Gel Electrophoresis in Prenatal Testing: Future Directions
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Gel Electrophoresis Evolution and Objectives
Gel electrophoresis has been a cornerstone technique in molecular biology since its inception in the 1960s. Initially developed for separating proteins, it quickly found applications in DNA analysis, revolutionizing genetic research. The evolution of gel electrophoresis in prenatal testing has been particularly significant, offering non-invasive methods for detecting genetic abnormalities in fetuses.
The primary objective of gel electrophoresis in prenatal testing is to provide accurate, reliable, and timely information about the genetic makeup of a fetus. This technique aims to identify chromosomal abnormalities, genetic disorders, and potential health risks without endangering the pregnancy. As the field progresses, the goals have expanded to include earlier detection, higher resolution, and broader spectrum analysis of genetic material.
Over the years, gel electrophoresis has undergone several transformations to meet the growing demands of prenatal testing. The shift from agarose to polyacrylamide gels allowed for better resolution of smaller DNA fragments. The introduction of pulsed-field gel electrophoresis (PFGE) in the 1980s enabled the separation of larger DNA molecules, expanding the range of detectable genetic variations.
Recent advancements have focused on improving the speed, sensitivity, and automation of gel electrophoresis. The development of capillary electrophoresis and microfluidic devices has significantly reduced sample volumes and analysis times. These innovations have made it possible to perform rapid and high-throughput genetic screenings, crucial for early prenatal diagnosis.
The future directions of gel electrophoresis in prenatal testing are aimed at overcoming current limitations and expanding its capabilities. One key objective is to enhance the detection of sub-microscopic chromosomal abnormalities and single-gene disorders. This involves developing more sensitive electrophoresis techniques and combining them with advanced molecular biology methods like PCR and DNA sequencing.
Another important goal is to make prenatal genetic testing more accessible and less invasive. This includes refining methods for isolating and analyzing cell-free fetal DNA from maternal blood samples, potentially eliminating the need for invasive procedures like amniocentesis. The integration of gel electrophoresis with emerging technologies such as next-generation sequencing and artificial intelligence is expected to revolutionize prenatal diagnostics, offering more comprehensive and accurate genetic profiles.
As we look to the future, the objectives of gel electrophoresis in prenatal testing extend beyond mere genetic analysis. There is a growing emphasis on understanding the functional implications of genetic variations and their potential impact on fetal development and long-term health outcomes. This holistic approach aims to provide more meaningful and actionable information to healthcare providers and expectant parents, ultimately improving prenatal care and decision-making.
The primary objective of gel electrophoresis in prenatal testing is to provide accurate, reliable, and timely information about the genetic makeup of a fetus. This technique aims to identify chromosomal abnormalities, genetic disorders, and potential health risks without endangering the pregnancy. As the field progresses, the goals have expanded to include earlier detection, higher resolution, and broader spectrum analysis of genetic material.
Over the years, gel electrophoresis has undergone several transformations to meet the growing demands of prenatal testing. The shift from agarose to polyacrylamide gels allowed for better resolution of smaller DNA fragments. The introduction of pulsed-field gel electrophoresis (PFGE) in the 1980s enabled the separation of larger DNA molecules, expanding the range of detectable genetic variations.
Recent advancements have focused on improving the speed, sensitivity, and automation of gel electrophoresis. The development of capillary electrophoresis and microfluidic devices has significantly reduced sample volumes and analysis times. These innovations have made it possible to perform rapid and high-throughput genetic screenings, crucial for early prenatal diagnosis.
The future directions of gel electrophoresis in prenatal testing are aimed at overcoming current limitations and expanding its capabilities. One key objective is to enhance the detection of sub-microscopic chromosomal abnormalities and single-gene disorders. This involves developing more sensitive electrophoresis techniques and combining them with advanced molecular biology methods like PCR and DNA sequencing.
