How to Leverage Gel Electrophoresis in Proteomic Analysis?
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Gel Electrophoresis in Proteomics: Background and Objectives
Gel electrophoresis has been a cornerstone technique in molecular biology and biochemistry for decades, playing a crucial role in the separation and analysis of proteins. In the context of proteomics, this technique has evolved to become an indispensable tool for researchers seeking to unravel the complexities of the proteome. The background of gel electrophoresis in proteomics is rooted in the fundamental principle of separating proteins based on their molecular weight and charge.
The development of gel electrophoresis for protein analysis can be traced back to the 1960s, with the introduction of polyacrylamide gel electrophoresis (PAGE) by Raymond and Weintraub. This technique quickly gained traction due to its ability to provide high-resolution separation of proteins. The subsequent addition of sodium dodecyl sulfate (SDS) to create SDS-PAGE further enhanced the method's utility by allowing for the separation of proteins solely based on their molecular weight.
As the field of proteomics emerged in the 1990s, gel electrophoresis techniques underwent significant refinements to meet the demands of large-scale protein analysis. Two-dimensional gel electrophoresis (2-DE) became a powerful tool, combining isoelectric focusing (IEF) with SDS-PAGE to separate proteins based on both their isoelectric point and molecular weight. This advancement greatly increased the resolving power and allowed for the visualization of thousands of proteins simultaneously.
The primary objective of leveraging gel electrophoresis in proteomic analysis is to achieve comprehensive protein separation and characterization. This technique aims to provide a detailed map of the proteome, allowing researchers to identify and quantify individual proteins within complex biological samples. By separating proteins based on their physical properties, gel electrophoresis enables the detection of post-translational modifications, protein isoforms, and changes in protein expression levels.
Furthermore, gel electrophoresis serves as a preparative technique for subsequent mass spectrometry analysis, a critical step in modern proteomics workflows. The ability to isolate specific protein spots or bands from gels facilitates targeted protein identification and characterization. This integration of gel-based separation with mass spectrometry has significantly enhanced the depth and breadth of proteomic analyses.
In recent years, the objectives of gel electrophoresis in proteomics have expanded to include the study of protein-protein interactions, the analysis of membrane proteins, and the investigation of post-translational modifications. Researchers are continually developing new variations of gel electrophoresis techniques to address these evolving needs, such as blue native PAGE for studying protein complexes and differential gel electrophoresis (DIGE) for quantitative proteomics.
The development of gel electrophoresis for protein analysis can be traced back to the 1960s, with the introduction of polyacrylamide gel electrophoresis (PAGE) by Raymond and Weintraub. This technique quickly gained traction due to its ability to provide high-resolution separation of proteins. The subsequent addition of sodium dodecyl sulfate (SDS) to create SDS-PAGE further enhanced the method's utility by allowing for the separation of proteins solely based on their molecular weight.
As the field of proteomics emerged in the 1990s, gel electrophoresis techniques underwent significant refinements to meet the demands of large-scale protein analysis. Two-dimensional gel electrophoresis (2-DE) became a powerful tool, combining isoelectric focusing (IEF) with SDS-PAGE to separate proteins based on both their isoelectric point and molecular weight. This advancement greatly increased the resolving power and allowed for the visualization of thousands of proteins simultaneously.
The primary objective of leveraging gel electrophoresis in proteomic analysis is to achieve comprehensive protein separation and characterization. This technique aims to provide a detailed map of the proteome, allowing researchers to identify and quantify individual proteins within complex biological samples. By separating proteins based on their physical properties, gel electrophoresis enables the detection of post-translational modifications, protein isoforms, and changes in protein expression levels.
Furthermore, gel electrophoresis serves as a preparative technique for subsequent mass spectrometry analysis, a critical step in modern proteomics workflows. The ability to isolate specific protein spots or bands from gels facilitates targeted protein identification and characterization. This integration of gel-based separation with mass spectrometry has significantly enhanced the depth and breadth of proteomic analyses.
In recent years, the objectives of gel electrophoresis in proteomics have expanded to include the study of protein-protein interactions, the analysis of membrane proteins, and the investigation of post-translational modifications. Researchers are continually developing new variations of gel electrophoresis techniques to address these evolving needs, such as blue native PAGE for studying protein complexes and differential gel electrophoresis (DIGE) for quantitative proteomics.
Market Demand for Proteomic Analysis Tools
The market demand for proteomic analysis tools has been experiencing significant growth in recent years, driven by advancements in life sciences research and the increasing focus on personalized medicine. Gel electrophoresis, as a fundamental technique in proteomic analysis, continues to play a crucial role in this expanding market.
