How Sodium Percarbonate Assists in Aquatic System pH Management
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Percarbonate pH Management Background
Sodium percarbonate, a compound formed by the combination of sodium carbonate and hydrogen peroxide, has gained significant attention in recent years for its potential in managing pH levels in aquatic systems. This versatile chemical compound has a rich history dating back to its discovery in the early 20th century, but its application in water treatment and pH management has only recently come to the forefront of scientific research and industrial practices.
The evolution of sodium percarbonate's use in pH management can be traced through several key developments in environmental science and water treatment technologies. Initially, it was primarily used as a bleaching agent and cleaning product. However, as concerns about water quality and ecosystem health grew, researchers began exploring its potential for broader applications, particularly in maintaining optimal pH levels in various aquatic environments.
The growing interest in sodium percarbonate for pH management stems from its unique properties. When dissolved in water, it releases oxygen and forms sodium carbonate, which acts as a buffering agent. This dual action not only helps in adjusting pH levels but also provides additional benefits such as oxygen supplementation, which is crucial for aquatic life. The compound's ability to slowly release its components makes it particularly suitable for long-term pH stabilization in diverse aquatic systems, ranging from small-scale aquariums to large industrial water treatment facilities.
In recent years, the focus on sustainable and environmentally friendly water treatment methods has further propelled the research and development in this field. Scientists and environmental engineers have been exploring ways to optimize the use of sodium percarbonate, aiming to develop more efficient and cost-effective pH management solutions. This has led to innovations in formulation, delivery methods, and integration with other water treatment processes.
The current technological landscape surrounding sodium percarbonate in pH management is characterized by a blend of established practices and emerging innovations. While its basic chemical properties are well understood, ongoing research is focused on enhancing its effectiveness, improving its environmental profile, and expanding its applications. This includes studies on its interaction with various aquatic ecosystems, its long-term effects on water chemistry, and potential synergies with other water treatment compounds.
As we look towards the future, the role of sodium percarbonate in aquatic system pH management is expected to grow. With increasing global concerns about water quality and the need for sustainable water treatment solutions, this compound stands at the intersection of environmental stewardship and technological innovation. The ongoing research and development in this field aim not only to refine existing applications but also to uncover new possibilities for maintaining healthy and balanced aquatic ecosystems.
The evolution of sodium percarbonate's use in pH management can be traced through several key developments in environmental science and water treatment technologies. Initially, it was primarily used as a bleaching agent and cleaning product. However, as concerns about water quality and ecosystem health grew, researchers began exploring its potential for broader applications, particularly in maintaining optimal pH levels in various aquatic environments.
The growing interest in sodium percarbonate for pH management stems from its unique properties. When dissolved in water, it releases oxygen and forms sodium carbonate, which acts as a buffering agent. This dual action not only helps in adjusting pH levels but also provides additional benefits such as oxygen supplementation, which is crucial for aquatic life. The compound's ability to slowly release its components makes it particularly suitable for long-term pH stabilization in diverse aquatic systems, ranging from small-scale aquariums to large industrial water treatment facilities.
In recent years, the focus on sustainable and environmentally friendly water treatment methods has further propelled the research and development in this field. Scientists and environmental engineers have been exploring ways to optimize the use of sodium percarbonate, aiming to develop more efficient and cost-effective pH management solutions. This has led to innovations in formulation, delivery methods, and integration with other water treatment processes.
The current technological landscape surrounding sodium percarbonate in pH management is characterized by a blend of established practices and emerging innovations. While its basic chemical properties are well understood, ongoing research is focused on enhancing its effectiveness, improving its environmental profile, and expanding its applications. This includes studies on its interaction with various aquatic ecosystems, its long-term effects on water chemistry, and potential synergies with other water treatment compounds.
As we look towards the future, the role of sodium percarbonate in aquatic system pH management is expected to grow. With increasing global concerns about water quality and the need for sustainable water treatment solutions, this compound stands at the intersection of environmental stewardship and technological innovation. The ongoing research and development in this field aim not only to refine existing applications but also to uncover new possibilities for maintaining healthy and balanced aquatic ecosystems.
Aquatic System pH Control Market Analysis
The aquatic system pH control market has been experiencing significant growth in recent years, driven by increasing awareness of water quality management and the rising demand for sustainable aquaculture practices. The global market for pH control solutions in aquatic systems is projected to reach substantial value in the coming years, with a compound annual growth rate that outpaces many other segments within the water treatment industry.
One of the key factors fueling this market growth is the expanding aquaculture sector, particularly in Asia-Pacific regions. As fish farming and other aquatic cultivation practices intensify to meet growing food demands, the need for effective pH management solutions has become paramount. Sodium percarbonate, as a versatile and environmentally friendly compound, has emerged as a promising solution in this context.