Another important goal is to make prenatal genetic testing more accessible and less invasive. This includes refining methods for isolating and analyzing cell-free fetal DNA from maternal blood samples, potentially eliminating the need for invasive procedures like amniocentesis. The integration of gel electrophoresis with emerging technologies such as next-generation sequencing and artificial intelligence is expected to revolutionize prenatal diagnostics, offering more comprehensive and accurate genetic profiles.
As we look to the future, the objectives of gel electrophoresis in prenatal testing extend beyond mere genetic analysis. There is a growing emphasis on understanding the functional implications of genetic variations and their potential impact on fetal development and long-term health outcomes. This holistic approach aims to provide more meaningful and actionable information to healthcare providers and expectant parents, ultimately improving prenatal care and decision-making.
Prenatal Testing Market Analysis
The prenatal testing market has experienced significant growth in recent years, driven by increasing maternal age, advancements in genetic screening technologies, and growing awareness of genetic disorders. The global prenatal testing market was valued at approximately $3.5 billion in 2020 and is projected to reach $6.7 billion by 2027, with a compound annual growth rate (CAGR) of 9.8% during the forecast period.
Gel electrophoresis, a key technique in prenatal testing, plays a crucial role in various applications such as non-invasive prenatal testing (NIPT), chromosomal abnormality detection, and genetic disorder screening. The demand for gel electrophoresis in prenatal testing is expected to grow steadily due to its reliability, cost-effectiveness, and ability to provide accurate results.
The market for prenatal testing is segmented based on test type, technology, and end-user. NIPT holds the largest market share, accounting for over 40% of the total market revenue. This segment is expected to maintain its dominance due to its non-invasive nature and high accuracy in detecting chromosomal abnormalities.
Geographically, North America leads the prenatal testing market, followed by Europe and Asia-Pacific. The United States, in particular, holds the largest market share due to advanced healthcare infrastructure, high adoption rates of new technologies, and favorable reimbursement policies. However, the Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by increasing healthcare expenditure, rising awareness, and improving access to prenatal care in countries like China and India.
Key market players in the prenatal testing industry include Illumina, Inc., Natera, Inc., Laboratory Corporation of America Holdings, Quest Diagnostics, and Roche Diagnostics. These companies are focusing on research and development activities to introduce innovative products and expand their market presence.
The future of gel electrophoresis in prenatal testing looks promising, with ongoing technological advancements aimed at improving accuracy, reducing turnaround time, and expanding the range of detectable genetic abnormalities. Integration of gel electrophoresis with other advanced technologies, such as next-generation sequencing and microarray analysis, is expected to further enhance its capabilities and market potential in prenatal testing applications.
However, the market faces challenges such as ethical concerns surrounding prenatal genetic testing, stringent regulatory requirements, and the high cost of advanced testing methods. Despite these challenges, the growing emphasis on early detection and prevention of genetic disorders is expected to drive the continued growth and innovation in the prenatal testing market, with gel electrophoresis remaining a critical component of this evolving landscape.
Gel electrophoresis, a key technique in prenatal testing, plays a crucial role in various applications such as non-invasive prenatal testing (NIPT), chromosomal abnormality detection, and genetic disorder screening. The demand for gel electrophoresis in prenatal testing is expected to grow steadily due to its reliability, cost-effectiveness, and ability to provide accurate results.
The market for prenatal testing is segmented based on test type, technology, and end-user. NIPT holds the largest market share, accounting for over 40% of the total market revenue. This segment is expected to maintain its dominance due to its non-invasive nature and high accuracy in detecting chromosomal abnormalities.
Geographically, North America leads the prenatal testing market, followed by Europe and Asia-Pacific. The United States, in particular, holds the largest market share due to advanced healthcare infrastructure, high adoption rates of new technologies, and favorable reimbursement policies. However, the Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by increasing healthcare expenditure, rising awareness, and improving access to prenatal care in countries like China and India.
Key market players in the prenatal testing industry include Illumina, Inc., Natera, Inc., Laboratory Corporation of America Holdings, Quest Diagnostics, and Roche Diagnostics. These companies are focusing on research and development activities to introduce innovative products and expand their market presence.