The global proteomics market, which includes gel electrophoresis tools and related technologies, is projected to reach substantial market value in the coming years. This growth is fueled by the rising prevalence of chronic diseases, the need for early disease diagnosis, and the increasing investments in proteomics research by both public and private sectors.
Pharmaceutical and biotechnology companies are major contributors to the demand for proteomic analysis tools, including gel electrophoresis systems. These organizations rely heavily on proteomics for drug discovery and development processes, driving the need for more advanced and efficient analytical techniques.
Academic and research institutions also represent a significant portion of the market demand. As proteomics research continues to expand, universities and research centers are investing in state-of-the-art equipment to support their studies. This includes both traditional gel electrophoresis systems and more advanced variations that offer higher resolution and throughput.
The healthcare sector is another key driver of market demand for proteomic analysis tools. With the growing emphasis on precision medicine and biomarker discovery, hospitals and diagnostic laboratories are increasingly adopting proteomic technologies to improve patient care and treatment outcomes.
Emerging applications in fields such as agriculture, food safety, and environmental monitoring are also contributing to the expanding market for proteomic analysis tools. These sectors are leveraging proteomics techniques, including gel electrophoresis, for various purposes such as crop improvement, food quality control, and environmental impact assessment.
Geographically, North America and Europe currently dominate the market for proteomic analysis tools, owing to their well-established research infrastructure and significant investments in life sciences. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing research activities and government initiatives to boost biotechnology and healthcare sectors.
Despite the growing popularity of newer technologies like mass spectrometry, gel electrophoresis remains a fundamental technique in proteomic analysis due to its reliability, cost-effectiveness, and ability to separate complex protein mixtures. The market continues to see demand for both traditional and advanced gel electrophoresis systems, with innovations focusing on improving resolution, automation, and integration with other analytical techniques.
The global proteomics market, which includes gel electrophoresis tools and related technologies, is projected to reach substantial market value in the coming years. This growth is fueled by the rising prevalence of chronic diseases, the need for early disease diagnosis, and the increasing investments in proteomics research by both public and private sectors.
Pharmaceutical and biotechnology companies are major contributors to the demand for proteomic analysis tools, including gel electrophoresis systems. These organizations rely heavily on proteomics for drug discovery and development processes, driving the need for more advanced and efficient analytical techniques.
Academic and research institutions also represent a significant portion of the market demand. As proteomics research continues to expand, universities and research centers are investing in state-of-the-art equipment to support their studies. This includes both traditional gel electrophoresis systems and more advanced variations that offer higher resolution and throughput.
The healthcare sector is another key driver of market demand for proteomic analysis tools. With the growing emphasis on precision medicine and biomarker discovery, hospitals and diagnostic laboratories are increasingly adopting proteomic technologies to improve patient care and treatment outcomes.
Emerging applications in fields such as agriculture, food safety, and environmental monitoring are also contributing to the expanding market for proteomic analysis tools. These sectors are leveraging proteomics techniques, including gel electrophoresis, for various purposes such as crop improvement, food quality control, and environmental impact assessment.
Geographically, North America and Europe currently dominate the market for proteomic analysis tools, owing to their well-established research infrastructure and significant investments in life sciences. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by increasing research activities and government initiatives to boost biotechnology and healthcare sectors.
Despite the growing popularity of newer technologies like mass spectrometry, gel electrophoresis remains a fundamental technique in proteomic analysis due to its reliability, cost-effectiveness, and ability to separate complex protein mixtures. The market continues to see demand for both traditional and advanced gel electrophoresis systems, with innovations focusing on improving resolution, automation, and integration with other analytical techniques.
Current State and Challenges in Gel Electrophoresis
Gel electrophoresis remains a cornerstone technique in proteomic analysis, offering robust separation of proteins based on their molecular weight and charge. Currently, the method is widely employed in various forms, including one-dimensional (1D) and two-dimensional (2D) gel electrophoresis, with each variant providing unique advantages for specific applications.
The 1D gel electrophoresis, particularly sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), is extensively used for its simplicity and effectiveness in separating proteins solely based on molecular weight. This technique continues to be a standard method for protein characterization and quality control in both research and industrial settings.
2D gel electrophoresis, combining isoelectric focusing (IEF) with SDS-PAGE, offers higher resolution by separating proteins based on both their isoelectric point and molecular weight. This approach has been instrumental in comparative proteomics studies, enabling the visualization of complex protein mixtures and the detection of post-translational modifications.
Despite its widespread use, gel electrophoresis faces several challenges in the context of modern proteomic analysis. One significant limitation is the difficulty in detecting low-abundance proteins, which are often masked by highly abundant species. This issue is particularly problematic when analyzing complex biological samples, such as plasma or tissue extracts.
