standardized protocols across laboratories.
The limited dynamic range of protein detection in gels presents a further obstacle. The co-existence of highly abundant and rare proteins in biological samples often results in the masking of low-abundance species. This compression of the dynamic range can lead to incomplete proteome characterization and missed biological insights.
Integrating gel-based separations with downstream mass spectrometry analysis remains challenging. The process of extracting proteins or peptides from gel spots or bands for subsequent MS analysis can be inefficient and prone to sample loss. This interface between gel electrophoresis and MS often results in reduced sensitivity and incomplete protein identification.
Lastly, the analysis and interpretation of complex 2D gel images pose significant bioinformatic challenges. Accurate spot detection, matching across gels, and quantification require sophisticated software tools and expertise. The development of robust, user-friendly bioinformatic solutions for gel image analysis remains an ongoing challenge in the field of proteomics.
Another significant challenge is the difficulty in separating and visualizing proteins with extreme molecular weights or isoelectric points. Proteins that are very large, very small, highly acidic, or highly basic often fail to resolve properly on standard gels, leading to incomplete proteome coverage. This limitation can result in biased analyses and incomplete understanding of cellular processes.
The time-consuming nature of gel electrophoresis poses a substantial hurdle in high-throughput proteomics studies. The manual steps involved in gel preparation, running, staining, and image analysis can be labor-intensive and prone to variability. This not only reduces sample throughput but also introduces potential sources of error and inconsistency between experiments.
Reproducibility is another critical challenge facing gel electrophoresis in proteomics. Variations in gel composition, running conditions, and staining procedures can lead to significant differences in protein migration patterns and spot intensities between gels. This lack of reproducibility complicates comparative analyses and makes it difficult to establish standardized protocols across laboratories.
The limited dynamic range of protein detection in gels presents a further obstacle. The co-existence of highly abundant and rare proteins in biological samples often results in the masking of low-abundance species. This compression of the dynamic range can lead to incomplete proteome characterization and missed biological insights.
Integrating gel-based separations with downstream mass spectrometry analysis remains challenging. The process of extracting proteins or peptides from gel spots or bands for subsequent MS analysis can be inefficient and prone to sample loss. This interface between gel electrophoresis and MS often results in reduced sensitivity and incomplete protein identification.
Lastly, the analysis and interpretation of complex 2D gel images pose significant bioinformatic challenges. Accurate spot detection, matching across gels, and quantification require sophisticated software tools and expertise. The development of robust, user-friendly bioinformatic solutions for gel image analysis remains an ongoing challenge in the field of proteomics.
Current Gel Electrophoresis Methods in Proteomics
01 Optimization of gel composition
Improving separation efficiency in gel electrophoresis can be achieved by optimizing the gel composition. This includes adjusting the concentration and type of polymer used, as well as incorporating additives that enhance resolution. Tailoring the gel composition to the specific molecules being separated can significantly improve the overall separation efficiency.- Gel composition optimization: Improving separation efficiency in gel electrophoresis by optimizing the gel composition. This includes adjusting the concentration and type of polymers used, as well as incorporating additives to enhance resolution and reduce band diffusion. The optimized gel composition can lead to better separation of DNA, RNA, or protein molecules.
- Electric field manipulation: Enhancing separation efficiency through manipulation of the electric field applied during gel electrophoresis. This involves techniques such as pulsed-field gel electrophoresis, gradient gel electrophoresis, or the use of alternating current. These methods can improve the resolution of larger molecules or complex mixtures.
- Buffer system optimization: Improving separation efficiency by optimizing the buffer system used in gel electrophoresis. This includes adjusting buffer composition, pH, and ionic strength to enhance the separation of specific types of molecules. Optimized buffer systems can lead to sharper bands and improved resolution.
- Sample preparation techniques: Enhancing separation efficiency through improved sample preparation methods. This includes techniques for concentrating samples, removing interfering substances, and optimizing sample loading. Proper sample preparation can lead to clearer results and improved resolution in gel electrophoresis.
- Detection and imaging improvements: Improving separation efficiency by enhancing detection and imaging techniques in gel electrophoresis. This includes the use of fluorescent dyes, advanced imaging systems, and computer-aided analysis to improve the visualization and quantification of separated molecules. These improvements can lead to better sensitivity and accuracy in interpreting results.