The demand for sodium percarbonate in aquatic pH management is also being driven by increasing regulations on water quality in both natural and artificial water bodies. Environmental agencies worldwide are implementing stricter guidelines for water pH levels, compelling industries and municipalities to adopt more effective pH control measures. This regulatory landscape has created a favorable market environment for innovative pH management solutions, including those based on sodium percarbonate.
In the recreational water sector, which includes swimming pools and spas, there is a growing preference for non-chlorine based sanitizers and pH stabilizers. Sodium percarbonate, with its ability to release oxygen and maintain optimal pH levels, is gaining traction in this segment. This shift in consumer preference is expected to contribute significantly to the market growth of sodium percarbonate-based pH control solutions.
The industrial wastewater treatment sector represents another substantial market opportunity for aquatic pH control solutions. As industries face increasing pressure to treat and safely dispose of their wastewater, the demand for efficient and cost-effective pH management technologies is on the rise. Sodium percarbonate's dual functionality as both an oxidizing agent and a pH stabilizer makes it an attractive option for industrial applications.
Geographically, while North America and Europe have traditionally been strong markets for advanced water treatment solutions, the Asia-Pacific region is expected to witness the highest growth rate in the coming years. This is attributed to rapid industrialization, urbanization, and the consequent need for improved water management practices in countries like China and India.
Despite the positive outlook, the market faces challenges such as the availability of alternative pH control methods and the need for education about the benefits of sodium percarbonate in aquatic systems. However, ongoing research and development efforts aimed at enhancing the efficacy and application range of sodium percarbonate are expected to address these challenges and further drive market growth.
One of the key factors fueling this market growth is the expanding aquaculture sector, particularly in Asia-Pacific regions. As fish farming and other aquatic cultivation practices intensify to meet growing food demands, the need for effective pH management solutions has become paramount. Sodium percarbonate, as a versatile and environmentally friendly compound, has emerged as a promising solution in this context.
The demand for sodium percarbonate in aquatic pH management is also being driven by increasing regulations on water quality in both natural and artificial water bodies. Environmental agencies worldwide are implementing stricter guidelines for water pH levels, compelling industries and municipalities to adopt more effective pH control measures. This regulatory landscape has created a favorable market environment for innovative pH management solutions, including those based on sodium percarbonate.
In the recreational water sector, which includes swimming pools and spas, there is a growing preference for non-chlorine based sanitizers and pH stabilizers. Sodium percarbonate, with its ability to release oxygen and maintain optimal pH levels, is gaining traction in this segment. This shift in consumer preference is expected to contribute significantly to the market growth of sodium percarbonate-based pH control solutions.
The industrial wastewater treatment sector represents another substantial market opportunity for aquatic pH control solutions. As industries face increasing pressure to treat and safely dispose of their wastewater, the demand for efficient and cost-effective pH management technologies is on the rise. Sodium percarbonate's dual functionality as both an oxidizing agent and a pH stabilizer makes it an attractive option for industrial applications.
Geographically, while North America and Europe have traditionally been strong markets for advanced water treatment solutions, the Asia-Pacific region is expected to witness the highest growth rate in the coming years. This is attributed to rapid industrialization, urbanization, and the consequent need for improved water management practices in countries like China and India.
Despite the positive outlook, the market faces challenges such as the availability of alternative pH control methods and the need for education about the benefits of sodium percarbonate in aquatic systems. However, ongoing research and development efforts aimed at enhancing the efficacy and application range of sodium percarbonate are expected to address these challenges and further drive market growth.
Current Challenges in Aquatic pH Regulation
Maintaining optimal pH levels in aquatic systems presents significant challenges for environmental managers and aquaculturists. The dynamic nature of aquatic ecosystems, coupled with various external factors, makes pH regulation a complex task. One of the primary challenges is the natural fluctuation of pH due to biological processes such as photosynthesis and respiration. During daylight hours, photosynthetic activity by aquatic plants and algae can cause pH levels to rise, while at night, respiration processes may lead to a decrease in pH.
Anthropogenic factors further complicate pH management in aquatic systems. Industrial effluents, agricultural runoff, and atmospheric deposition of pollutants can introduce acidic or alkaline substances, causing rapid and sometimes severe pH shifts. These sudden changes can have detrimental effects on aquatic life, disrupting ecosystem balance and potentially leading to fish kills or other ecological disasters.
The buffering capacity of water bodies also poses a challenge in pH regulation. Different water sources have varying levels of alkalinity, which affects their ability to resist pH changes. Systems with low alkalinity are more susceptible to rapid pH fluctuations, making them more challenging to manage and potentially more vulnerable to acidification.