Another challenge lies in the reproducibility and standardization of gel-based techniques. Variations in sample preparation, gel casting, and running conditions can lead to inconsistencies between experiments, complicating data comparison and integration across different laboratories or studies.
The time-consuming nature of gel electrophoresis, especially for 2D gels, poses a bottleneck in high-throughput proteomic analyses. This limitation becomes more pronounced when dealing with large sample sets or time-sensitive experiments.
Quantification accuracy remains a concern, particularly for 2D gels where spot intensity may not always accurately reflect protein abundance due to factors such as protein solubility, isoelectric point, and molecular weight.
The dynamic range of gel-based methods is another area of challenge. While improvements have been made, the ability to simultaneously detect and quantify proteins across a wide range of concentrations is still limited compared to some mass spectrometry-based approaches.
Efforts to address these challenges have led to the development of improved techniques such as difference gel electrophoresis (DIGE) for better quantification, and the integration of gel-based methods with mass spectrometry for enhanced protein identification and characterization.
Despite these challenges, gel electrophoresis continues to evolve and adapt, maintaining its relevance in proteomic analysis. Ongoing research focuses on enhancing sensitivity, reproducibility, and throughput, ensuring that this classic technique remains a valuable tool in the proteomics toolkit.
The 1D gel electrophoresis, particularly sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), is extensively used for its simplicity and effectiveness in separating proteins solely based on molecular weight. This technique continues to be a standard method for protein characterization and quality control in both research and industrial settings.
2D gel electrophoresis, combining isoelectric focusing (IEF) with SDS-PAGE, offers higher resolution by separating proteins based on both their isoelectric point and molecular weight. This approach has been instrumental in comparative proteomics studies, enabling the visualization of complex protein mixtures and the detection of post-translational modifications.
Despite its widespread use, gel electrophoresis faces several challenges in the context of modern proteomic analysis. One significant limitation is the difficulty in detecting low-abundance proteins, which are often masked by highly abundant species. This issue is particularly problematic when analyzing complex biological samples, such as plasma or tissue extracts.
Another challenge lies in the reproducibility and standardization of gel-based techniques. Variations in sample preparation, gel casting, and running conditions can lead to inconsistencies between experiments, complicating data comparison and integration across different laboratories or studies.
The time-consuming nature of gel electrophoresis, especially for 2D gels, poses a bottleneck in high-throughput proteomic analyses. This limitation becomes more pronounced when dealing with large sample sets or time-sensitive experiments.
Quantification accuracy remains a concern, particularly for 2D gels where spot intensity may not always accurately reflect protein abundance due to factors such as protein solubility, isoelectric point, and molecular weight.
The dynamic range of gel-based methods is another area of challenge. While improvements have been made, the ability to simultaneously detect and quantify proteins across a wide range of concentrations is still limited compared to some mass spectrometry-based approaches.
Efforts to address these challenges have led to the development of improved techniques such as difference gel electrophoresis (DIGE) for better quantification, and the integration of gel-based methods with mass spectrometry for enhanced protein identification and characterization.
Despite these challenges, gel electrophoresis continues to evolve and adapt, maintaining its relevance in proteomic analysis. Ongoing research focuses on enhancing sensitivity, reproducibility, and throughput, ensuring that this classic technique remains a valuable tool in the proteomics toolkit.
Existing Gel Electrophoresis Methods in Proteomics
01 Gel composition for protein separation
Various gel compositions are used in electrophoresis for protein separation. These gels can be made from different materials and concentrations to optimize separation based on protein size and charge. Some gels incorporate specific additives to enhance resolution or modify separation characteristics.- Gel composition for protein separation: Various gel compositions are used in electrophoresis for protein separation. These gels can be made from different materials and concentrations to optimize separation based on protein size and charge. Some gels incorporate specific additives or cross-linking agents to enhance resolution and separation efficiency.
- Electrophoresis apparatus design: Specialized apparatus designs for gel electrophoresis improve protein separation. These designs may include innovative electrode configurations, buffer circulation systems, or temperature control mechanisms to enhance separation quality and reproducibility.
- Sample preparation techniques: Effective sample preparation is crucial for successful protein separation. Techniques may include protein extraction, purification, and denaturation methods to ensure optimal loading and separation in the gel. Some methods focus on maintaining protein stability or removing interfering substances.
- Detection and visualization methods: Various methods are employed to detect and visualize separated proteins in gels. These may include staining techniques, fluorescent labeling, or advanced imaging systems that enhance sensitivity and allow for quantitative analysis of separated proteins.
- Two-dimensional gel electrophoresis: Two-dimensional gel electrophoresis techniques separate proteins based on two different properties, typically isoelectric point and molecular weight. This approach provides higher resolution for complex protein mixtures and can be coupled with mass spectrometry for protein identification.
