Gel Electrophoresis: Precision Tools for Genome Mapping
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Gel Electrophoresis Evolution and Objectives
Gel electrophoresis has evolved significantly since its inception in the 1930s, becoming an indispensable tool in molecular biology and genomics. Initially developed for protein separation, it has since been adapted for DNA and RNA analysis, playing a crucial role in genome mapping. The technique's evolution has been driven by the increasing demand for higher resolution, faster separation, and the ability to handle larger DNA fragments.
In the 1970s, the introduction of agarose gel electrophoresis marked a significant milestone, allowing for the separation of DNA fragments based on size. This development was pivotal in early genome mapping efforts, enabling researchers to visualize and analyze DNA restriction fragments. The subsequent invention of pulsed-field gel electrophoresis (PFGE) in the 1980s further expanded the technique's capabilities, permitting the separation of much larger DNA fragments, up to several megabases in size.
The advent of capillary gel electrophoresis in the 1990s revolutionized the field, offering higher resolution, faster analysis times, and the potential for automation. This innovation was instrumental in accelerating genome sequencing projects, including the Human Genome Project. Concurrently, the development of two-dimensional gel electrophoresis enhanced protein separation and analysis, contributing to the growth of proteomics.
Recent advancements have focused on miniaturization and integration with other technologies. Microchip-based gel electrophoresis systems have emerged, offering portability and reduced sample requirements. These systems are particularly valuable for point-of-care diagnostics and field-based genomic studies. Additionally, the integration of gel electrophoresis with mass spectrometry has opened new avenues for high-throughput protein identification and characterization.
The primary objectives of current research in gel electrophoresis for genome mapping are multifaceted. Researchers aim to enhance resolution and sensitivity, enabling the detection and separation of increasingly smaller DNA fragments and low-abundance molecules. There is also a push towards developing more sustainable and environmentally friendly electrophoresis methods, including the use of alternative gel materials and buffer systems.
Another key goal is to improve the speed and throughput of gel electrophoresis techniques, particularly for large-scale genomic studies. This includes the development of parallel processing systems and the integration of artificial intelligence for data analysis. Furthermore, there is a growing emphasis on creating user-friendly, automated systems that can be easily adopted in clinical settings for diagnostic applications.
As genomics continues to advance, gel electrophoresis techniques are evolving to meet the demands of emerging fields such as single-cell genomics and long-read sequencing. The ultimate objective is to establish gel electrophoresis as a versatile, high-precision tool capable of supporting a wide range of genomic applications, from basic research to personalized medicine.
In the 1970s, the introduction of agarose gel electrophoresis marked a significant milestone, allowing for the separation of DNA fragments based on size. This development was pivotal in early genome mapping efforts, enabling researchers to visualize and analyze DNA restriction fragments. The subsequent invention of pulsed-field gel electrophoresis (PFGE) in the 1980s further expanded the technique's capabilities, permitting the separation of much larger DNA fragments, up to several megabases in size.
The advent of capillary gel electrophoresis in the 1990s revolutionized the field, offering higher resolution, faster analysis times, and the potential for automation. This innovation was instrumental in accelerating genome sequencing projects, including the Human Genome Project. Concurrently, the development of two-dimensional gel electrophoresis enhanced protein separation and analysis, contributing to the growth of proteomics.
Recent advancements have focused on miniaturization and integration with other technologies. Microchip-based gel electrophoresis systems have emerged, offering portability and reduced sample requirements. These systems are particularly valuable for point-of-care diagnostics and field-based genomic studies. Additionally, the integration of gel electrophoresis with mass spectrometry has opened new avenues for high-throughput protein identification and characterization.
The primary objectives of current research in gel electrophoresis for genome mapping are multifaceted. Researchers aim to enhance resolution and sensitivity, enabling the detection and separation of increasingly smaller DNA fragments and low-abundance molecules. There is also a push towards developing more sustainable and environmentally friendly electrophoresis methods, including the use of alternative gel materials and buffer systems.