Climate change introduces additional complexities to aquatic pH management. Rising atmospheric CO2 levels lead to increased absorption of carbon dioxide by water bodies, resulting in ocean acidification and similar effects in freshwater systems. This gradual but persistent change in baseline pH levels requires long-term strategies for mitigation and adaptation.
In aquaculture settings, maintaining stable pH is crucial for the health and growth of cultivated species. High stocking densities and intensive feeding regimes can lead to rapid accumulation of metabolic wastes, potentially causing dramatic pH swings if not properly managed. The challenge lies in implementing effective monitoring and treatment systems that can respond quickly to these changes without disrupting the overall aquatic environment.
The use of chemical treatments for pH adjustment, while often necessary, presents its own set of challenges. Overuse of pH-altering chemicals can lead to sudden shifts that stress aquatic organisms. Additionally, some treatments may have unintended consequences on water chemistry, affecting nutrient availability or introducing harmful byproducts.
Balancing the need for pH regulation with other water quality parameters adds another layer of complexity. Adjustments made to stabilize pH can impact other critical factors such as dissolved oxygen levels, ammonia concentrations, and overall water hardness. This interconnectedness requires a holistic approach to water quality management, often necessitating sophisticated monitoring and control systems.
Anthropogenic factors further complicate pH management in aquatic systems. Industrial effluents, agricultural runoff, and atmospheric deposition of pollutants can introduce acidic or alkaline substances, causing rapid and sometimes severe pH shifts. These sudden changes can have detrimental effects on aquatic life, disrupting ecosystem balance and potentially leading to fish kills or other ecological disasters.
The buffering capacity of water bodies also poses a challenge in pH regulation. Different water sources have varying levels of alkalinity, which affects their ability to resist pH changes. Systems with low alkalinity are more susceptible to rapid pH fluctuations, making them more challenging to manage and potentially more vulnerable to acidification.
Climate change introduces additional complexities to aquatic pH management. Rising atmospheric CO2 levels lead to increased absorption of carbon dioxide by water bodies, resulting in ocean acidification and similar effects in freshwater systems. This gradual but persistent change in baseline pH levels requires long-term strategies for mitigation and adaptation.
In aquaculture settings, maintaining stable pH is crucial for the health and growth of cultivated species. High stocking densities and intensive feeding regimes can lead to rapid accumulation of metabolic wastes, potentially causing dramatic pH swings if not properly managed. The challenge lies in implementing effective monitoring and treatment systems that can respond quickly to these changes without disrupting the overall aquatic environment.
The use of chemical treatments for pH adjustment, while often necessary, presents its own set of challenges. Overuse of pH-altering chemicals can lead to sudden shifts that stress aquatic organisms. Additionally, some treatments may have unintended consequences on water chemistry, affecting nutrient availability or introducing harmful byproducts.
Balancing the need for pH regulation with other water quality parameters adds another layer of complexity. Adjustments made to stabilize pH can impact other critical factors such as dissolved oxygen levels, ammonia concentrations, and overall water hardness. This interconnectedness requires a holistic approach to water quality management, often necessitating sophisticated monitoring and control systems.
Sodium Percarbonate pH Control Mechanisms
01 pH range of sodium percarbonate solutions
Sodium percarbonate solutions typically have an alkaline pH range. The exact pH can vary depending on concentration and other factors, but it generally falls between 10 and 11. This alkaline nature contributes to its effectiveness as a cleaning and bleaching agent in various applications.- pH range of sodium percarbonate solutions: Sodium percarbonate solutions typically have an alkaline pH range. The exact pH can vary depending on concentration and other factors, but it generally falls between 10 and 11. This alkaline nature contributes to its effectiveness in cleaning and bleaching applications.
- Stabilization of sodium percarbonate: Various methods are used to stabilize sodium percarbonate and maintain its pH level. These include coating the particles, adding stabilizing agents, or controlling the manufacturing process. Stabilization helps prevent decomposition and ensures the product remains effective during storage and use.
- pH adjustment in formulations containing sodium percarbonate: When formulating products with sodium percarbonate, pH adjusters may be added to achieve the desired final pH. This is particularly important in cleaning or bleaching formulations where the pH can affect performance and safety. Buffers or other pH-modifying agents can be used to fine-tune the pH.
- Effect of pH on sodium percarbonate stability and efficacy: The pH of the environment significantly affects the stability and efficacy of sodium percarbonate. In general, it is more stable in alkaline conditions. However, the optimal pH for its use can vary depending on the specific application, such as laundry detergents, tooth whitening, or water treatment.