02 Electrophoresis apparatus design
Specialized apparatus designs for gel electrophoresis improve protein separation efficiency. These designs may include features for temperature control, uniform electric field distribution, or integrated detection systems. Some apparatuses are optimized for specific types of protein samples or separation requirements.Expand Specific Solutions03 Sample preparation techniques
Effective sample preparation is crucial for successful protein separation in gel electrophoresis. Techniques may include protein extraction, purification, concentration, and denaturation methods. Some approaches focus on maintaining protein stability or removing interfering substances to improve separation quality.Expand Specific Solutions04 Detection and analysis methods
Various detection and analysis methods are employed to visualize and quantify separated proteins. These may include staining techniques, fluorescent labeling, or advanced imaging systems. Some methods allow for real-time monitoring of protein migration during electrophoresis.Expand Specific Solutions05 Specialized electrophoresis techniques
Specialized electrophoresis techniques have been developed for specific protein separation challenges. These may include two-dimensional electrophoresis, pulsed-field gel electrophoresis, or capillary electrophoresis. Some techniques are designed to separate proteins with similar properties or to achieve ultra-high resolution.Expand Specific Solutions
Key Players in Proteomic Analysis Industry
The gel electrophoresis market for proteomic analysis is in a growth phase, driven by increasing demand for protein research in life sciences. The global market size is estimated to be in the hundreds of millions of dollars, with steady expansion projected. Technologically, while gel electrophoresis is a mature technique, innovations in 2D gel electrophoresis and integration with mass spectrometry are advancing its capabilities. Key players like Agilent Technologies, Thermo Fisher Scientific (through Applied Biosystems), and Bio-Rad Laboratories are leading development efforts, with academic institutions like Caltech and Fudan University contributing to research advancements. The competitive landscape is characterized by established companies investing in R&D to enhance resolution, automation, and data analysis capabilities.
Agilent Technologies, Inc.
Technical Solution: Agilent Technologies has developed advanced gel electrophoresis systems for proteomic analysis, such as the 2100 Bioanalyzer. This system utilizes microfluidic technology to perform automated electrophoresis, providing high-resolution protein separation and quantification[1]. The company has also introduced the Agilent 4150 TapeStation system, which offers automated gel electrophoresis with minimal sample consumption and rapid analysis times[2]. Agilent's gel electrophoresis solutions incorporate innovative features like LabChip technology, which allows for the integration of multiple analytical steps on a single microfluidic chip, enhancing efficiency and reproducibility in proteomic studies[3].
Strengths: High automation, minimal sample requirements, and rapid analysis times. Weaknesses: Higher cost compared to traditional gel electrophoresis methods and potential limitations in separating very large proteins.
Thermo Finnigan Corp.
Technical Solution: Thermo Finnigan, now part of Thermo Fisher Scientific, has developed innovative approaches to leverage gel electrophoresis in proteomic analysis. Their technology integrates gel electrophoresis with mass spectrometry, allowing for more comprehensive protein identification and characterization[4]. The company's Orbitrap mass spectrometers, when coupled with gel-based separation techniques, enable high-resolution protein analysis and post-translational modification studies[5]. Thermo Fisher has also introduced the Invitrogen Novex gel electrophoresis system, which offers pre-cast gels and optimized buffers for consistent and reproducible protein separation in proteomic research[6].
Strengths: Integration of gel electrophoresis with mass spectrometry for comprehensive analysis. Weaknesses: Complex instrumentation may require specialized training and higher initial investment.