The future of gel electrophoresis in prenatal testing looks promising, with ongoing technological advancements aimed at improving accuracy, reducing turnaround time, and expanding the range of detectable genetic abnormalities. Integration of gel electrophoresis with other advanced technologies, such as next-generation sequencing and microarray analysis, is expected to further enhance its capabilities and market potential in prenatal testing applications.
However, the market faces challenges such as ethical concerns surrounding prenatal genetic testing, stringent regulatory requirements, and the high cost of advanced testing methods. Despite these challenges, the growing emphasis on early detection and prevention of genetic disorders is expected to drive the continued growth and innovation in the prenatal testing market, with gel electrophoresis remaining a critical component of this evolving landscape.
Gel Electrophoresis Challenges in Prenatal Diagnostics
Gel electrophoresis has been a cornerstone technique in prenatal diagnostics for decades, offering valuable insights into fetal genetic health. However, as the field of prenatal testing advances, several challenges have emerged that limit the effectiveness and applicability of traditional gel electrophoresis methods in this critical area of healthcare.
One of the primary challenges is the limited resolution and sensitivity of conventional gel electrophoresis techniques when dealing with the minute quantities of fetal DNA present in maternal blood samples. The low concentration of cell-free fetal DNA (cffDNA) in maternal plasma, typically ranging from 3% to 13%, poses significant difficulties in accurate detection and analysis using standard gel-based methods.
Another hurdle is the time-consuming nature of gel electrophoresis procedures. In prenatal diagnostics, rapid results are often crucial for timely decision-making and intervention. The lengthy process of sample preparation, gel running, and subsequent analysis can delay critical information, potentially impacting patient care and outcomes.
The inability to detect a wide range of genetic abnormalities simultaneously is another limitation of traditional gel electrophoresis in prenatal testing. As our understanding of fetal genetics expands, there is an increasing need for comprehensive screening methods that can identify multiple genetic markers and chromosomal abnormalities in a single test.
Furthermore, the interpretation of gel electrophoresis results in prenatal diagnostics can be subjective and prone to human error. The visual analysis of band patterns requires expertise and can lead to inconsistencies between different observers, potentially resulting in misdiagnosis or inconclusive results.
The challenge of integrating gel electrophoresis data with other prenatal testing modalities, such as ultrasound findings and maternal serum screening, also presents difficulties in providing a holistic view of fetal health. The lack of standardized protocols for combining these diverse data sources hampers the development of more accurate and comprehensive prenatal risk assessments.
Additionally, the limited automation capabilities of traditional gel electrophoresis techniques pose challenges in scaling up prenatal testing to meet the growing demand for non-invasive prenatal testing (NIPT). As the popularity of NIPT increases, there is a need for high-throughput methods that can process large numbers of samples efficiently and accurately.
Lastly, the disposal of hazardous materials used in gel electrophoresis, such as ethidium bromide for DNA staining, raises environmental and safety concerns. Finding safer alternatives that maintain the same level of sensitivity and reliability is an ongoing challenge in the field of prenatal diagnostics.
One of the primary challenges is the limited resolution and sensitivity of conventional gel electrophoresis techniques when dealing with the minute quantities of fetal DNA present in maternal blood samples. The low concentration of cell-free fetal DNA (cffDNA) in maternal plasma, typically ranging from 3% to 13%, poses significant difficulties in accurate detection and analysis using standard gel-based methods.
Another hurdle is the time-consuming nature of gel electrophoresis procedures. In prenatal diagnostics, rapid results are often crucial for timely decision-making and intervention. The lengthy process of sample preparation, gel running, and subsequent analysis can delay critical information, potentially impacting patient care and outcomes.
The inability to detect a wide range of genetic abnormalities simultaneously is another limitation of traditional gel electrophoresis in prenatal testing. As our understanding of fetal genetics expands, there is an increasing need for comprehensive screening methods that can identify multiple genetic markers and chromosomal abnormalities in a single test.
Furthermore, the interpretation of gel electrophoresis results in prenatal diagnostics can be subjective and prone to human error. The visual analysis of band patterns requires expertise and can lead to inconsistencies between different observers, potentially resulting in misdiagnosis or inconclusive results.
