How to Optimize Buffer Systems in Gel Electrophoresis?
JUN 30, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Gel Electrophoresis Buffer Evolution and Objectives
Gel electrophoresis has been a cornerstone technique in molecular biology since its inception in the 1960s. The evolution of buffer systems in this field has been driven by the need for improved resolution, speed, and versatility in separating nucleic acids and proteins. Initially, simple Tris-based buffers were used, but as the demands of molecular biology research grew, so did the complexity and specificity of buffer systems.
The primary objective in optimizing buffer systems for gel electrophoresis is to enhance the separation of biomolecules while maintaining their stability. This involves balancing factors such as ionic strength, pH, and buffer capacity to create an environment that facilitates efficient and accurate separation. Over time, researchers have developed a variety of buffer systems tailored to specific applications, from standard TAE (Tris-acetate-EDTA) and TBE (Tris-borate-EDTA) buffers to more specialized formulations.
One significant trend in buffer evolution has been the development of low-conductivity buffers, which allow for higher voltage applications and faster run times without excessive heat generation. This advancement has been particularly important in the era of high-throughput genomics, where rapid analysis is crucial. Another key development has been the introduction of buffers optimized for specific DNA or protein sizes, enabling more precise separation of target molecules.
The advent of pulsed-field gel electrophoresis (PFGE) in the 1980s necessitated the creation of new buffer systems capable of maintaining DNA integrity during long separation times. This led to the development of buffers with enhanced ionic strength and the inclusion of chelating agents to prevent DNA degradation. Similarly, the rise of capillary electrophoresis has driven innovation in buffer composition to manage electroosmotic flow and improve resolution in microfluidic environments.
Recent objectives in buffer system optimization focus on sustainability and cost-effectiveness. Researchers are exploring eco-friendly alternatives to traditional buffer components and developing reusable buffer systems to reduce waste and expenses in laboratory settings. Additionally, there is a growing interest in buffers that can be used across a wider range of applications, simplifying laboratory protocols and improving reproducibility across different experimental setups.
Looking forward, the field aims to develop "smart" buffer systems that can dynamically adjust their properties during electrophoresis to optimize separation based on real-time feedback. This could involve the integration of nanomaterials or responsive polymers into buffer formulations. Another emerging goal is the creation of buffer systems compatible with downstream applications, such as mass spectrometry or next-generation sequencing, to streamline analytical workflows and reduce sample handling.
The primary objective in optimizing buffer systems for gel electrophoresis is to enhance the separation of biomolecules while maintaining their stability. This involves balancing factors such as ionic strength, pH, and buffer capacity to create an environment that facilitates efficient and accurate separation. Over time, researchers have developed a variety of buffer systems tailored to specific applications, from standard TAE (Tris-acetate-EDTA) and TBE (Tris-borate-EDTA) buffers to more specialized formulations.
One significant trend in buffer evolution has been the development of low-conductivity buffers, which allow for higher voltage applications and faster run times without excessive heat generation. This advancement has been particularly important in the era of high-throughput genomics, where rapid analysis is crucial. Another key development has been the introduction of buffers optimized for specific DNA or protein sizes, enabling more precise separation of target molecules.
The advent of pulsed-field gel electrophoresis (PFGE) in the 1980s necessitated the creation of new buffer systems capable of maintaining DNA integrity during long separation times. This led to the development of buffers with enhanced ionic strength and the inclusion of chelating agents to prevent DNA degradation. Similarly, the rise of capillary electrophoresis has driven innovation in buffer composition to manage electroosmotic flow and improve resolution in microfluidic environments.
Recent objectives in buffer system optimization focus on sustainability and cost-effectiveness. Researchers are exploring eco-friendly alternatives to traditional buffer components and developing reusable buffer systems to reduce waste and expenses in laboratory settings. Additionally, there is a growing interest in buffers that can be used across a wider range of applications, simplifying laboratory protocols and improving reproducibility across different experimental setups.