Innovations in Gel-Based Protein Separation
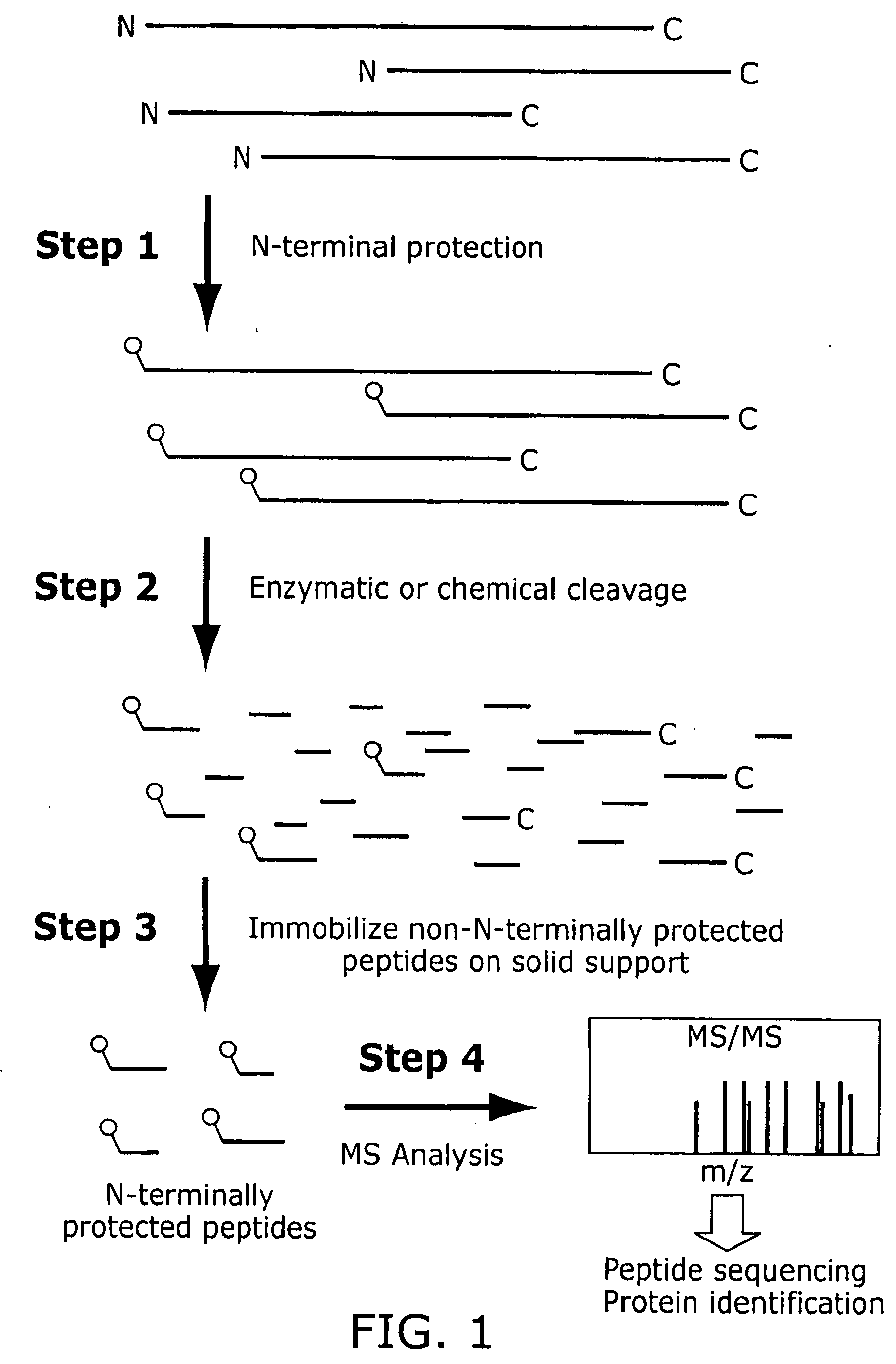
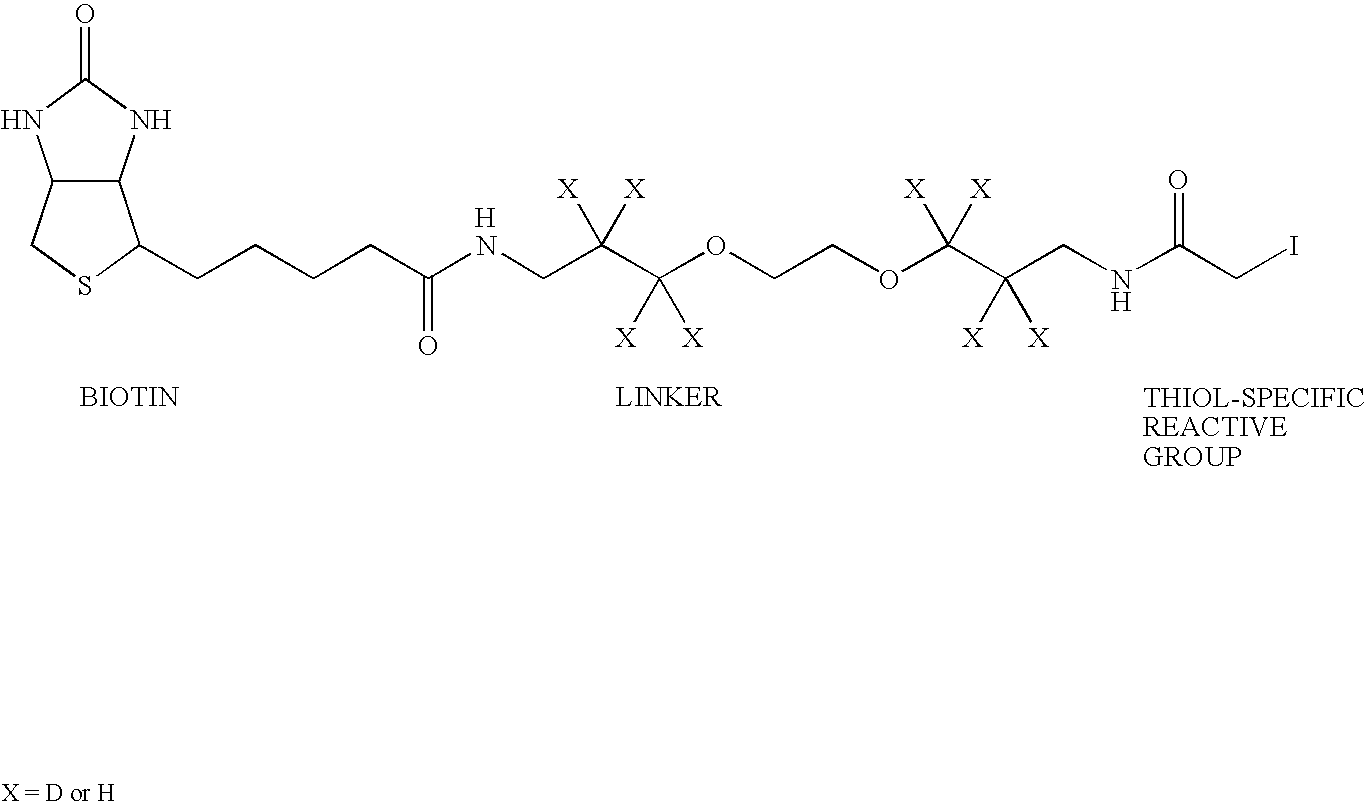
N-or C-terminal peptide selection method for proteomics
PatentInactiveUS20060134723A1
Innovation
- A method involving the selection and characterization of single N-terminal or C-terminal peptides from proteins, using protecting agents and cleaving agents to separate and identify peptides, reducing sample complexity and enabling efficient protein identification and quantification through mass spectrometry and database searches.
Automated two-dimensional gel electrophoresis
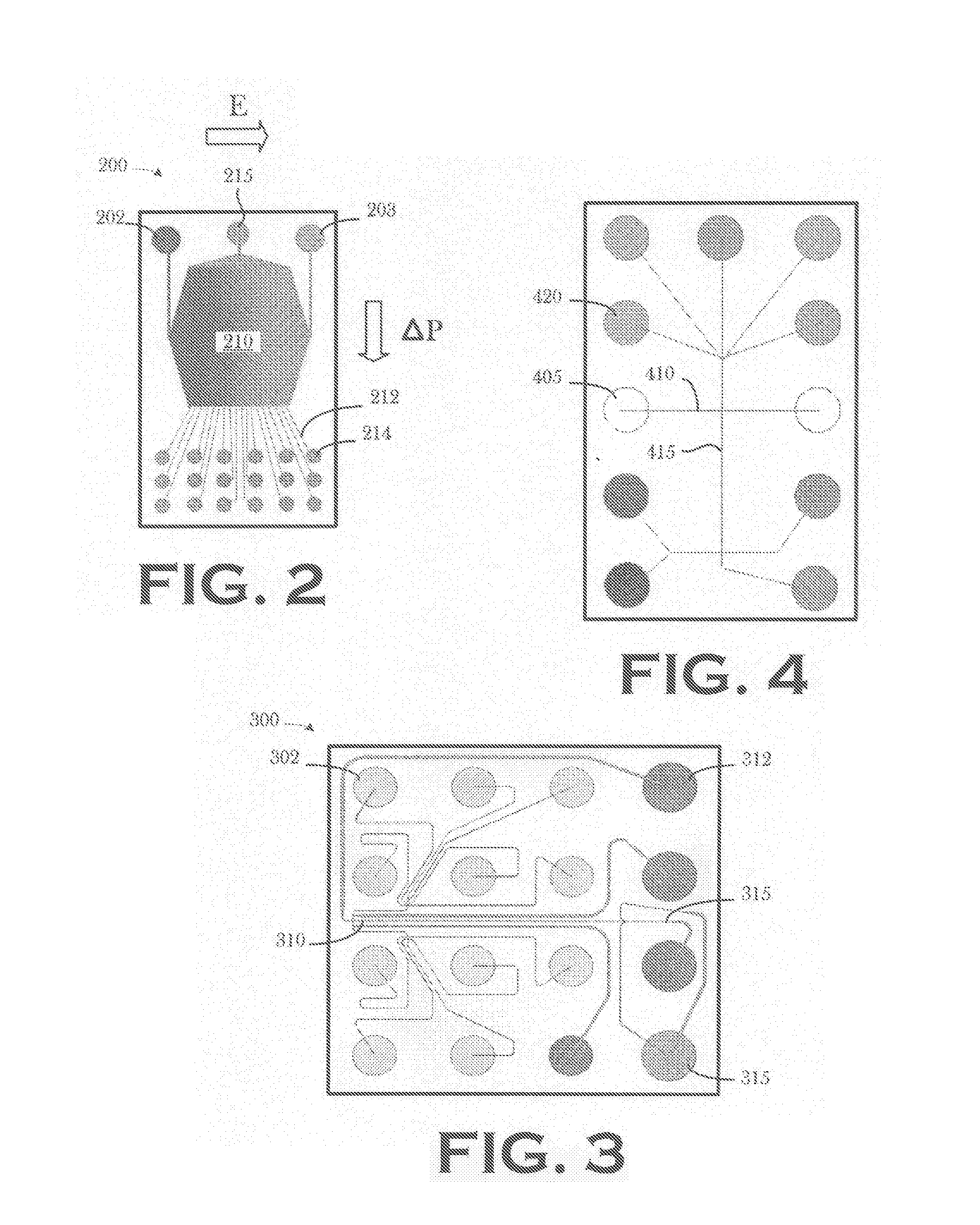
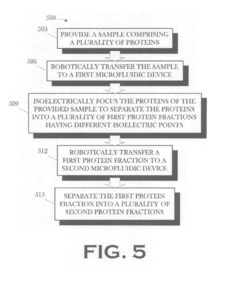
PatentInactiveUS20070199821A1
Innovation
- An automated, integrated macro and microfluidic platform that robotically transfers samples between cartridges for isoelectric focusing and size separation, reducing the need for manual handling and minimizing sample transfer steps.
Regulatory Considerations for Proteomic Analysis Tools
The regulatory landscape for proteomic analysis tools, including gel electrophoresis, is complex and evolving. In the United States, the Food and Drug Administration (FDA) oversees the regulation of these tools under the medical device framework. Gel electrophoresis systems used in proteomics are typically classified as Class I or Class II medical devices, depending on their intended use and risk profile.