The challenge of integrating gel electrophoresis data with other prenatal testing modalities, such as ultrasound findings and maternal serum screening, also presents difficulties in providing a holistic view of fetal health. The lack of standardized protocols for combining these diverse data sources hampers the development of more accurate and comprehensive prenatal risk assessments.
Additionally, the limited automation capabilities of traditional gel electrophoresis techniques pose challenges in scaling up prenatal testing to meet the growing demand for non-invasive prenatal testing (NIPT). As the popularity of NIPT increases, there is a need for high-throughput methods that can process large numbers of samples efficiently and accurately.
Lastly, the disposal of hazardous materials used in gel electrophoresis, such as ethidium bromide for DNA staining, raises environmental and safety concerns. Finding safer alternatives that maintain the same level of sensitivity and reliability is an ongoing challenge in the field of prenatal diagnostics.
Current Gel Electrophoresis Methods in Prenatal Testing
01 Gel composition and preparation
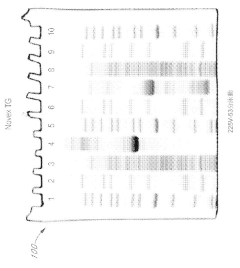
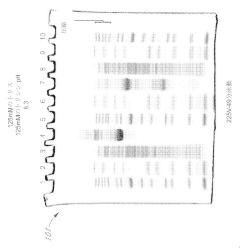
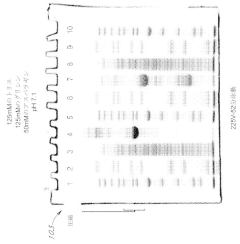
Various gel compositions and preparation methods are used in gel electrophoresis. These include specific formulations of agarose, polyacrylamide, and other polymers to create gels with desired properties for different applications. The composition and preparation of the gel can significantly affect the separation and resolution of molecules during electrophoresis.- Gel composition and preparation: Various gel compositions and preparation methods are used in gel electrophoresis. These include specific formulations of polymers, cross-linkers, and buffer solutions to create gels with desired properties for different separation applications. The composition and preparation of the gel matrix play a crucial role in determining the resolution and efficiency of the electrophoretic separation.
- Electrophoresis apparatus design: Innovations in electrophoresis apparatus design focus on improving separation efficiency, sample loading, and detection capabilities. These designs may include specialized electrodes, buffer chambers, and cooling systems to enhance performance and reproducibility. Some apparatus designs also incorporate features for automated operation or integration with other analytical techniques.
- Detection and imaging methods: Advanced detection and imaging methods are developed to visualize and analyze separated molecules in gel electrophoresis. These techniques may involve fluorescent labeling, staining protocols, or real-time monitoring of the separation process. Some methods focus on improving sensitivity, quantification accuracy, or the ability to detect specific molecules of interest.
- Sample preparation and loading techniques: Innovative sample preparation and loading techniques are crucial for improving the resolution and reproducibility of gel electrophoresis. These may include methods for concentrating samples, removing interfering substances, or precisely controlling the amount and distribution of sample loaded onto the gel. Some techniques focus on minimizing sample loss or degradation during the preparation process.
- Specialized electrophoresis applications: Gel electrophoresis techniques are adapted for specialized applications in various fields such as proteomics, genomics, and forensics. These applications may involve modifications to the gel composition, running conditions, or detection methods to optimize separation and analysis of specific types of molecules. Some specialized techniques focus on improving the resolution of closely related molecules or enabling the separation of complex mixtures.