Looking forward, the field aims to develop "smart" buffer systems that can dynamically adjust their properties during electrophoresis to optimize separation based on real-time feedback. This could involve the integration of nanomaterials or responsive polymers into buffer formulations. Another emerging goal is the creation of buffer systems compatible with downstream applications, such as mass spectrometry or next-generation sequencing, to streamline analytical workflows and reduce sample handling.
Market Analysis for Advanced Electrophoresis Buffers
The market for advanced electrophoresis buffers is experiencing significant growth, driven by the increasing demand for more efficient and precise separation techniques in various fields of life sciences. The global electrophoresis market, which includes buffers as a crucial component, was valued at approximately $2.7 billion in 2020 and is projected to reach $3.9 billion by 2026, growing at a CAGR of around 6.2% during the forecast period.
Advanced electrophoresis buffers play a vital role in optimizing gel electrophoresis processes, offering improved resolution, faster run times, and enhanced separation of biomolecules. The market for these specialized buffers is primarily fueled by the expanding applications of electrophoresis in genomics, proteomics, and clinical diagnostics. The rising prevalence of genetic disorders and the growing focus on personalized medicine have further boosted the demand for high-performance electrophoresis buffers.
The pharmaceutical and biotechnology sectors are the largest consumers of advanced electrophoresis buffers, accounting for approximately 40% of the market share. Academic and research institutions follow closely, contributing to about 35% of the market demand. The remaining market share is distributed among clinical laboratories, forensic laboratories, and other end-users.
Geographically, North America dominates the advanced electrophoresis buffer market, holding a market share of around 35%. This dominance is attributed to the presence of major biotechnology and pharmaceutical companies, well-established research infrastructure, and significant investments in genomics and proteomics research. Europe follows closely with a market share of approximately 30%, driven by the increasing adoption of advanced separation techniques in academic research and clinical diagnostics.
The Asia-Pacific region is emerging as the fastest-growing market for advanced electrophoresis buffers, with a projected CAGR of 8.5% during the forecast period. This growth is fueled by the rapid expansion of the biotechnology and pharmaceutical industries in countries like China and India, increasing research activities, and rising government investments in life sciences research.
Key market trends include the development of specialized buffers for specific applications, such as high-resolution separation of proteins or nucleic acids, and the introduction of ready-to-use buffer systems that offer convenience and reproducibility. There is also a growing demand for environmentally friendly and non-toxic buffer formulations, aligning with the increasing focus on sustainable laboratory practices.
The competitive landscape of the advanced electrophoresis buffer market is characterized by the presence of both established players and innovative start-ups. Major companies in this space are continuously investing in research and development to introduce novel buffer formulations that address the evolving needs of researchers and clinicians. Strategic collaborations and partnerships between buffer manufacturers and electrophoresis equipment providers are becoming increasingly common, aiming to offer integrated solutions that optimize overall electrophoresis performance.
Advanced electrophoresis buffers play a vital role in optimizing gel electrophoresis processes, offering improved resolution, faster run times, and enhanced separation of biomolecules. The market for these specialized buffers is primarily fueled by the expanding applications of electrophoresis in genomics, proteomics, and clinical diagnostics. The rising prevalence of genetic disorders and the growing focus on personalized medicine have further boosted the demand for high-performance electrophoresis buffers.
The pharmaceutical and biotechnology sectors are the largest consumers of advanced electrophoresis buffers, accounting for approximately 40% of the market share. Academic and research institutions follow closely, contributing to about 35% of the market demand. The remaining market share is distributed among clinical laboratories, forensic laboratories, and other end-users.
Geographically, North America dominates the advanced electrophoresis buffer market, holding a market share of around 35%. This dominance is attributed to the presence of major biotechnology and pharmaceutical companies, well-established research infrastructure, and significant investments in genomics and proteomics research. Europe follows closely with a market share of approximately 30%, driven by the increasing adoption of advanced separation techniques in academic research and clinical diagnostics.
The Asia-Pacific region is emerging as the fastest-growing market for advanced electrophoresis buffers, with a projected CAGR of 8.5% during the forecast period. This growth is fueled by the rapid expansion of the biotechnology and pharmaceutical industries in countries like China and India, increasing research activities, and rising government investments in life sciences research.