02 Electric field manipulation
Manipulating the electric field during gel electrophoresis can enhance separation efficiency. This may involve using pulsed field gel electrophoresis, alternating current, or gradient electric fields. These techniques can improve the resolution of larger molecules and allow for better separation of complex mixtures.Expand Specific Solutions03 Buffer system optimization
The choice and optimization of buffer systems play a crucial role in gel electrophoresis separation efficiency. Adjusting buffer composition, pH, and ionic strength can significantly impact the migration and resolution of molecules. Specialized buffer systems can be developed for specific applications to enhance overall separation performance.Expand Specific Solutions04 Sample preparation techniques
Improving sample preparation methods can lead to better separation efficiency in gel electrophoresis. This includes optimizing sample loading techniques, using pre-concentration methods, and developing novel sample clean-up procedures. Proper sample preparation can reduce interference and improve the overall quality of separation.Expand Specific Solutions05 Detection and imaging enhancements
Enhancing detection and imaging techniques can improve the apparent separation efficiency of gel electrophoresis. This includes developing more sensitive staining methods, implementing advanced imaging technologies, and utilizing computer-aided analysis. These improvements can lead to better visualization and quantification of separated molecules.Expand Specific Solutions
Key Players in Proteomics and Electrophoresis Industry
The field of Gel Electrophoresis for Proteomics is currently in a mature stage of development, with ongoing advancements in technology and applications. The global market for proteomics is substantial, estimated to reach $50 billion by 2027, driven by increasing demand in biomedical research and drug discovery. Technologically, gel electrophoresis remains a fundamental technique, but innovations are focused on improving resolution, sensitivity, and automation. Companies like Agilent Technologies, Roche Diagnostics, and NeoGenomics are at the forefront, developing advanced systems and reagents. Academic institutions such as Caltech and Fudan University contribute significantly to research and method development. The integration of gel electrophoresis with mass spectrometry and bioinformatics is enhancing its capabilities, making it an indispensable tool in proteomics research and clinical applications.
F. Hoffmann-La Roche Ltd.
Technical Solution: Roche has made significant strides in gel electrophoresis for proteomics through its subsidiary, Roche Diagnostics. They have developed the Roche SomaLogic platform, which combines gel electrophoresis with aptamer-based protein detection[3]. This innovative approach allows for the simultaneous measurement of thousands of proteins in a single sample. Additionally, Roche has introduced the cobas 8000 modular analyzer series, which incorporates gel electrophoresis techniques for high-throughput protein analysis in clinical settings[4].
Strengths: High-throughput capabilities, integration with other analytical techniques. Weaknesses: Complex system setup, requires specialized training for operation.
Agilent Technologies, Inc.
Technical Solution: Agilent has developed advanced gel electrophoresis systems for proteomics, including the 2100 Bioanalyzer. This microfluidic-based platform performs automated electrophoresis for protein analysis, offering high sensitivity and resolution[1]. The company has also introduced the Agilent 4150 TapeStation system, which utilizes a gel matrix in a capillary format for rapid protein separation and quantification[2]. These innovations have significantly improved the speed and accuracy of protein analysis in proteomics research.
Strengths: High automation, rapid analysis, and improved sensitivity. Weaknesses: Higher cost compared to traditional gel electrophoresis methods, limited to smaller sample volumes.
Innovations in Gel-Based Protein Separation
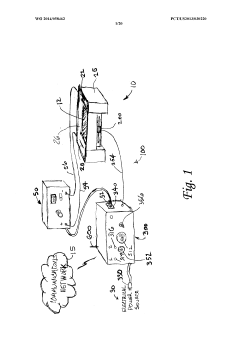
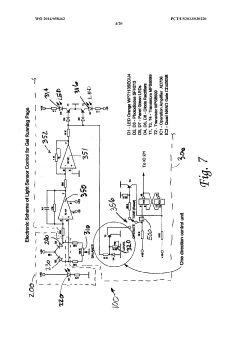
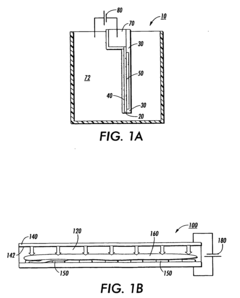
Electrophoresis controllers and methods for controlling electrophoresis process
PatentWO2014058462A1
Innovation
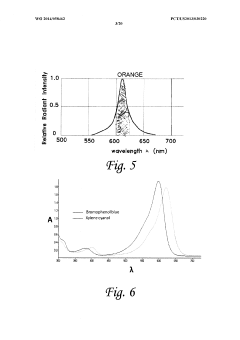
- An electrophoresis controller with a sensor and light source that emits light into the gel matrix, detecting the migration of a tracking dye and automatically shutting off the power supply when the dye reaches a predetermined point, accompanied by alerts to the user.