Another key goal is to improve the speed and throughput of gel electrophoresis techniques, particularly for large-scale genomic studies. This includes the development of parallel processing systems and the integration of artificial intelligence for data analysis. Furthermore, there is a growing emphasis on creating user-friendly, automated systems that can be easily adopted in clinical settings for diagnostic applications.
As genomics continues to advance, gel electrophoresis techniques are evolving to meet the demands of emerging fields such as single-cell genomics and long-read sequencing. The ultimate objective is to establish gel electrophoresis as a versatile, high-precision tool capable of supporting a wide range of genomic applications, from basic research to personalized medicine.
Genomic Research Market Analysis
The genomic research market has experienced significant growth in recent years, driven by advancements in sequencing technologies, increasing demand for personalized medicine, and growing applications in various fields such as agriculture, forensics, and drug discovery. The global genomics market size was valued at approximately $23.11 billion in 2020 and is projected to reach $62.9 billion by 2028, growing at a CAGR of 15.35% during the forecast period.
Gel electrophoresis, as a precision tool for genome mapping, plays a crucial role in this expanding market. The technique's ability to separate DNA fragments based on size has made it an indispensable tool in genomic research, particularly in applications such as genetic fingerprinting, mutation detection, and gene expression analysis. The gel electrophoresis segment of the genomics market is expected to maintain steady growth, with a CAGR of around 6.5% from 2021 to 2026.
The demand for gel electrophoresis in genome mapping is driven by several factors. Firstly, the increasing prevalence of genetic disorders and cancer has led to a surge in genomic research aimed at understanding disease mechanisms and developing targeted therapies. Secondly, the growing adoption of personalized medicine approaches requires precise genomic analysis, where gel electrophoresis serves as a cost-effective and reliable tool for DNA fragment separation and analysis.
In the academic and research sector, gel electrophoresis continues to be a staple technique in molecular biology laboratories. The technique's simplicity, affordability, and versatility make it an attractive option for both small-scale research projects and large-scale genomic studies. Additionally, the integration of gel electrophoresis with other genomic technologies, such as next-generation sequencing and PCR, has further expanded its applications and market potential.
The pharmaceutical and biotechnology industries are significant contributors to the growth of the gel electrophoresis market for genome mapping. These sectors rely heavily on genomic research for drug discovery, development of biomarkers, and clinical trials. The increasing investment in R&D activities by pharmaceutical companies, coupled with the rising number of drug candidates in clinical trials, is expected to drive the demand for gel electrophoresis techniques in the coming years.
Geographically, North America dominates the genomic research market, including the gel electrophoresis segment, due to the presence of well-established research infrastructure, high R&D investments, and early adoption of advanced genomic technologies. However, the Asia-Pacific region is anticipated to witness the fastest growth in the coming years, driven by increasing government initiatives to promote genomic research, rising healthcare expenditure, and growing awareness about personalized medicine.
Gel electrophoresis, as a precision tool for genome mapping, plays a crucial role in this expanding market. The technique's ability to separate DNA fragments based on size has made it an indispensable tool in genomic research, particularly in applications such as genetic fingerprinting, mutation detection, and gene expression analysis. The gel electrophoresis segment of the genomics market is expected to maintain steady growth, with a CAGR of around 6.5% from 2021 to 2026.
The demand for gel electrophoresis in genome mapping is driven by several factors. Firstly, the increasing prevalence of genetic disorders and cancer has led to a surge in genomic research aimed at understanding disease mechanisms and developing targeted therapies. Secondly, the growing adoption of personalized medicine approaches requires precise genomic analysis, where gel electrophoresis serves as a cost-effective and reliable tool for DNA fragment separation and analysis.
In the academic and research sector, gel electrophoresis continues to be a staple technique in molecular biology laboratories. The technique's simplicity, affordability, and versatility make it an attractive option for both small-scale research projects and large-scale genomic studies. Additionally, the integration of gel electrophoresis with other genomic technologies, such as next-generation sequencing and PCR, has further expanded its applications and market potential.