Key market trends include the development of specialized buffers for specific applications, such as high-resolution separation of proteins or nucleic acids, and the introduction of ready-to-use buffer systems that offer convenience and reproducibility. There is also a growing demand for environmentally friendly and non-toxic buffer formulations, aligning with the increasing focus on sustainable laboratory practices.
The competitive landscape of the advanced electrophoresis buffer market is characterized by the presence of both established players and innovative start-ups. Major companies in this space are continuously investing in research and development to introduce novel buffer formulations that address the evolving needs of researchers and clinicians. Strategic collaborations and partnerships between buffer manufacturers and electrophoresis equipment providers are becoming increasingly common, aiming to offer integrated solutions that optimize overall electrophoresis performance.
Current Challenges in Buffer System Optimization
Optimizing buffer systems in gel electrophoresis remains a significant challenge in molecular biology and biochemistry. One of the primary issues is maintaining consistent pH levels throughout the electrophoresis process. As the current flows, electrolysis of water occurs at the electrodes, leading to the production of H+ and OH- ions. This can cause pH gradients within the gel, potentially affecting the migration and separation of molecules.
Another challenge is the generation of heat during electrophoresis, known as Joule heating. This can cause temperature gradients within the gel, leading to uneven migration rates and band distortion. High ionic strength buffers, while providing good conductivity, exacerbate this problem by increasing current flow and heat production. Conversely, low ionic strength buffers may result in insufficient conductivity and poor separation.
The choice of buffer components also presents challenges. Traditional Tris-based buffers, while widely used, have limitations. Tris has a relatively high temperature coefficient, meaning its pH can change significantly with temperature fluctuations. This can lead to inconsistent results, especially in longer runs or when comparing data from different laboratories with varying ambient temperatures.
Maintaining buffer capacity throughout the run is another critical issue. As electrophoresis progresses, the buffer can become depleted, particularly near the electrodes. This depletion can lead to changes in pH and ionic strength, affecting the separation quality and reproducibility of results. In vertical gel systems, differences in buffer composition between the upper and lower chambers can occur, further complicating the optimization process.
The interaction between buffer components and the molecules being separated adds another layer of complexity. Some buffers may interact with certain proteins or nucleic acids, affecting their migration patterns or even their structure. This is particularly problematic when working with sensitive biomolecules or when trying to maintain native conformations.
Scaling up electrophoresis systems for larger gels or high-throughput applications presents additional challenges in buffer optimization. Ensuring uniform electric fields and consistent buffer conditions across larger gel areas becomes increasingly difficult, often requiring more sophisticated buffer systems or gel designs.
Lastly, the environmental impact and safety concerns associated with some traditional buffer components are driving the need for more sustainable and less toxic alternatives. Developing new buffer systems that are both effective and environmentally friendly is an ongoing challenge in the field of gel electrophoresis.
Another challenge is the generation of heat during electrophoresis, known as Joule heating. This can cause temperature gradients within the gel, leading to uneven migration rates and band distortion. High ionic strength buffers, while providing good conductivity, exacerbate this problem by increasing current flow and heat production. Conversely, low ionic strength buffers may result in insufficient conductivity and poor separation.
The choice of buffer components also presents challenges. Traditional Tris-based buffers, while widely used, have limitations. Tris has a relatively high temperature coefficient, meaning its pH can change significantly with temperature fluctuations. This can lead to inconsistent results, especially in longer runs or when comparing data from different laboratories with varying ambient temperatures.
Maintaining buffer capacity throughout the run is another critical issue. As electrophoresis progresses, the buffer can become depleted, particularly near the electrodes. This depletion can lead to changes in pH and ionic strength, affecting the separation quality and reproducibility of results. In vertical gel systems, differences in buffer composition between the upper and lower chambers can occur, further complicating the optimization process.
The interaction between buffer components and the molecules being separated adds another layer of complexity. Some buffers may interact with certain proteins or nucleic acids, affecting their migration patterns or even their structure. This is particularly problematic when working with sensitive biomolecules or when trying to maintain native conformations.