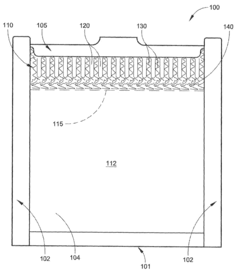
02 Electrophoresis apparatus design
Innovations in electrophoresis apparatus design focus on improving efficiency, resolution, and ease of use. These designs may include novel electrode configurations, buffer systems, or integrated cooling mechanisms. Some apparatus designs also incorporate features for automated sample loading or real-time monitoring of the electrophoresis process.Expand Specific Solutions03 Detection and analysis methods
Advanced detection and analysis methods are developed to enhance the sensitivity and accuracy of gel electrophoresis results. These may include fluorescence-based detection, image analysis software, or integration with mass spectrometry. Some methods focus on real-time monitoring of the separation process or automated data interpretation.Expand Specific Solutions04 Specialized electrophoresis techniques
Specialized electrophoresis techniques are developed for specific applications or to overcome limitations of traditional methods. These may include pulsed-field gel electrophoresis, two-dimensional electrophoresis, or capillary electrophoresis. Such techniques can offer improved resolution, separation of larger molecules, or analysis of complex mixtures.Expand Specific Solutions05 Sample preparation and loading
Innovations in sample preparation and loading aim to improve the efficiency and reproducibility of gel electrophoresis. These may include automated sample preparation systems, novel buffer formulations, or methods for concentrating samples. Some techniques focus on minimizing sample degradation or improving the uniformity of sample application to the gel.Expand Specific Solutions
Key Players in Prenatal Diagnostics
The gel electrophoresis market for prenatal testing is in a growth phase, driven by increasing demand for non-invasive prenatal testing and advancements in genetic analysis technologies. The global market size is expanding, with projections indicating substantial growth in the coming years. Technologically, the field is evolving rapidly, with companies like Life Technologies Corp., Natera, Inc., and Bio-Rad Laboratories leading innovation. These firms are developing more sensitive and accurate gel electrophoresis techniques, integrating them with advanced genomic analysis platforms. While the technology is well-established, ongoing refinements in resolution, speed, and automation are enhancing its applicability in prenatal diagnostics, indicating a mature yet still-advancing technological landscape.
Natera, Inc.
Technical Solution: Natera has developed advanced cell-free DNA (cfDNA) technology for prenatal testing, incorporating gel electrophoresis in their workflow. Their Panorama test uses single nucleotide polymorphism (SNP)-based technology combined with gel electrophoresis for size selection of cfDNA fragments[1]. This approach allows for highly accurate detection of fetal chromosomal abnormalities. Natera's method involves amplifying specific SNPs across the genome, followed by gel electrophoresis to isolate the desired DNA fragment sizes. The company has also explored the use of microfluidic-based gel electrophoresis systems to improve resolution and automation in their prenatal testing pipeline[2].
Strengths: High accuracy in detecting chromosomal abnormalities, ability to perform tests as early as 9 weeks gestation. Weaknesses: Relatively high cost compared to traditional screening methods, potential for false positives in certain scenarios.
Cytiva Sweden AB
Technical Solution: Cytiva, formerly part of GE Healthcare Life Sciences, has developed innovative gel electrophoresis solutions for prenatal testing applications. Their Amersham ECL Gel system offers high-sensitivity detection of DNA fragments, which is particularly useful for analyzing low-abundance fetal DNA in maternal blood samples[10]. Cytiva has also introduced the Whatman FTA technology, which combines sample collection, DNA extraction, and preparation for gel electrophoresis in a single card format. This streamlines the workflow for prenatal genetic testing, especially in resource-limited settings. Additionally, their PhastSystem automated electrophoresis platform has been adapted for rapid separation and analysis of fetal DNA fragments in prenatal diagnostics[11].
Strengths: Innovative sample preparation technologies, high-sensitivity detection systems. Weaknesses: Some specialized consumables may have higher costs compared to generic alternatives.
Innovative Gel Electrophoresis Techniques for Prenatal Screening
Electrophoresis gels with extended shelf life and high performance
PatentActiveJP2018530758A
Innovation
- Formulations of polyacrylamide gels with a near-neutral pH (6.5 to 7.5) using gel amine buffers, primary gel ampholytes, and conjugated gel ampholytes such as threonine and serine, which maintain gel stability and improve separation efficiency.
Electrophoresis Gel and Method of Making Same
PatentInactiveUS20100236932A1
Innovation
- Incorporating insoluble pigmented materials into the loading area of the electrophoresis gel, such as dry powder-coat paint or colored plastic beads, to create a visually distinct area that is easy to see, without interfering with the electrophoretic separation.
