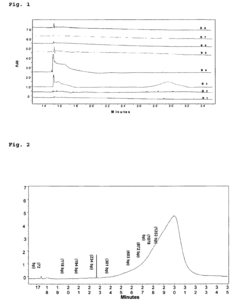
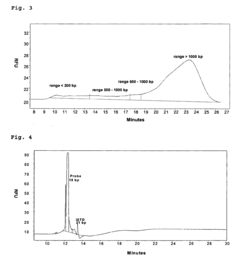
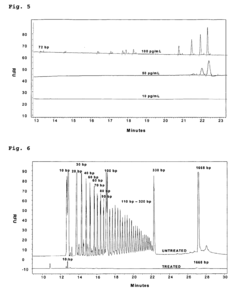
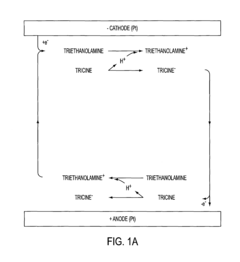
Gel Electrophoresis Techniques for Vaccine Development
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Vaccine Gel Electrophoresis Background and Objectives
Gel electrophoresis has been a cornerstone technique in molecular biology and biochemistry for decades, playing a crucial role in vaccine development. This method, which separates molecules based on their size and electrical charge, has evolved significantly since its inception in the 1930s. The technique has become increasingly sophisticated, allowing for more precise analysis of nucleic acids and proteins, which are fundamental components in vaccine research and production.
The primary objective of utilizing gel electrophoresis in vaccine development is to analyze and characterize vaccine components, assess their purity, and evaluate immune responses. This technique enables researchers to separate and identify specific proteins or nucleic acids, crucial for understanding the molecular basis of vaccine efficacy and safety. As vaccine development has become more complex, particularly with the advent of recombinant and nucleic acid-based vaccines, the role of gel electrophoresis has expanded to encompass quality control, stability testing, and batch-to-batch consistency assessments.
Recent technological advancements have led to the development of high-resolution gel electrophoresis techniques, such as pulsed-field gel electrophoresis (PFGE) and capillary electrophoresis, which offer enhanced separation capabilities. These innovations have significantly improved the ability to analyze larger DNA fragments and complex protein mixtures, critical for the development of modern vaccines targeting emerging pathogens.
The ongoing COVID-19 pandemic has further highlighted the importance of gel electrophoresis in rapid vaccine development. The technique has been instrumental in characterizing the SARS-CoV-2 spike protein and assessing the quality of mRNA vaccines. This application demonstrates the adaptability of gel electrophoresis to new challenges in vaccine research and production.
Looking forward, the integration of gel electrophoresis with other analytical techniques, such as mass spectrometry and next-generation sequencing, is expected to provide more comprehensive insights into vaccine composition and efficacy. Additionally, the development of automated and high-throughput electrophoresis systems is anticipated to accelerate vaccine research and production processes, potentially reducing the time from concept to market for new vaccines.
As we continue to face global health challenges, the objectives for advancing gel electrophoresis techniques in vaccine development include improving resolution, increasing sensitivity, and enhancing automation. These advancements aim to facilitate the rapid development of safe and effective vaccines against a wide range of pathogens, including those responsible for emerging infectious diseases.
The primary objective of utilizing gel electrophoresis in vaccine development is to analyze and characterize vaccine components, assess their purity, and evaluate immune responses. This technique enables researchers to separate and identify specific proteins or nucleic acids, crucial for understanding the molecular basis of vaccine efficacy and safety. As vaccine development has become more complex, particularly with the advent of recombinant and nucleic acid-based vaccines, the role of gel electrophoresis has expanded to encompass quality control, stability testing, and batch-to-batch consistency assessments.
Recent technological advancements have led to the development of high-resolution gel electrophoresis techniques, such as pulsed-field gel electrophoresis (PFGE) and capillary electrophoresis, which offer enhanced separation capabilities. These innovations have significantly improved the ability to analyze larger DNA fragments and complex protein mixtures, critical for the development of modern vaccines targeting emerging pathogens.