Scaling up electrophoresis systems for larger gels or high-throughput applications presents additional challenges in buffer optimization. Ensuring uniform electric fields and consistent buffer conditions across larger gel areas becomes increasingly difficult, often requiring more sophisticated buffer systems or gel designs.
Lastly, the environmental impact and safety concerns associated with some traditional buffer components are driving the need for more sustainable and less toxic alternatives. Developing new buffer systems that are both effective and environmentally friendly is an ongoing challenge in the field of gel electrophoresis.
Existing Buffer Optimization Techniques
01 Optimization of buffer systems in integrated circuits
Buffer systems in integrated circuits can be optimized to improve performance and reduce power consumption. This involves adjusting buffer sizes, placement, and timing to meet design constraints and enhance overall circuit efficiency. Techniques may include buffer insertion, buffer resizing, and buffer tree construction to optimize signal propagation and minimize delay.- Optimization of buffer systems in integrated circuits: Buffer systems in integrated circuits can be optimized to improve performance and reduce power consumption. This involves adjusting buffer sizes, placement, and timing to meet design constraints and enhance overall circuit efficiency.
- Buffer optimization in network communication: In network communication systems, buffer optimization techniques are employed to enhance data transfer efficiency and reduce latency. This includes adjusting buffer sizes, implementing adaptive buffering algorithms, and optimizing buffer allocation strategies.
- Memory buffer optimization in computing systems: Optimizing memory buffer systems in computing devices involves techniques such as cache optimization, dynamic buffer allocation, and intelligent prefetching. These methods aim to improve memory access times and overall system performance.
- Buffer optimization in audio/video processing: In audio and video processing applications, buffer optimization techniques are used to manage data flow, reduce jitter, and ensure smooth playback. This includes implementing adaptive buffering strategies and optimizing buffer sizes based on content characteristics.
- Chemical buffer system optimization: Optimization of chemical buffer systems involves adjusting the composition and concentration of buffer components to maintain desired pH levels and chemical stability. This is crucial in various applications, including pharmaceutical formulations and industrial processes.
02 Buffer optimization in network communication systems
In network communication systems, buffer optimization is crucial for managing data flow and reducing latency. This involves adjusting buffer sizes, implementing intelligent buffer allocation algorithms, and optimizing buffer management policies to handle varying network conditions and traffic patterns effectively. The goal is to minimize packet loss, improve throughput, and enhance overall network performance.Expand Specific Solutions03 Memory buffer optimization in computing systems
Optimizing memory buffer systems in computing environments involves techniques to improve data access speeds and reduce memory bottlenecks. This may include implementing intelligent caching strategies, optimizing buffer allocation and deallocation, and fine-tuning buffer sizes based on application requirements. The aim is to enhance system performance and reduce latency in data-intensive operations.Expand Specific Solutions04 Buffer optimization in audio/video processing
In audio and video processing applications, buffer optimization is essential for smooth playback and real-time processing. This involves techniques such as adaptive buffer sizing, implementing efficient buffer underrun/overrun prevention mechanisms, and optimizing buffer management for various media formats and streaming conditions. The goal is to minimize jitter, reduce latency, and ensure high-quality audio/video output.Expand Specific Solutions05 Chemical buffer system optimization
Optimization of chemical buffer systems involves adjusting the composition and concentration of buffer components to maintain desired pH levels in various applications. This may include selecting appropriate buffer pairs, fine-tuning buffer capacity, and considering factors such as temperature and ionic strength. The aim is to enhance stability, improve reaction efficiency, and maintain optimal conditions in chemical and biological processes.Expand Specific Solutions
Key Players in Electrophoresis Buffer Industry
The optimization of buffer systems in gel electrophoresis is a mature field within the broader context of molecular biology and biochemical analysis. The market for electrophoresis equipment and consumables is well-established, with a steady growth trajectory driven by ongoing research in genomics and proteomics. Key players in this space include Life Technologies Corp., Applied Biosystems LLC, and Bio-Rad Laboratories, Inc., who offer comprehensive solutions for gel electrophoresis. These companies have developed advanced buffer systems and related products, leveraging their extensive R&D capabilities and market presence. The technology's maturity is evident in the wide adoption across academic, pharmaceutical, and biotechnology sectors, with ongoing innovations focusing on improving resolution, speed, and automation in electrophoretic separations.