Ethical Considerations in Prenatal Testing
The ethical considerations surrounding prenatal testing, particularly in the context of gel electrophoresis, are complex and multifaceted. As this technology continues to advance, it is crucial to address the ethical implications that arise from its use in prenatal diagnostics.
One of the primary ethical concerns is the potential for discrimination based on genetic information. As gel electrophoresis techniques become more sophisticated, they may reveal an increasing range of genetic variations and potential health risks. This raises questions about how this information should be used and whether it could lead to unfair treatment or stigmatization of individuals based on their genetic makeup.
The issue of informed consent is another critical ethical consideration. Pregnant women and their partners must be fully informed about the nature of the tests, the potential results, and the implications of those results. This includes understanding the limitations of the technology, the possibility of false positives or negatives, and the potential emotional and psychological impact of receiving certain results.
Privacy and confidentiality of genetic information obtained through gel electrophoresis are also significant concerns. As prenatal testing becomes more comprehensive, there is a need to establish robust safeguards to protect this sensitive data from unauthorized access or misuse. This includes considerations about who should have access to the information and under what circumstances.
The potential for prenatal testing to lead to increased termination of pregnancies based on genetic information is a contentious ethical issue. This raises questions about the value placed on different human lives and the potential for eugenics-like practices. It is essential to consider the societal implications of widespread genetic screening and its impact on diversity and inclusion.
Another ethical consideration is the equitable access to prenatal testing technologies. As gel electrophoresis techniques become more advanced, there is a risk that they may become prohibitively expensive or only available in certain geographic areas. This could exacerbate existing health disparities and raise questions of justice and fairness in healthcare provision.
The rapid pace of technological advancement in this field also raises ethical questions about the appropriate limits of prenatal testing. As it becomes possible to detect an ever-increasing range of genetic variations, there is a need to consider which tests are truly necessary and beneficial, and which may cause undue anxiety or lead to difficult decision-making without clear medical benefit.
Lastly, there are ethical considerations surrounding the long-term psychological impact of prenatal testing on families. The availability of detailed genetic information about a fetus may alter the experience of pregnancy and parenting, potentially creating anxiety or changing the parent-child relationship before birth. It is important to consider the support and counseling needs of families undergoing these tests.
One of the primary ethical concerns is the potential for discrimination based on genetic information. As gel electrophoresis techniques become more sophisticated, they may reveal an increasing range of genetic variations and potential health risks. This raises questions about how this information should be used and whether it could lead to unfair treatment or stigmatization of individuals based on their genetic makeup.
The issue of informed consent is another critical ethical consideration. Pregnant women and their partners must be fully informed about the nature of the tests, the potential results, and the implications of those results. This includes understanding the limitations of the technology, the possibility of false positives or negatives, and the potential emotional and psychological impact of receiving certain results.
Privacy and confidentiality of genetic information obtained through gel electrophoresis are also significant concerns. As prenatal testing becomes more comprehensive, there is a need to establish robust safeguards to protect this sensitive data from unauthorized access or misuse. This includes considerations about who should have access to the information and under what circumstances.
The potential for prenatal testing to lead to increased termination of pregnancies based on genetic information is a contentious ethical issue. This raises questions about the value placed on different human lives and the potential for eugenics-like practices. It is essential to consider the societal implications of widespread genetic screening and its impact on diversity and inclusion.
Another ethical consideration is the equitable access to prenatal testing technologies. As gel electrophoresis techniques become more advanced, there is a risk that they may become prohibitively expensive or only available in certain geographic areas. This could exacerbate existing health disparities and raise questions of justice and fairness in healthcare provision.
The rapid pace of technological advancement in this field also raises ethical questions about the appropriate limits of prenatal testing. As it becomes possible to detect an ever-increasing range of genetic variations, there is a need to consider which tests are truly necessary and beneficial, and which may cause undue anxiety or lead to difficult decision-making without clear medical benefit.