The pharmaceutical and biotechnology industries are significant contributors to the growth of the gel electrophoresis market for genome mapping. These sectors rely heavily on genomic research for drug discovery, development of biomarkers, and clinical trials. The increasing investment in R&D activities by pharmaceutical companies, coupled with the rising number of drug candidates in clinical trials, is expected to drive the demand for gel electrophoresis techniques in the coming years.
Geographically, North America dominates the genomic research market, including the gel electrophoresis segment, due to the presence of well-established research infrastructure, high R&D investments, and early adoption of advanced genomic technologies. However, the Asia-Pacific region is anticipated to witness the fastest growth in the coming years, driven by increasing government initiatives to promote genomic research, rising healthcare expenditure, and growing awareness about personalized medicine.
Current Challenges in Gel Electrophoresis Techniques
Despite the widespread use of gel electrophoresis in genome mapping, several challenges persist that limit its precision and applicability. One of the primary issues is the resolution of DNA fragments, particularly for large genomic regions. As the size of DNA fragments increases, the ability to distinguish between closely sized fragments diminishes, leading to potential inaccuracies in genome mapping.
Another significant challenge is the non-linear migration of DNA molecules through the gel matrix. This non-linearity can result in distorted band patterns, making it difficult to accurately determine fragment sizes and relative positions within the genome. The phenomenon is especially pronounced for larger DNA molecules, which can adopt complex conformations and interact unpredictably with the gel matrix.
The time-consuming nature of gel electrophoresis techniques also poses a challenge, particularly when dealing with high-throughput genomic studies. The process of gel preparation, sample loading, and electrophoresis can take several hours to days, depending on the size of fragments being separated. This time factor can be a significant bottleneck in large-scale genome mapping projects.
Reproducibility is another critical issue facing gel electrophoresis techniques. Variations in gel composition, running conditions, and even environmental factors can lead to inconsistencies in results between different experiments or laboratories. This lack of standardization can complicate the comparison and integration of genomic data from multiple sources.
The limited sensitivity of traditional staining methods used in gel electrophoresis is also a concern. While ethidium bromide has been widely used, its sensitivity is not sufficient for detecting very low concentrations of DNA. This limitation can result in the loss of important genetic information, especially when dealing with rare genomic variants or low-copy-number sequences.
Furthermore, the manual nature of many gel electrophoresis processes introduces the potential for human error. Tasks such as gel loading, band visualization, and size estimation are often performed manually, which can lead to inconsistencies and subjective interpretations of results.
Lastly, the challenge of analyzing complex genomic structures, such as repetitive sequences or structural variations, remains a significant hurdle for gel electrophoresis techniques. These genomic elements can produce ambiguous or misleading band patterns, making it difficult to accurately map their positions and characteristics within the genome.
Another significant challenge is the non-linear migration of DNA molecules through the gel matrix. This non-linearity can result in distorted band patterns, making it difficult to accurately determine fragment sizes and relative positions within the genome. The phenomenon is especially pronounced for larger DNA molecules, which can adopt complex conformations and interact unpredictably with the gel matrix.
The time-consuming nature of gel electrophoresis techniques also poses a challenge, particularly when dealing with high-throughput genomic studies. The process of gel preparation, sample loading, and electrophoresis can take several hours to days, depending on the size of fragments being separated. This time factor can be a significant bottleneck in large-scale genome mapping projects.
Reproducibility is another critical issue facing gel electrophoresis techniques. Variations in gel composition, running conditions, and even environmental factors can lead to inconsistencies in results between different experiments or laboratories. This lack of standardization can complicate the comparison and integration of genomic data from multiple sources.
The limited sensitivity of traditional staining methods used in gel electrophoresis is also a concern. While ethidium bromide has been widely used, its sensitivity is not sufficient for detecting very low concentrations of DNA. This limitation can result in the loss of important genetic information, especially when dealing with rare genomic variants or low-copy-number sequences.