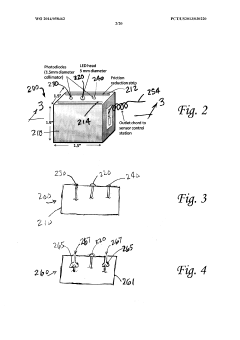
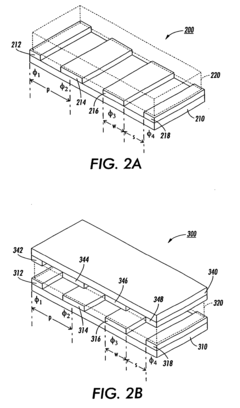
Distributed multi-segmented reconfigurable traveling wave grids for separation of proteins in gel electrophoresis
PatentInactiveEP1486782B1
Innovation
- A 2D electrophoretic system utilizing a distributed multi-segmented traveling wave grid with optimized electrode configurations and voltage patterns to enhance protein loading and transport, allowing for rapid separation and focusing of biomolecules using electrostatic traveling waves, reducing voltage requirements and increasing transport velocities up to ten times compared to conventional methods.
Regulatory Considerations for Proteomics Technologies
The regulatory landscape for proteomics technologies, including gel electrophoresis, is complex and evolving. Regulatory bodies such as the FDA in the United States and the EMA in Europe play crucial roles in overseeing the development, validation, and implementation of these technologies in clinical and research settings.
For gel electrophoresis in proteomics, regulatory considerations primarily focus on ensuring the accuracy, reproducibility, and safety of the technique. Manufacturers of gel electrophoresis equipment and reagents must adhere to quality control standards and provide detailed documentation on their products' performance characteristics.
In clinical applications, the use of gel electrophoresis for proteomics is subject to stringent regulations. The FDA's guidance on in vitro diagnostic devices applies to proteomics technologies used for patient diagnosis or treatment decisions. This includes requirements for analytical and clinical validation, as well as demonstration of clinical utility.
Research laboratories using gel electrophoresis for proteomics must comply with good laboratory practice (GLP) guidelines. These guidelines ensure the quality and integrity of non-clinical laboratory studies and data. Additionally, laboratories may need to adhere to ISO standards, particularly ISO 15189 for medical laboratories.
Data privacy and security regulations, such as GDPR in Europe and HIPAA in the United States, also impact proteomics research. These regulations govern the collection, storage, and sharing of personal health information derived from proteomic analyses.
As proteomics technologies advance, regulatory frameworks are adapting to keep pace. The FDA's approach to regulating next-generation sequencing and other omics technologies provides a model for how gel electrophoresis and other proteomics methods may be regulated in the future. This includes a focus on analytical validity, clinical validity, and clinical utility.
Standardization efforts in proteomics, led by organizations such as the Human Proteome Organization (HUPO), are influencing regulatory approaches. These efforts aim to establish common protocols and quality metrics for proteomics experiments, which may eventually be incorporated into regulatory guidelines.
Researchers and manufacturers must stay informed about evolving regulations and engage with regulatory bodies to ensure compliance. This proactive approach can help streamline the development and implementation of new proteomics technologies while maintaining high standards of safety and efficacy.
For gel electrophoresis in proteomics, regulatory considerations primarily focus on ensuring the accuracy, reproducibility, and safety of the technique. Manufacturers of gel electrophoresis equipment and reagents must adhere to quality control standards and provide detailed documentation on their products' performance characteristics.
In clinical applications, the use of gel electrophoresis for proteomics is subject to stringent regulations. The FDA's guidance on in vitro diagnostic devices applies to proteomics technologies used for patient diagnosis or treatment decisions. This includes requirements for analytical and clinical validation, as well as demonstration of clinical utility.
Research laboratories using gel electrophoresis for proteomics must comply with good laboratory practice (GLP) guidelines. These guidelines ensure the quality and integrity of non-clinical laboratory studies and data. Additionally, laboratories may need to adhere to ISO standards, particularly ISO 15189 for medical laboratories.
Data privacy and security regulations, such as GDPR in Europe and HIPAA in the United States, also impact proteomics research. These regulations govern the collection, storage, and sharing of personal health information derived from proteomic analyses.
As proteomics technologies advance, regulatory frameworks are adapting to keep pace. The FDA's approach to regulating next-generation sequencing and other omics technologies provides a model for how gel electrophoresis and other proteomics methods may be regulated in the future. This includes a focus on analytical validity, clinical validity, and clinical utility.