Lastly, there are ethical considerations surrounding the long-term psychological impact of prenatal testing on families. The availability of detailed genetic information about a fetus may alter the experience of pregnancy and parenting, potentially creating anxiety or changing the parent-child relationship before birth. It is important to consider the support and counseling needs of families undergoing these tests.
Regulatory Framework for Prenatal Diagnostic Technologies
The regulatory framework for prenatal diagnostic technologies, including gel electrophoresis, is a complex and evolving landscape that significantly impacts the development, implementation, and accessibility of these crucial tools. In the United States, the Food and Drug Administration (FDA) plays a central role in overseeing the safety and efficacy of prenatal diagnostic technologies. The agency has established specific guidelines for the validation and approval of these tests, ensuring they meet stringent quality standards before entering the market.
Internationally, regulatory bodies such as the European Medicines Agency (EMA) and the World Health Organization (WHO) have also developed comprehensive frameworks for prenatal diagnostic technologies. These organizations work to harmonize standards across different countries, facilitating global research collaboration and market access while maintaining high safety and efficacy standards.
One of the key challenges in regulating prenatal diagnostic technologies is balancing innovation with patient safety. As new techniques like non-invasive prenatal testing (NIPT) emerge, regulatory bodies must adapt their frameworks to accommodate these advancements while ensuring rigorous evaluation processes. This often involves close collaboration between regulatory agencies, research institutions, and industry stakeholders to develop appropriate guidelines and standards.
Ethical considerations play a significant role in shaping the regulatory landscape for prenatal diagnostic technologies. Many countries have implemented specific laws and regulations addressing the ethical implications of prenatal testing, including issues related to genetic privacy, informed consent, and the potential for discrimination based on genetic information. These ethical frameworks often inform the development of regulatory policies and guidelines.
The regulatory environment also influences the commercialization and market access of prenatal diagnostic technologies. Companies developing new tests or improving existing ones must navigate complex approval processes, which can impact the timeline and cost of bringing innovations to market. This regulatory burden can sometimes act as a barrier to entry for smaller companies or startups, potentially limiting diversity in the field.
As gel electrophoresis and other prenatal diagnostic technologies continue to advance, regulatory frameworks will need to evolve to keep pace with technological progress. This may include developing new guidelines for emerging technologies, streamlining approval processes for incremental improvements, and addressing the challenges posed by the increasing use of artificial intelligence and machine learning in diagnostic tools.
Internationally, regulatory bodies such as the European Medicines Agency (EMA) and the World Health Organization (WHO) have also developed comprehensive frameworks for prenatal diagnostic technologies. These organizations work to harmonize standards across different countries, facilitating global research collaboration and market access while maintaining high safety and efficacy standards.
One of the key challenges in regulating prenatal diagnostic technologies is balancing innovation with patient safety. As new techniques like non-invasive prenatal testing (NIPT) emerge, regulatory bodies must adapt their frameworks to accommodate these advancements while ensuring rigorous evaluation processes. This often involves close collaboration between regulatory agencies, research institutions, and industry stakeholders to develop appropriate guidelines and standards.
Ethical considerations play a significant role in shaping the regulatory landscape for prenatal diagnostic technologies. Many countries have implemented specific laws and regulations addressing the ethical implications of prenatal testing, including issues related to genetic privacy, informed consent, and the potential for discrimination based on genetic information. These ethical frameworks often inform the development of regulatory policies and guidelines.
The regulatory environment also influences the commercialization and market access of prenatal diagnostic technologies. Companies developing new tests or improving existing ones must navigate complex approval processes, which can impact the timeline and cost of bringing innovations to market. This regulatory burden can sometimes act as a barrier to entry for smaller companies or startups, potentially limiting diversity in the field.
As gel electrophoresis and other prenatal diagnostic technologies continue to advance, regulatory frameworks will need to evolve to keep pace with technological progress. This may include developing new guidelines for emerging technologies, streamlining approval processes for incremental improvements, and addressing the challenges posed by the increasing use of artificial intelligence and machine learning in diagnostic tools.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!