Life Technologies Corp.
Technical Solution: Life Technologies (now part of Thermo Fisher Scientific) has developed a range of optimized buffer systems for various electrophoresis applications. Their approach includes the development of the Novex™ line of pre-cast gels and corresponding optimized buffers, which offer consistent performance and reduced preparation time[10]. They have introduced innovations such as the NuPAGE™ Bis-Tris buffer system, which provides a neutral pH environment during electrophoresis, resulting in improved protein stability and sharper band resolution[11]. Life Technologies has also focused on developing buffers for specific applications, such as their E-Gel™ system, which uses a proprietary buffer formulation enclosed within pre-cast agarose gels for rapid DNA electrophoresis[12]. Their buffer optimization strategies often involve the use of antioxidants and metal chelators to prevent protein modifications during separation.
Strengths: Wide range of application-specific buffer systems, integration with pre-cast gel technologies for ease of use. Weaknesses: Some proprietary buffer systems may be tied to specific gel or instrument platforms, potentially limiting flexibility.
Applied Biosystems LLC
Technical Solution: Applied Biosystems (now part of Thermo Fisher Scientific) has contributed significantly to buffer system optimization in gel electrophoresis, particularly for DNA sequencing and fragment analysis. Their approach includes the development of performance-optimized polymer (POP) buffers for capillary electrophoresis, which offer improved resolution and faster separation times for DNA fragments[13]. They have also introduced specialized buffer systems for their genetic analyzers, such as the 3500 Series, which use a multi-component buffer system to enhance separation quality and increase run-to-run consistency[14]. Applied Biosystems' buffer optimization strategies often involve the use of denaturants and viscosity modifiers to improve DNA separation and reduce secondary structure formation. Their latest innovations include buffer systems designed for next-generation sequencing library preparation, which aim to minimize bias and improve sequencing quality[15].
Strengths: Highly optimized buffer systems for DNA analysis, particularly in automated sequencing platforms. Weaknesses: Some buffer systems may be instrument-specific, potentially limiting their use with other electrophoresis systems.
Innovative Buffer Formulations and Their Impact
Expanding cam lock for sealing slab gels in an electrophoresis apparatus
PatentInactiveUS20120167383A1
Innovation
- An expanding cam lock system that securely seals multiple gel cassettes by slidingly engaging a base plate and follower plate between buffer core assemblies, using a cam to apply consistent pressure and a buffer displacement dam to reduce buffer volume requirements.
Buffer system for a long-lasting precast electrophoresis gel
PatentInactiveUS20100264031A1
Innovation
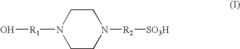
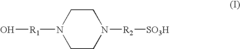
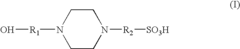
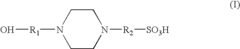
- A precast polyacrylamide gel composition using Tris, sulfonated amines, and ampholytes at specific pH and concentration ranges (5.5-7.5) to stabilize the gel without compromising electrophoresis performance, including the use of HEPES and amino acids like glycine and asparagine to maintain gel stability and separation quality.
Environmental Impact of Electrophoresis Buffers
The environmental impact of electrophoresis buffers is a critical consideration in the optimization of gel electrophoresis systems. These buffers, while essential for the separation of biomolecules, can have significant ecological consequences if not properly managed. The most commonly used buffers, such as Tris-Borate-EDTA (TBE) and Tris-Acetate-EDTA (TAE), contain chemicals that can be harmful to aquatic ecosystems when discharged into water bodies.