Standardization efforts in proteomics, led by organizations such as the Human Proteome Organization (HUPO), are influencing regulatory approaches. These efforts aim to establish common protocols and quality metrics for proteomics experiments, which may eventually be incorporated into regulatory guidelines.
Researchers and manufacturers must stay informed about evolving regulations and engage with regulatory bodies to ensure compliance. This proactive approach can help streamline the development and implementation of new proteomics technologies while maintaining high standards of safety and efficacy.
Environmental Impact of Gel Electrophoresis Methods
Gel electrophoresis, a fundamental technique in proteomics, has undergone significant advancements in recent years. However, these developments have also raised concerns about their environmental impact. The traditional gel electrophoresis methods often involve the use of toxic chemicals and generate substantial waste, posing potential risks to the environment.
One of the primary environmental concerns is the use of acrylamide, a neurotoxin and potential carcinogen, in polyacrylamide gels. While essential for protein separation, acrylamide can contaminate water sources if not properly disposed of. Recent efforts have focused on developing alternative gel materials that are less toxic and more environmentally friendly.
The disposal of used gels and buffers also presents environmental challenges. These materials often contain heavy metals, dyes, and other potentially harmful substances. Improper disposal can lead to soil and water pollution, affecting ecosystems and potentially entering the food chain.
Energy consumption is another significant environmental factor. Gel electrophoresis systems, particularly those used in large-scale proteomics studies, require substantial electrical power. This contributes to increased carbon footprints and energy-related environmental impacts.
Recent developments have aimed to address these environmental concerns. The introduction of more efficient and miniaturized gel systems has reduced both material consumption and energy usage. Some researchers have explored the use of biodegradable gel materials, which can significantly reduce the environmental impact of gel disposal.
Advancements in recycling and waste management protocols specific to gel electrophoresis have also emerged. These include methods for safely neutralizing and disposing of acrylamide-containing waste, as well as techniques for recovering and reusing certain gel components.
The shift towards more sustainable laboratory practices has led to the development of greener alternatives to traditional staining methods. For instance, fluorescent protein labeling techniques reduce the need for toxic staining agents, thereby minimizing chemical waste.
As the field of proteomics continues to grow, there is an increasing emphasis on developing more environmentally friendly gel electrophoresis methods. This includes research into completely gel-free proteomics techniques, which could potentially eliminate many of the environmental concerns associated with traditional gel-based methods.
In conclusion, while gel electrophoresis remains a crucial tool in proteomics, its environmental impact is a growing concern. The latest developments in this field are not only focused on improving technical performance but also on reducing environmental footprints, aligning with broader sustainability goals in scientific research.
One of the primary environmental concerns is the use of acrylamide, a neurotoxin and potential carcinogen, in polyacrylamide gels. While essential for protein separation, acrylamide can contaminate water sources if not properly disposed of. Recent efforts have focused on developing alternative gel materials that are less toxic and more environmentally friendly.
The disposal of used gels and buffers also presents environmental challenges. These materials often contain heavy metals, dyes, and other potentially harmful substances. Improper disposal can lead to soil and water pollution, affecting ecosystems and potentially entering the food chain.
Energy consumption is another significant environmental factor. Gel electrophoresis systems, particularly those used in large-scale proteomics studies, require substantial electrical power. This contributes to increased carbon footprints and energy-related environmental impacts.
Recent developments have aimed to address these environmental concerns. The introduction of more efficient and miniaturized gel systems has reduced both material consumption and energy usage. Some researchers have explored the use of biodegradable gel materials, which can significantly reduce the environmental impact of gel disposal.
Advancements in recycling and waste management protocols specific to gel electrophoresis have also emerged. These include methods for safely neutralizing and disposing of acrylamide-containing waste, as well as techniques for recovering and reusing certain gel components.
The shift towards more sustainable laboratory practices has led to the development of greener alternatives to traditional staining methods. For instance, fluorescent protein labeling techniques reduce the need for toxic staining agents, thereby minimizing chemical waste.
As the field of proteomics continues to grow, there is an increasing emphasis on developing more environmentally friendly gel electrophoresis methods. This includes research into completely gel-free proteomics techniques, which could potentially eliminate many of the environmental concerns associated with traditional gel-based methods.
In conclusion, while gel electrophoresis remains a crucial tool in proteomics, its environmental impact is a growing concern. The latest developments in this field are not only focused on improving technical performance but also on reducing environmental footprints, aligning with broader sustainability goals in scientific research.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!