The ongoing COVID-19 pandemic has further highlighted the importance of gel electrophoresis in rapid vaccine development. The technique has been instrumental in characterizing the SARS-CoV-2 spike protein and assessing the quality of mRNA vaccines. This application demonstrates the adaptability of gel electrophoresis to new challenges in vaccine research and production.
Looking forward, the integration of gel electrophoresis with other analytical techniques, such as mass spectrometry and next-generation sequencing, is expected to provide more comprehensive insights into vaccine composition and efficacy. Additionally, the development of automated and high-throughput electrophoresis systems is anticipated to accelerate vaccine research and production processes, potentially reducing the time from concept to market for new vaccines.
As we continue to face global health challenges, the objectives for advancing gel electrophoresis techniques in vaccine development include improving resolution, increasing sensitivity, and enhancing automation. These advancements aim to facilitate the rapid development of safe and effective vaccines against a wide range of pathogens, including those responsible for emerging infectious diseases.
Market Analysis for Gel Electrophoresis in Vaccines
The gel electrophoresis market in vaccine development has experienced significant growth in recent years, driven by the increasing demand for vaccines and the need for advanced analytical techniques in their production. This market segment is expected to continue its upward trajectory, with a compound annual growth rate projected to remain strong over the next five years.
The primary factors fueling this growth include the rising prevalence of infectious diseases, the ongoing COVID-19 pandemic, and the growing focus on preventive healthcare measures. Governments and healthcare organizations worldwide are investing heavily in vaccine research and development, which directly impacts the demand for gel electrophoresis techniques.
Geographically, North America currently dominates the market, owing to its advanced healthcare infrastructure and substantial investments in biotechnology research. However, the Asia-Pacific region is anticipated to witness the fastest growth, attributed to increasing healthcare expenditure, improving research facilities, and a growing emphasis on vaccine production in countries like India and China.
The market is segmented based on product type, with polyacrylamide gel electrophoresis (PAGE) holding the largest share due to its widespread use in protein analysis. Agarose gel electrophoresis, while less dominant, remains crucial for nucleic acid separation in vaccine development processes.
Key players in the gel electrophoresis market for vaccine development include Bio-Rad Laboratories, Thermo Fisher Scientific, and GE Healthcare. These companies are continuously innovating to improve the efficiency and accuracy of gel electrophoresis techniques, driving market growth and competitiveness.
The increasing adoption of personalized medicine and the development of novel vaccine platforms, such as mRNA vaccines, are creating new opportunities for gel electrophoresis in vaccine research. These trends are expected to further expand the market size and diversify its applications in the coming years.
However, the market faces challenges such as the high cost of advanced gel electrophoresis systems and the technical expertise required for their operation. Additionally, the emergence of alternative technologies like capillary electrophoresis and next-generation sequencing may pose a threat to traditional gel electrophoresis methods in certain applications.
Despite these challenges, the overall outlook for the gel electrophoresis market in vaccine development remains positive. The continuous need for reliable and efficient analytical tools in vaccine production, coupled with ongoing technological advancements, is expected to sustain market growth and drive innovation in the field.
The primary factors fueling this growth include the rising prevalence of infectious diseases, the ongoing COVID-19 pandemic, and the growing focus on preventive healthcare measures. Governments and healthcare organizations worldwide are investing heavily in vaccine research and development, which directly impacts the demand for gel electrophoresis techniques.
Geographically, North America currently dominates the market, owing to its advanced healthcare infrastructure and substantial investments in biotechnology research. However, the Asia-Pacific region is anticipated to witness the fastest growth, attributed to increasing healthcare expenditure, improving research facilities, and a growing emphasis on vaccine production in countries like India and China.
The market is segmented based on product type, with polyacrylamide gel electrophoresis (PAGE) holding the largest share due to its widespread use in protein analysis. Agarose gel electrophoresis, while less dominant, remains crucial for nucleic acid separation in vaccine development processes.
Key players in the gel electrophoresis market for vaccine development include Bio-Rad Laboratories, Thermo Fisher Scientific, and GE Healthcare. These companies are continuously innovating to improve the efficiency and accuracy of gel electrophoresis techniques, driving market growth and competitiveness.