Furthermore, the manual nature of many gel electrophoresis processes introduces the potential for human error. Tasks such as gel loading, band visualization, and size estimation are often performed manually, which can lead to inconsistencies and subjective interpretations of results.
Lastly, the challenge of analyzing complex genomic structures, such as repetitive sequences or structural variations, remains a significant hurdle for gel electrophoresis techniques. These genomic elements can produce ambiguous or misleading band patterns, making it difficult to accurately map their positions and characteristics within the genome.
State-of-the-Art Gel Electrophoresis Protocols
01 Improved gel composition for electrophoresis
Advancements in gel composition can enhance the precision of gel electrophoresis. These improvements include the use of specific polymers, cross-linking agents, and additives that create a more uniform and stable gel matrix. This results in better resolution and separation of molecules, leading to more accurate and reproducible results.- Improved gel composition for electrophoresis: Advancements in gel composition can enhance the precision of gel electrophoresis. These improvements include optimizing polymer concentrations, incorporating novel monomers, and adding specific additives to enhance separation and resolution of biomolecules. Such modifications can lead to sharper bands, better separation of closely related molecules, and increased overall precision of the technique.
- Enhanced detection and imaging systems: Precision in gel electrophoresis can be significantly improved through advanced detection and imaging systems. These may include high-resolution cameras, fluorescence-based detection methods, and sophisticated image analysis software. Such systems allow for more accurate quantification of bands, detection of faint signals, and overall improvement in data quality and reproducibility.
- Microfluidic and miniaturized electrophoresis systems: Miniaturization of gel electrophoresis systems, including microfluidic devices, can lead to increased precision. These systems often require smaller sample volumes, provide faster separation times, and offer better control over experimental conditions. The reduced scale can minimize sample diffusion and improve resolution, resulting in more precise and reproducible results.
- Automated sample loading and running: Automation in gel electrophoresis can significantly improve precision by reducing human error and ensuring consistency across experiments. Automated systems can precisely control sample loading volumes, apply consistent voltage, and maintain optimal running conditions throughout the electrophoresis process. This leads to more reproducible results and increased overall precision of the technique.
- Specialized buffer systems and running conditions: The development of specialized buffer systems and optimized running conditions can enhance the precision of gel electrophoresis. This includes the use of gradient gels, pulsed-field electrophoresis techniques, and buffer additives that improve separation of specific types of molecules. By tailoring the electrophoresis conditions to the specific sample and analysis requirements, researchers can achieve higher resolution and more precise results.
02 Enhanced detection and quantification methods
Innovative detection and quantification techniques have been developed to increase the precision of gel electrophoresis. These methods include improved staining protocols, fluorescent labeling, and advanced imaging systems. By enhancing the visibility and measurement of separated molecules, these techniques contribute to more accurate analysis and interpretation of results.Expand Specific Solutions03 Automated gel electrophoresis systems
Automation of gel electrophoresis processes has significantly improved precision by reducing human error and increasing reproducibility. These systems can control various parameters such as voltage, temperature, and run time with high accuracy. Additionally, automated sample loading and gel imaging contribute to more consistent and reliable results.Expand Specific Solutions04 Microfluidic devices for electrophoresis
The development of microfluidic devices for gel electrophoresis has led to increased precision in sample handling and analysis. These miniaturized systems allow for better control over sample volumes, reduced sample consumption, and improved heat dissipation. The result is enhanced resolution and reproducibility, particularly for small sample sizes.Expand Specific Solutions05 Specialized buffer systems and electrodes
Advancements in buffer compositions and electrode designs have contributed to improved precision in gel electrophoresis. Specialized buffer systems can enhance separation and reduce band distortion, while optimized electrode configurations ensure more uniform electric fields. These improvements result in better resolution and more accurate molecular weight determinations.Expand Specific Solutions
Key Players in Genomic Mapping Industry
The gel electrophoresis market for genome mapping is in a growth phase, driven by increasing applications in genomics research and personalized medicine. The global market size is expanding, with projections indicating significant growth in the coming years. Technologically, gel electrophoresis is mature but continues to evolve with innovations in precision and automation. Key players like Life Technologies, QIAGEN, and Bio-Rad Laboratories are leading the field, offering advanced systems and consumables. Emerging companies such as Sage Science are introducing specialized instruments, while established firms like Hitachi and 3M are leveraging their expertise to develop improved solutions. Academic institutions, including ETH Zurich and the University of California, contribute significantly to research and development in this area.