For research use only (RUO) gel electrophoresis systems, the regulatory burden is relatively low. However, when these tools are intended for diagnostic or clinical applications, they fall under stricter regulatory scrutiny. Manufacturers must comply with Quality System Regulations (QSR) and obtain premarket approval or clearance before commercialization.
In the European Union, gel electrophoresis systems used in proteomics are regulated under the In Vitro Diagnostic Medical Devices Regulation (IVDR). This regulation, which replaced the previous In Vitro Diagnostic Directive (IVDD) in 2022, introduces more stringent requirements for performance evaluation, risk management, and post-market surveillance.
Globally, regulatory bodies are increasingly focusing on the validation and standardization of proteomic analysis methods. This trend is driven by the growing importance of proteomics in personalized medicine and biomarker discovery. Manufacturers and researchers must demonstrate the reproducibility and reliability of their gel electrophoresis-based proteomic analysis methods.
Data privacy and security regulations also play a crucial role in proteomic analysis. As gel electrophoresis generates sensitive biological data, compliance with regulations such as the General Data Protection Regulation (GDPR) in the EU and the Health Insurance Portability and Accountability Act (HIPAA) in the US is essential.
Looking ahead, regulatory considerations for proteomic analysis tools are likely to become more stringent. Emerging technologies, such as automated gel electrophoresis systems and integrated proteomics platforms, may face new regulatory challenges. Manufacturers and researchers should stay informed about evolving regulations and engage proactively with regulatory bodies to ensure compliance and facilitate innovation in this rapidly advancing field.
For research use only (RUO) gel electrophoresis systems, the regulatory burden is relatively low. However, when these tools are intended for diagnostic or clinical applications, they fall under stricter regulatory scrutiny. Manufacturers must comply with Quality System Regulations (QSR) and obtain premarket approval or clearance before commercialization.
In the European Union, gel electrophoresis systems used in proteomics are regulated under the In Vitro Diagnostic Medical Devices Regulation (IVDR). This regulation, which replaced the previous In Vitro Diagnostic Directive (IVDD) in 2022, introduces more stringent requirements for performance evaluation, risk management, and post-market surveillance.
Globally, regulatory bodies are increasingly focusing on the validation and standardization of proteomic analysis methods. This trend is driven by the growing importance of proteomics in personalized medicine and biomarker discovery. Manufacturers and researchers must demonstrate the reproducibility and reliability of their gel electrophoresis-based proteomic analysis methods.