The increasing adoption of personalized medicine and the development of novel vaccine platforms, such as mRNA vaccines, are creating new opportunities for gel electrophoresis in vaccine research. These trends are expected to further expand the market size and diversify its applications in the coming years.
However, the market faces challenges such as the high cost of advanced gel electrophoresis systems and the technical expertise required for their operation. Additionally, the emergence of alternative technologies like capillary electrophoresis and next-generation sequencing may pose a threat to traditional gel electrophoresis methods in certain applications.
Despite these challenges, the overall outlook for the gel electrophoresis market in vaccine development remains positive. The continuous need for reliable and efficient analytical tools in vaccine production, coupled with ongoing technological advancements, is expected to sustain market growth and drive innovation in the field.
Current Challenges in Gel Electrophoresis for Vaccines
Gel electrophoresis remains a critical technique in vaccine development, yet it faces several challenges that hinder its full potential. One of the primary issues is the limited resolution for complex protein mixtures, particularly when dealing with vaccine antigens that may have similar molecular weights. This limitation can lead to difficulties in accurately identifying and characterizing specific components within a vaccine formulation.
Another significant challenge is the time-consuming nature of traditional gel electrophoresis methods. The lengthy process of sample preparation, gel casting, running, and analysis can slow down the vaccine development pipeline, especially in situations where rapid screening and characterization are crucial, such as during pandemic responses.
Reproducibility and standardization across different laboratories and batches pose additional hurdles. Variations in gel composition, running conditions, and staining procedures can lead to inconsistent results, making it challenging to compare data across different studies or vaccine candidates. This lack of standardization can impede collaborative efforts and slow down the overall progress in vaccine research.
The detection sensitivity of conventional gel electrophoresis techniques is another area of concern. As vaccine formulations often contain low concentrations of antigens or adjuvants, there is a need for more sensitive detection methods to accurately quantify and characterize these components. This limitation can affect the ability to detect trace impurities or degradation products that may impact vaccine safety and efficacy.
Furthermore, the analysis of post-translational modifications (PTMs) in vaccine antigens remains challenging with traditional gel electrophoresis. PTMs can significantly influence the immunogenicity and stability of vaccine components, yet their detection and characterization often require additional techniques beyond standard gel-based methods.
The integration of gel electrophoresis with high-throughput screening platforms is another challenge facing the field. As vaccine development increasingly relies on rapid screening of multiple candidates, there is a need for more automated and scalable electrophoresis techniques that can be seamlessly incorporated into high-throughput workflows.
Lastly, the environmental impact of gel electrophoresis, particularly the use of toxic staining agents and the generation of non-biodegradable waste, is becoming a growing concern. Developing more sustainable and eco-friendly alternatives without compromising analytical performance is an ongoing challenge in the field of vaccine development.
Another significant challenge is the time-consuming nature of traditional gel electrophoresis methods. The lengthy process of sample preparation, gel casting, running, and analysis can slow down the vaccine development pipeline, especially in situations where rapid screening and characterization are crucial, such as during pandemic responses.
Reproducibility and standardization across different laboratories and batches pose additional hurdles. Variations in gel composition, running conditions, and staining procedures can lead to inconsistent results, making it challenging to compare data across different studies or vaccine candidates. This lack of standardization can impede collaborative efforts and slow down the overall progress in vaccine research.
The detection sensitivity of conventional gel electrophoresis techniques is another area of concern. As vaccine formulations often contain low concentrations of antigens or adjuvants, there is a need for more sensitive detection methods to accurately quantify and characterize these components. This limitation can affect the ability to detect trace impurities or degradation products that may impact vaccine safety and efficacy.
Furthermore, the analysis of post-translational modifications (PTMs) in vaccine antigens remains challenging with traditional gel electrophoresis. PTMs can significantly influence the immunogenicity and stability of vaccine components, yet their detection and characterization often require additional techniques beyond standard gel-based methods.