Life Technologies Corp.
Technical Solution: Life Technologies has developed advanced gel electrophoresis systems for genome mapping, including the E-Gel™ platform. This system utilizes pre-cast agarose gels with integrated electrodes, allowing for rapid and consistent DNA separation[1]. Their technology incorporates a digital imaging system for real-time visualization and analysis of DNA fragments. The company has also introduced the E-Gel™ NGS system, specifically designed for next-generation sequencing library preparation, which can separate DNA fragments as small as 10 base pairs with high resolution[2]. Additionally, Life Technologies offers automated gel electrophoresis systems that can handle high-throughput genome mapping projects, improving efficiency and reproducibility in large-scale genomic studies[3].
Strengths: High-throughput capabilities, integrated imaging systems, and specialized products for NGS. Weaknesses: Reliance on proprietary consumables, potentially higher cost compared to traditional methods.
QIAGEN Sciences LLC
Technical Solution: QIAGEN has developed the QIAxcel Advanced system, a capillary gel electrophoresis platform that automates DNA fragment analysis for genome mapping. This system uses a unique gel cartridge technology that eliminates the need for traditional slab gels, reducing hands-on time and improving reproducibility[4]. The QIAxcel can analyze fragments ranging from 15 bp to 10 kb with high resolution, making it suitable for a wide range of genomic applications. QIAGEN's ScreenGel software accompanies the system, providing automated data analysis and reporting features. The company has also introduced the QIAxcel DNA High Resolution Kit, which can distinguish DNA fragments that differ by as little as 3-5 base pairs, enhancing precision in genome mapping studies[5].
Strengths: Automated system with high-resolution capabilities, reduced hands-on time. Weaknesses: Limited to smaller fragment sizes compared to pulsed-field gel electrophoresis systems.
Breakthrough Technologies in DNA Separation
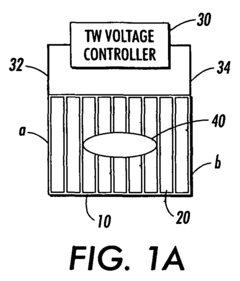
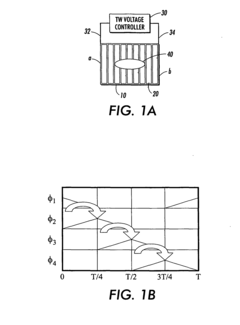
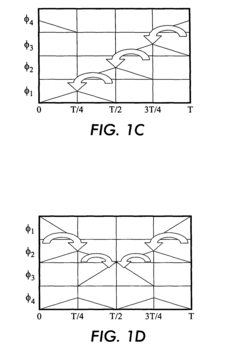
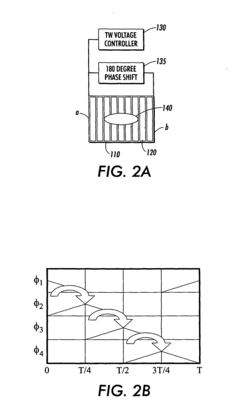
Travelling wave algorithms to focus and concentrate proteins in gel electrophoresis
PatentInactiveEP1486781A1
Innovation
- The use of electrostatic traveling waves in a gel electrophoretic system with a grid of closely spaced parallel electrodes and a voltage controller generating bi-directional traveling waves to compact protein bands, reducing band broadening and increasing resolution by applying low voltage and short processing times.