Data privacy and security regulations also play a crucial role in proteomic analysis. As gel electrophoresis generates sensitive biological data, compliance with regulations such as the General Data Protection Regulation (GDPR) in the EU and the Health Insurance Portability and Accountability Act (HIPAA) in the US is essential.
Looking ahead, regulatory considerations for proteomic analysis tools are likely to become more stringent. Emerging technologies, such as automated gel electrophoresis systems and integrated proteomics platforms, may face new regulatory challenges. Manufacturers and researchers should stay informed about evolving regulations and engage proactively with regulatory bodies to ensure compliance and facilitate innovation in this rapidly advancing field.
Integration with Other Proteomic Techniques
Gel electrophoresis, a cornerstone technique in proteomics, can be effectively integrated with other proteomic methods to enhance the overall analysis of complex protein samples. This integration allows for a more comprehensive and accurate characterization of proteins, their modifications, and interactions within biological systems.
One of the primary ways gel electrophoresis complements other proteomic techniques is through its combination with mass spectrometry (MS). After protein separation by gel electrophoresis, individual protein bands or spots can be excised and subjected to in-gel digestion, followed by MS analysis. This approach, known as gel-based proteomics, enables the identification and quantification of proteins with high sensitivity and specificity.
Furthermore, gel electrophoresis can be coupled with Western blotting to provide additional information about specific proteins of interest. This combination allows for the detection and quantification of target proteins within complex mixtures, as well as the analysis of post-translational modifications and protein-protein interactions.
Two-dimensional gel electrophoresis (2-DE) can be integrated with differential in-gel electrophoresis (DIGE) to enhance quantitative proteomics studies. DIGE involves labeling different protein samples with fluorescent dyes before separation, allowing for the direct comparison of multiple samples on a single gel. This integration improves the accuracy and reproducibility of quantitative proteomic analyses.
Gel electrophoresis can also be used in conjunction with liquid chromatography (LC) to achieve higher resolution protein separation. The combination of gel-based and LC-based separation methods, known as GeLC-MS/MS, provides enhanced proteome coverage and improved identification of low-abundance proteins.
Moreover, gel electrophoresis can be integrated with protein microarray technologies for high-throughput proteomic analysis. Proteins separated by gel electrophoresis can be transferred onto microarray platforms, enabling the simultaneous analysis of multiple proteins and their interactions.
The integration of gel electrophoresis with bioinformatics tools and databases further enhances proteomic analysis. Software packages for gel image analysis, protein identification, and data interpretation can be used in conjunction with gel electrophoresis to extract meaningful biological insights from complex proteomic datasets.
In conclusion, the integration of gel electrophoresis with other proteomic techniques creates a powerful toolset for comprehensive protein analysis. This synergistic approach enables researchers to overcome the limitations of individual methods and obtain a more complete understanding of protein dynamics in biological systems.
One of the primary ways gel electrophoresis complements other proteomic techniques is through its combination with mass spectrometry (MS). After protein separation by gel electrophoresis, individual protein bands or spots can be excised and subjected to in-gel digestion, followed by MS analysis. This approach, known as gel-based proteomics, enables the identification and quantification of proteins with high sensitivity and specificity.
Furthermore, gel electrophoresis can be coupled with Western blotting to provide additional information about specific proteins of interest. This combination allows for the detection and quantification of target proteins within complex mixtures, as well as the analysis of post-translational modifications and protein-protein interactions.
Two-dimensional gel electrophoresis (2-DE) can be integrated with differential in-gel electrophoresis (DIGE) to enhance quantitative proteomics studies. DIGE involves labeling different protein samples with fluorescent dyes before separation, allowing for the direct comparison of multiple samples on a single gel. This integration improves the accuracy and reproducibility of quantitative proteomic analyses.
Gel electrophoresis can also be used in conjunction with liquid chromatography (LC) to achieve higher resolution protein separation. The combination of gel-based and LC-based separation methods, known as GeLC-MS/MS, provides enhanced proteome coverage and improved identification of low-abundance proteins.
Moreover, gel electrophoresis can be integrated with protein microarray technologies for high-throughput proteomic analysis. Proteins separated by gel electrophoresis can be transferred onto microarray platforms, enabling the simultaneous analysis of multiple proteins and their interactions.
The integration of gel electrophoresis with bioinformatics tools and databases further enhances proteomic analysis. Software packages for gel image analysis, protein identification, and data interpretation can be used in conjunction with gel electrophoresis to extract meaningful biological insights from complex proteomic datasets.
In conclusion, the integration of gel electrophoresis with other proteomic techniques creates a powerful toolset for comprehensive protein analysis. This synergistic approach enables researchers to overcome the limitations of individual methods and obtain a more complete understanding of protein dynamics in biological systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!