The integration of gel electrophoresis with high-throughput screening platforms is another challenge facing the field. As vaccine development increasingly relies on rapid screening of multiple candidates, there is a need for more automated and scalable electrophoresis techniques that can be seamlessly incorporated into high-throughput workflows.
Lastly, the environmental impact of gel electrophoresis, particularly the use of toxic staining agents and the generation of non-biodegradable waste, is becoming a growing concern. Developing more sustainable and eco-friendly alternatives without compromising analytical performance is an ongoing challenge in the field of vaccine development.
Existing Gel Electrophoresis Techniques for Vaccines
01 Gel composition and preparation
Various gel compositions and preparation methods are used in gel electrophoresis. These include specific formulations of agarose, polyacrylamide, and other polymers to create gels with desired properties for different applications. The composition and preparation of the gel can significantly affect the separation and resolution of molecules during electrophoresis.- Gel composition and preparation: Various gel compositions and preparation methods are used in gel electrophoresis. These include specific formulations of agarose or polyacrylamide gels, as well as techniques for creating gradient gels. The composition and preparation of the gel matrix are crucial for achieving optimal separation of biomolecules.
- Electrophoresis apparatus design: Innovations in electrophoresis apparatus design focus on improving efficiency and reproducibility. These include specialized chambers, electrode configurations, and cooling systems. Advanced designs aim to enhance resolution, reduce run times, and allow for multiple sample analysis.
- Detection and imaging techniques: Various detection and imaging techniques are employed to visualize and analyze separated biomolecules. These include fluorescence detection, UV absorption, and staining methods. Advanced imaging systems and software are used for quantitative analysis and documentation of electrophoresis results.
- Sample preparation and loading: Techniques for sample preparation and loading are critical for successful gel electrophoresis. This includes methods for concentrating samples, removing interfering substances, and ensuring uniform sample application. Specialized loading devices and buffer systems are used to improve resolution and reproducibility.
- Specialized electrophoresis techniques: Various specialized electrophoresis techniques have been developed for specific applications. These include pulsed-field gel electrophoresis, capillary electrophoresis, and two-dimensional gel electrophoresis. Each technique offers unique advantages for separating different types of biomolecules or addressing specific analytical challenges.
02 Electrophoresis apparatus design
Innovations in electrophoresis apparatus design focus on improving efficiency, resolution, and ease of use. These designs may include novel electrode configurations, buffer systems, or integrated cooling mechanisms. Some apparatuses are designed for specific applications or to handle multiple samples simultaneously.Expand Specific Solutions03 Detection and analysis methods
Various detection and analysis methods are employed in gel electrophoresis to visualize and quantify separated molecules. These may include fluorescence-based detection, staining techniques, or integration with mass spectrometry. Advanced image analysis software and algorithms are also used to interpret electrophoresis results.Expand Specific Solutions04 Microfluidic and miniaturized systems
Miniaturized gel electrophoresis systems, often integrated into microfluidic devices, offer advantages such as reduced sample volume, faster analysis times, and potential for automation. These systems may incorporate novel fabrication techniques or materials to achieve high-resolution separations at a microscale.Expand Specific Solutions05 Specialized applications and modifications
Gel electrophoresis techniques are modified for specialized applications, such as DNA sequencing, protein analysis, or separation of specific biomolecules. These modifications may involve changes in gel composition, running conditions, or integration with other analytical techniques to enhance separation or detection of target molecules.Expand Specific Solutions
Key Players in Vaccine Gel Electrophoresis Industry
The gel electrophoresis techniques for vaccine development market is in a growth phase, driven by increasing demand for advanced vaccine research tools. The global market size is estimated to be in the hundreds of millions of dollars, with steady expansion projected. Technologically, the field is moderately mature but continues to evolve with innovations in high-resolution separation and analysis methods. Key players like Life Technologies Corp., GlaxoSmithKline Biologicals SA, and Novartis AG are investing in R&D to enhance gel electrophoresis capabilities for vaccine applications. Emerging companies such as Affinivax and MaxCyte are also contributing novel approaches, indicating a competitive and innovation-driven landscape.