One of the primary concerns is the presence of EDTA (Ethylenediaminetetraacetic acid) in these buffers. EDTA is a chelating agent that can bind to metal ions in the environment, potentially altering the bioavailability of essential nutrients for aquatic organisms. This can lead to disruptions in local ecosystems, affecting the growth and reproduction of various species. Additionally, the high salt concentrations in electrophoresis buffers can contribute to increased salinity in freshwater systems if not properly treated before disposal.
The production and disposal of electrophoresis buffers also contribute to the overall environmental footprint of laboratory practices. The chemicals used in buffer preparation often require energy-intensive manufacturing processes, contributing to greenhouse gas emissions. Furthermore, the plastic containers used for buffer storage and disposal add to the growing problem of plastic waste in the environment.
To mitigate these environmental impacts, researchers and laboratories are exploring more sustainable alternatives. One approach is the development of biodegradable buffer systems that break down more readily in the environment. These eco-friendly buffers aim to maintain the efficiency of electrophoresis while reducing the long-term ecological impact. Another strategy involves the implementation of buffer recycling systems, which can significantly reduce the volume of waste generated and minimize the need for new buffer production.
Proper waste management protocols are also crucial in minimizing the environmental impact of electrophoresis buffers. This includes neutralization of buffers before disposal, appropriate dilution, and treatment through specialized waste handling facilities. Some institutions are implementing on-site treatment systems to process laboratory waste, including electrophoresis buffers, before release into the general waste stream.
The scientific community is increasingly recognizing the importance of green chemistry principles in laboratory practices. This has led to research into alternative buffer systems that maintain or improve electrophoresis performance while reducing environmental impact. For instance, some studies have explored the use of organic compounds derived from renewable sources as potential replacements for traditional buffer components.
As the field of gel electrophoresis continues to evolve, the optimization of buffer systems must consider not only performance metrics but also environmental sustainability. This holistic approach to buffer system design and usage is essential for developing more eco-friendly laboratory practices and reducing the overall environmental footprint of scientific research.
One of the primary concerns is the presence of EDTA (Ethylenediaminetetraacetic acid) in these buffers. EDTA is a chelating agent that can bind to metal ions in the environment, potentially altering the bioavailability of essential nutrients for aquatic organisms. This can lead to disruptions in local ecosystems, affecting the growth and reproduction of various species. Additionally, the high salt concentrations in electrophoresis buffers can contribute to increased salinity in freshwater systems if not properly treated before disposal.
The production and disposal of electrophoresis buffers also contribute to the overall environmental footprint of laboratory practices. The chemicals used in buffer preparation often require energy-intensive manufacturing processes, contributing to greenhouse gas emissions. Furthermore, the plastic containers used for buffer storage and disposal add to the growing problem of plastic waste in the environment.
To mitigate these environmental impacts, researchers and laboratories are exploring more sustainable alternatives. One approach is the development of biodegradable buffer systems that break down more readily in the environment. These eco-friendly buffers aim to maintain the efficiency of electrophoresis while reducing the long-term ecological impact. Another strategy involves the implementation of buffer recycling systems, which can significantly reduce the volume of waste generated and minimize the need for new buffer production.
Proper waste management protocols are also crucial in minimizing the environmental impact of electrophoresis buffers. This includes neutralization of buffers before disposal, appropriate dilution, and treatment through specialized waste handling facilities. Some institutions are implementing on-site treatment systems to process laboratory waste, including electrophoresis buffers, before release into the general waste stream.
The scientific community is increasingly recognizing the importance of green chemistry principles in laboratory practices. This has led to research into alternative buffer systems that maintain or improve electrophoresis performance while reducing environmental impact. For instance, some studies have explored the use of organic compounds derived from renewable sources as potential replacements for traditional buffer components.
As the field of gel electrophoresis continues to evolve, the optimization of buffer systems must consider not only performance metrics but also environmental sustainability. This holistic approach to buffer system design and usage is essential for developing more eco-friendly laboratory practices and reducing the overall environmental footprint of scientific research.
Regulatory Compliance for Lab Chemicals
Regulatory compliance for lab chemicals is a critical aspect of gel electrophoresis optimization, particularly concerning buffer systems. Laboratories must adhere to strict guidelines and regulations to ensure the safe handling, storage, and disposal of chemicals used in electrophoresis experiments. These regulations are designed to protect researchers, the environment, and the integrity of scientific results.