Method of determining the nucleotide sequence of oligonucleotides and DNA molecules
PatentInactiveUS20170321269A1
Innovation
- A novel method called 'reactive sequencing' that detects DNA polymerase-catalyzed incorporation of deoxynucleoside monophosphates individually and serially, eliminating the need for electrophoretic separation, using techniques like fluorescence, chemiluminescence, or microcalorimetry to monitor and correct sequencing errors, allowing direct readout of nucleotide sequences without gel separation.
Regulatory Framework for Genomic Research Tools
The regulatory framework for genomic research tools, including gel electrophoresis for genome mapping, is a complex and evolving landscape. In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the development and use of these tools. The FDA's Center for Devices and Radiological Health (CDRH) is responsible for regulating medical devices, including those used in genomic research.
For gel electrophoresis systems used in genome mapping, the FDA typically classifies them as Class I or Class II medical devices, depending on their intended use and risk profile. Class I devices are subject to general controls, while Class II devices require special controls and premarket notification (510(k)) submission. Manufacturers must demonstrate that their devices are substantially equivalent to a legally marketed predicate device.
The Clinical Laboratory Improvement Amendments (CLIA) of 1988 also impact the use of gel electrophoresis in clinical settings. Laboratories using these tools for diagnostic purposes must comply with CLIA regulations, which ensure the accuracy and reliability of test results.
Internationally, regulatory frameworks vary. The European Union's In Vitro Diagnostic Regulation (IVDR) came into full effect in May 2022, introducing more stringent requirements for genomic research tools. Under the IVDR, gel electrophoresis systems may be classified as Class B or C devices, depending on their intended use and risk level.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) oversees the regulation of genomic research tools. The Japanese regulatory system classifies medical devices into four classes, with gel electrophoresis systems typically falling into Class I or II.
Ethical considerations also play a significant role in the regulatory framework. Many countries have established bioethics committees to address the ethical implications of genomic research. These committees often provide guidelines on issues such as informed consent, data privacy, and the responsible use of genetic information.
As genomic research advances, regulatory bodies are continually adapting their frameworks to keep pace with technological developments. This includes addressing emerging challenges such as the integration of artificial intelligence in genomic analysis and the increasing use of next-generation sequencing technologies alongside traditional methods like gel electrophoresis.
For gel electrophoresis systems used in genome mapping, the FDA typically classifies them as Class I or Class II medical devices, depending on their intended use and risk profile. Class I devices are subject to general controls, while Class II devices require special controls and premarket notification (510(k)) submission. Manufacturers must demonstrate that their devices are substantially equivalent to a legally marketed predicate device.
The Clinical Laboratory Improvement Amendments (CLIA) of 1988 also impact the use of gel electrophoresis in clinical settings. Laboratories using these tools for diagnostic purposes must comply with CLIA regulations, which ensure the accuracy and reliability of test results.
Internationally, regulatory frameworks vary. The European Union's In Vitro Diagnostic Regulation (IVDR) came into full effect in May 2022, introducing more stringent requirements for genomic research tools. Under the IVDR, gel electrophoresis systems may be classified as Class B or C devices, depending on their intended use and risk level.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) oversees the regulation of genomic research tools. The Japanese regulatory system classifies medical devices into four classes, with gel electrophoresis systems typically falling into Class I or II.
Ethical considerations also play a significant role in the regulatory framework. Many countries have established bioethics committees to address the ethical implications of genomic research. These committees often provide guidelines on issues such as informed consent, data privacy, and the responsible use of genetic information.
As genomic research advances, regulatory bodies are continually adapting their frameworks to keep pace with technological developments. This includes addressing emerging challenges such as the integration of artificial intelligence in genomic analysis and the increasing use of next-generation sequencing technologies alongside traditional methods like gel electrophoresis.