GlaxoSmithKline Biologicals SA
Technical Solution: GlaxoSmithKline Biologicals SA has pioneered the use of microfluidic gel electrophoresis techniques in vaccine development. Their approach integrates lab-on-a-chip technology with traditional gel electrophoresis, allowing for miniaturization and automation of the separation process[4]. This innovation has significantly reduced sample volume requirements and increased throughput, enabling rapid screening of vaccine candidates. GSK has also developed a novel agarose gel electrophoresis method optimized for the analysis of virus-like particles (VLPs), a key component in many modern vaccines[5]. Their technique incorporates specialized staining protocols to enhance the visualization of VLPs and other nanoparticle-based vaccine components. Additionally, GSK has implemented capillary gel electrophoresis with laser-induced fluorescence detection for high-sensitivity analysis of vaccine polysaccharides and glycoconjugates[6].
Strengths: Miniaturization, high-throughput capabilities, and specialized techniques for nanoparticle-based vaccines. Weaknesses: May require significant investment in new equipment and training.
Novartis AG
Technical Solution: Novartis AG has developed advanced gel electrophoresis techniques for vaccine development, focusing on high-resolution protein separation and analysis. Their approach utilizes 2D gel electrophoresis combined with mass spectrometry for comprehensive proteomic analysis of vaccine candidates[1]. This method allows for the identification and characterization of potential antigens, enabling more targeted vaccine design. Novartis has also implemented capillary gel electrophoresis for rapid and high-throughput analysis of vaccine components, improving quality control processes[2]. Their innovative use of pulsed-field gel electrophoresis has enhanced the ability to separate large DNA fragments, crucial for analyzing genomic stability in live attenuated vaccines[3].
Strengths: Comprehensive protein analysis, high-throughput capabilities, and improved quality control. Weaknesses: Potentially time-consuming for complex samples, requires specialized equipment and expertise.
Innovative Gel Electrophoresis Methods for Vaccines
Analysis of DNA by means of cappillary electrophoresis
PatentInactiveEP2105736A1
Innovation
- A capillary gel electrophoresis method with hydrodynamic and electrokinetic injections, using a separation buffer with intercalating dyes like EnhanCE for laser-induced fluorescence detection, which allows for sensitive and reliable analysis of nucleic acids by adjusting injection pressures, voltages, and separation conditions to achieve detection limits down to 9 pg/ml.
pK-matched running buffers for gel electrophoresis
PatentInactiveUS7141153B2
Innovation
- Development of pK-matched buffers comprising a mixture of a weak acid and a weak base with pKa values within 0.3 units of each other, providing high resolution, stability, and a wide pH range, suitable for gel electrophoresis of nucleic acids or polypeptides.
Regulatory Considerations for Vaccine Development
Regulatory considerations play a crucial role in the development and approval of vaccines, including those utilizing gel electrophoresis techniques. The regulatory landscape for vaccine development is complex and multifaceted, involving various agencies and guidelines to ensure safety, efficacy, and quality.
In the United States, the Food and Drug Administration (FDA) is the primary regulatory body overseeing vaccine development and approval. The Center for Biologics Evaluation and Research (CBER) within the FDA is responsible for reviewing vaccine applications and ensuring compliance with regulatory requirements. Manufacturers must adhere to Good Manufacturing Practices (GMP) and Good Laboratory Practices (GLP) throughout the development process.
The European Medicines Agency (EMA) serves a similar function in the European Union, providing guidelines and regulations for vaccine development and approval. Other countries have their own regulatory bodies, such as the Medicines and Healthcare products Regulatory Agency (MHRA) in the United Kingdom and the Therapeutic Goods Administration (TGA) in Australia.
Regulatory considerations for gel electrophoresis techniques in vaccine development include validation of the method, reproducibility, and standardization. Manufacturers must demonstrate that their gel electrophoresis methods are suitable for their intended use, providing accurate and consistent results in analyzing vaccine components and impurities.
Quality control and quality assurance measures are essential aspects of regulatory compliance. This includes establishing and maintaining standard operating procedures (SOPs) for gel electrophoresis techniques, as well as implementing robust documentation practices to ensure traceability and data integrity.