One of the primary regulatory bodies overseeing laboratory chemical safety is the Occupational Safety and Health Administration (OSHA). OSHA mandates that laboratories maintain Safety Data Sheets (SDS) for all chemicals used, including buffer components. These documents provide crucial information on chemical properties, hazards, and proper handling procedures. Researchers must be trained in interpreting and accessing SDS information to ensure compliance and safety.
The Environmental Protection Agency (EPA) also plays a significant role in regulating lab chemicals. The EPA's Resource Conservation and Recovery Act (RCRA) governs the disposal of hazardous waste, including spent electrophoresis buffers. Laboratories must implement proper waste management protocols, such as segregating and labeling different types of chemical waste, to comply with EPA regulations.
In addition to federal regulations, state and local authorities may impose additional requirements for chemical handling and disposal. It is essential for laboratories to stay informed about these region-specific regulations and adjust their practices accordingly. This may involve obtaining specific permits or following stricter disposal guidelines.
When optimizing buffer systems for gel electrophoresis, researchers must consider the regulatory implications of their choices. For instance, the use of certain buffer components may be restricted or require special handling procedures. Tris-borate-EDTA (TBE) and Tris-acetate-EDTA (TAE) buffers, commonly used in electrophoresis, contain chemicals that are subject to specific regulatory requirements.
Compliance also extends to the procurement and inventory management of chemicals. Laboratories must maintain accurate records of chemical purchases, usage, and disposal. This documentation is crucial for regulatory inspections and internal audits. Implementing a robust chemical inventory system can help ensure compliance and facilitate efficient buffer system optimization.
Furthermore, laboratories must establish and follow standard operating procedures (SOPs) that incorporate regulatory requirements. These SOPs should cover all aspects of buffer preparation, use, and disposal, ensuring consistency and compliance across experiments. Regular training sessions for laboratory personnel on regulatory compliance and safe chemical handling practices are essential to maintain a culture of safety and adherence to regulations.
One of the primary regulatory bodies overseeing laboratory chemical safety is the Occupational Safety and Health Administration (OSHA). OSHA mandates that laboratories maintain Safety Data Sheets (SDS) for all chemicals used, including buffer components. These documents provide crucial information on chemical properties, hazards, and proper handling procedures. Researchers must be trained in interpreting and accessing SDS information to ensure compliance and safety.
The Environmental Protection Agency (EPA) also plays a significant role in regulating lab chemicals. The EPA's Resource Conservation and Recovery Act (RCRA) governs the disposal of hazardous waste, including spent electrophoresis buffers. Laboratories must implement proper waste management protocols, such as segregating and labeling different types of chemical waste, to comply with EPA regulations.
In addition to federal regulations, state and local authorities may impose additional requirements for chemical handling and disposal. It is essential for laboratories to stay informed about these region-specific regulations and adjust their practices accordingly. This may involve obtaining specific permits or following stricter disposal guidelines.
When optimizing buffer systems for gel electrophoresis, researchers must consider the regulatory implications of their choices. For instance, the use of certain buffer components may be restricted or require special handling procedures. Tris-borate-EDTA (TBE) and Tris-acetate-EDTA (TAE) buffers, commonly used in electrophoresis, contain chemicals that are subject to specific regulatory requirements.
Compliance also extends to the procurement and inventory management of chemicals. Laboratories must maintain accurate records of chemical purchases, usage, and disposal. This documentation is crucial for regulatory inspections and internal audits. Implementing a robust chemical inventory system can help ensure compliance and facilitate efficient buffer system optimization.
Furthermore, laboratories must establish and follow standard operating procedures (SOPs) that incorporate regulatory requirements. These SOPs should cover all aspects of buffer preparation, use, and disposal, ensuring consistency and compliance across experiments. Regular training sessions for laboratory personnel on regulatory compliance and safe chemical handling practices are essential to maintain a culture of safety and adherence to regulations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!