Ethical Implications of Precision Genome Mapping
The advent of precision genome mapping through gel electrophoresis has raised significant ethical considerations that warrant careful examination. As this technology becomes more refined and accessible, it brings forth a range of ethical implications that society must address.
One primary concern is the potential for genetic discrimination. As genome mapping becomes more precise, it may reveal an individual's predisposition to certain diseases or conditions. This information could be misused by employers, insurance companies, or other entities to discriminate against individuals based on their genetic makeup. Safeguarding genetic privacy and preventing such discrimination are crucial ethical challenges that need to be addressed through robust legal frameworks and societal norms.
The ability to map genomes with high precision also raises questions about genetic enhancement and designer babies. As our understanding of the genome improves, there may be increasing pressure to use this knowledge for non-medical purposes, such as selecting for specific traits in offspring. This raises complex ethical questions about the limits of parental choice, the nature of human identity, and the potential for exacerbating societal inequalities.
Furthermore, the use of precision genome mapping in prenatal testing presents ethical dilemmas. While it can provide valuable information about potential genetic disorders, it also raises questions about the value of human life and the ethics of selective abortion. Balancing the benefits of early detection with the potential for increased termination of pregnancies based on genetic information is a challenging ethical issue.
The ownership and control of genetic information is another critical ethical consideration. As genome mapping becomes more prevalent, questions arise about who owns this data, how it should be stored and protected, and who should have access to it. Ensuring informed consent and protecting individual autonomy in the face of powerful commercial and research interests is paramount.
Lastly, the global implications of precision genome mapping must be considered. The technology's potential to exacerbate existing health disparities between developed and developing nations raises ethical concerns about equitable access to advanced medical technologies. Addressing these disparities and ensuring that the benefits of precision genome mapping are accessible to all populations is an important ethical imperative.
In conclusion, while precision genome mapping through gel electrophoresis offers tremendous potential for advancing medical science and improving human health, it also presents a complex array of ethical challenges. Addressing these issues requires ongoing dialogue between scientists, ethicists, policymakers, and the public to ensure that this powerful technology is used responsibly and for the benefit of all humanity.
One primary concern is the potential for genetic discrimination. As genome mapping becomes more precise, it may reveal an individual's predisposition to certain diseases or conditions. This information could be misused by employers, insurance companies, or other entities to discriminate against individuals based on their genetic makeup. Safeguarding genetic privacy and preventing such discrimination are crucial ethical challenges that need to be addressed through robust legal frameworks and societal norms.
The ability to map genomes with high precision also raises questions about genetic enhancement and designer babies. As our understanding of the genome improves, there may be increasing pressure to use this knowledge for non-medical purposes, such as selecting for specific traits in offspring. This raises complex ethical questions about the limits of parental choice, the nature of human identity, and the potential for exacerbating societal inequalities.
Furthermore, the use of precision genome mapping in prenatal testing presents ethical dilemmas. While it can provide valuable information about potential genetic disorders, it also raises questions about the value of human life and the ethics of selective abortion. Balancing the benefits of early detection with the potential for increased termination of pregnancies based on genetic information is a challenging ethical issue.
The ownership and control of genetic information is another critical ethical consideration. As genome mapping becomes more prevalent, questions arise about who owns this data, how it should be stored and protected, and who should have access to it. Ensuring informed consent and protecting individual autonomy in the face of powerful commercial and research interests is paramount.
Lastly, the global implications of precision genome mapping must be considered. The technology's potential to exacerbate existing health disparities between developed and developing nations raises ethical concerns about equitable access to advanced medical technologies. Addressing these disparities and ensuring that the benefits of precision genome mapping are accessible to all populations is an important ethical imperative.
In conclusion, while precision genome mapping through gel electrophoresis offers tremendous potential for advancing medical science and improving human health, it also presents a complex array of ethical challenges. Addressing these issues requires ongoing dialogue between scientists, ethicists, policymakers, and the public to ensure that this powerful technology is used responsibly and for the benefit of all humanity.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!