Regulatory agencies also require comprehensive data on the safety and efficacy of vaccines. This includes results from preclinical studies, clinical trials, and post-marketing surveillance. Gel electrophoresis data may be used to support various aspects of these studies, such as characterizing vaccine antigens or detecting potential contaminants.
International harmonization efforts, such as the International Conference on Harmonisation (ICH) guidelines, aim to streamline regulatory requirements across different regions. These guidelines provide a framework for consistent quality standards and regulatory expectations, which can help facilitate global vaccine development and distribution.
As vaccine development technologies evolve, regulatory agencies must adapt their guidelines and requirements accordingly. This includes considering new applications of gel electrophoresis techniques and their potential impact on vaccine safety and efficacy assessments. Manufacturers should engage in early and frequent communication with regulatory authorities to ensure alignment on expectations and requirements throughout the development process.
In the United States, the Food and Drug Administration (FDA) is the primary regulatory body overseeing vaccine development and approval. The Center for Biologics Evaluation and Research (CBER) within the FDA is responsible for reviewing vaccine applications and ensuring compliance with regulatory requirements. Manufacturers must adhere to Good Manufacturing Practices (GMP) and Good Laboratory Practices (GLP) throughout the development process.
The European Medicines Agency (EMA) serves a similar function in the European Union, providing guidelines and regulations for vaccine development and approval. Other countries have their own regulatory bodies, such as the Medicines and Healthcare products Regulatory Agency (MHRA) in the United Kingdom and the Therapeutic Goods Administration (TGA) in Australia.
Regulatory considerations for gel electrophoresis techniques in vaccine development include validation of the method, reproducibility, and standardization. Manufacturers must demonstrate that their gel electrophoresis methods are suitable for their intended use, providing accurate and consistent results in analyzing vaccine components and impurities.
Quality control and quality assurance measures are essential aspects of regulatory compliance. This includes establishing and maintaining standard operating procedures (SOPs) for gel electrophoresis techniques, as well as implementing robust documentation practices to ensure traceability and data integrity.
Regulatory agencies also require comprehensive data on the safety and efficacy of vaccines. This includes results from preclinical studies, clinical trials, and post-marketing surveillance. Gel electrophoresis data may be used to support various aspects of these studies, such as characterizing vaccine antigens or detecting potential contaminants.
International harmonization efforts, such as the International Conference on Harmonisation (ICH) guidelines, aim to streamline regulatory requirements across different regions. These guidelines provide a framework for consistent quality standards and regulatory expectations, which can help facilitate global vaccine development and distribution.
As vaccine development technologies evolve, regulatory agencies must adapt their guidelines and requirements accordingly. This includes considering new applications of gel electrophoresis techniques and their potential impact on vaccine safety and efficacy assessments. Manufacturers should engage in early and frequent communication with regulatory authorities to ensure alignment on expectations and requirements throughout the development process.
Safety and Quality Control in Gel Electrophoresis
Safety and quality control are paramount in gel electrophoresis techniques for vaccine development. The process involves several critical steps that require stringent monitoring and adherence to established protocols to ensure the integrity and reliability of results.
One of the primary safety concerns in gel electrophoresis is the use of potentially hazardous chemicals and materials. Acrylamide, a common component in polyacrylamide gels, is a neurotoxin in its unpolymerized form. Proper handling, storage, and disposal of acrylamide and other toxic substances are essential to protect laboratory personnel and the environment. Implementing robust safety measures, including the use of personal protective equipment (PPE) and well-ventilated workspaces, is crucial.
Quality control in gel electrophoresis begins with the preparation of gels and buffers. Consistency in gel composition, including acrylamide concentration and cross-linking, is vital for reproducible results. Regular calibration and maintenance of equipment, such as power supplies and electrophoresis chambers, ensure optimal performance and minimize variability between runs.
Sample preparation is another critical aspect of quality control. Proper sample handling, including consistent protein or nucleic acid quantification and loading, is essential for accurate analysis. The use of standardized protocols for sample preparation helps maintain consistency across different batches and experiments.
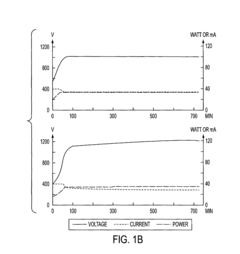
During the electrophoresis process, monitoring and controlling various parameters is crucial. Voltage, current, and run time must be carefully regulated to achieve consistent and reproducible separation of molecules. Temperature control is also important, as excessive heat generation can lead to band distortion or sample degradation.
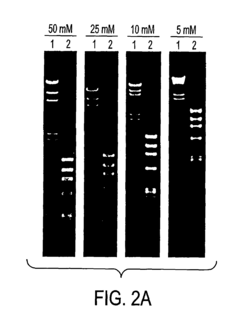
Post-electrophoresis steps, such as staining and imaging, require careful attention to quality control. The use of appropriate staining techniques and standardized imaging protocols ensures accurate visualization and quantification of separated molecules. Regular calibration of imaging equipment and the use of molecular weight markers are essential for reliable data interpretation.
Documentation and traceability are fundamental aspects of quality control in gel electrophoresis. Detailed record-keeping of all experimental parameters, including gel composition, running conditions, and sample information, is crucial for troubleshooting and reproducing results. Implementing a robust laboratory information management system (LIMS) can greatly enhance traceability and data integrity.
In the context of vaccine development, additional quality control measures may be necessary. These may include validation of gel electrophoresis methods for specific vaccine components, such as antigens or adjuvants. Establishing acceptance criteria for gel electrophoresis results, based on regulatory guidelines and product specifications, is essential for ensuring the consistency and quality of vaccine batches.
Regular proficiency testing and inter-laboratory comparisons can help maintain high standards of gel electrophoresis techniques across different research groups or manufacturing sites involved in vaccine development. This approach helps identify and address any systematic errors or inconsistencies in the methodology.
One of the primary safety concerns in gel electrophoresis is the use of potentially hazardous chemicals and materials. Acrylamide, a common component in polyacrylamide gels, is a neurotoxin in its unpolymerized form. Proper handling, storage, and disposal of acrylamide and other toxic substances are essential to protect laboratory personnel and the environment. Implementing robust safety measures, including the use of personal protective equipment (PPE) and well-ventilated workspaces, is crucial.
Quality control in gel electrophoresis begins with the preparation of gels and buffers. Consistency in gel composition, including acrylamide concentration and cross-linking, is vital for reproducible results. Regular calibration and maintenance of equipment, such as power supplies and electrophoresis chambers, ensure optimal performance and minimize variability between runs.
Sample preparation is another critical aspect of quality control. Proper sample handling, including consistent protein or nucleic acid quantification and loading, is essential for accurate analysis. The use of standardized protocols for sample preparation helps maintain consistency across different batches and experiments.
During the electrophoresis process, monitoring and controlling various parameters is crucial. Voltage, current, and run time must be carefully regulated to achieve consistent and reproducible separation of molecules. Temperature control is also important, as excessive heat generation can lead to band distortion or sample degradation.
Post-electrophoresis steps, such as staining and imaging, require careful attention to quality control. The use of appropriate staining techniques and standardized imaging protocols ensures accurate visualization and quantification of separated molecules. Regular calibration of imaging equipment and the use of molecular weight markers are essential for reliable data interpretation.
Documentation and traceability are fundamental aspects of quality control in gel electrophoresis. Detailed record-keeping of all experimental parameters, including gel composition, running conditions, and sample information, is crucial for troubleshooting and reproducing results. Implementing a robust laboratory information management system (LIMS) can greatly enhance traceability and data integrity.
In the context of vaccine development, additional quality control measures may be necessary. These may include validation of gel electrophoresis methods for specific vaccine components, such as antigens or adjuvants. Establishing acceptance criteria for gel electrophoresis results, based on regulatory guidelines and product specifications, is essential for ensuring the consistency and quality of vaccine batches.
Regular proficiency testing and inter-laboratory comparisons can help maintain high standards of gel electrophoresis techniques across different research groups or manufacturing sites involved in vaccine development. This approach helps identify and address any systematic errors or inconsistencies in the methodology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!