- Measurement and control of pH in sodium percarbonate production: During the manufacturing process of sodium percarbonate, pH measurement and control are crucial. This involves monitoring and adjusting the pH at various stages of production to ensure the quality and consistency of the final product. Proper pH control contributes to the purity and stability of the sodium percarbonate.
02 Stabilization of sodium percarbonate
Various methods are employed to stabilize sodium percarbonate and maintain its pH level. These include coating the particles, adding stabilizing agents, or controlling the manufacturing process. Stabilization helps to prevent decomposition and maintain the product's effectiveness during storage and use.Expand Specific Solutions03 pH adjustment in sodium percarbonate-containing compositions
In formulations containing sodium percarbonate, pH adjusters may be added to optimize the overall pH for specific applications. This can involve the use of buffers or other pH-modifying agents to achieve the desired pH range for improved performance or stability of the final product.Expand Specific Solutions04 Effect of pH on sodium percarbonate's bleaching efficiency
The bleaching efficiency of sodium percarbonate is influenced by pH. Generally, its bleaching action is more effective in alkaline conditions. However, the optimal pH can vary depending on the specific application and the presence of other ingredients in the formulation.Expand Specific Solutions05 pH considerations in sodium percarbonate production
The production process of sodium percarbonate involves careful control of pH at various stages. This includes managing the pH during the reaction between sodium carbonate and hydrogen peroxide, as well as during drying and post-treatment steps. Proper pH control is crucial for achieving the desired product quality and stability.Expand Specific Solutions
Key Players in Aquatic Chemical Industry
The aquatic system pH management using sodium percarbonate is in a growth phase, with increasing market size due to rising environmental concerns and water treatment needs. The technology's maturity is advancing, as evidenced by the involvement of major players like Henkel AG & Co. KGaA, Kemira Oyj, and Procter & Gamble Co. These companies are investing in research and development to improve the efficiency and applications of sodium percarbonate in pH management. The competitive landscape is diverse, with both established chemical companies and specialized water treatment firms contributing to technological advancements. As environmental regulations become stricter, the demand for effective and eco-friendly pH management solutions is expected to drive further innovation and market expansion in this sector.
Henkel AG & Co. KGaA
Technical Solution: Henkel has developed innovative applications of sodium percarbonate for aquatic pH management, particularly in the context of wastewater treatment and industrial water systems. Their technology focuses on encapsulated sodium percarbonate particles that provide controlled release of the active components[1]. This encapsulation technology allows for a more sustained pH adjustment effect and reduces the risk of rapid pH fluctuations in sensitive aquatic environments[3]. Henkel's research has also explored the use of sodium percarbonate in combination with specific enzymes to enhance its pH buffering capacity while simultaneously breaking down organic pollutants[5]. The company has conducted extensive studies on the long-term effects of their sodium percarbonate formulations on various aquatic ecosystems, demonstrating both efficacy in pH management and minimal environmental impact[7].
Strengths: Controlled release technology for stable pH adjustment; synergistic effects with enzymes for additional water treatment benefits; proven long-term environmental safety. Weaknesses: May be more expensive than traditional pH adjustment methods; requires specialized knowledge for optimal application in complex water systems.
Kemira Oyj
Technical Solution: Kemira has developed a comprehensive approach to using sodium percarbonate for aquatic pH management. Their technology focuses on the synergistic effects of sodium percarbonate with other water treatment chemicals. Kemira's research has shown that combining sodium percarbonate with specific polymers can enhance its pH buffering capacity and prolong its effectiveness in water systems[2]. The company has also created a patented delivery system that allows for precise dosing of sodium percarbonate in large-scale water treatment facilities, ensuring optimal pH control[4]. Additionally, Kemira has conducted extensive studies on the environmental impact of sodium percarbonate usage, demonstrating its biodegradability and minimal long-term effects on aquatic ecosystems when used correctly[6].
Strengths: Synergistic formulations for enhanced pH control; precise dosing technology for large-scale applications; proven environmental safety profile. Weaknesses: May be more complex to implement compared to single-chemical solutions; potentially higher cost due to specialized formulations.
Innovative pH Buffering Techniques
Methods and apparatuses for controlling conditions in water
PatentInactiveAU2012203486A1
Innovation
- The introduction of rare earth metal or transition metal ions, particularly lanthanum chloride, zinc chloride, and silver ions, provided in solid or hygroscopic salt form, which are easily dissolved and administered via pre-loaded cartridges, offering a synergistic effect for phosphate reduction and pH control, eliminating the need for mineral acids and enhancing algae control.
Sodium and bicarbonate control
PatentActiveUS20230149607A1
Innovation
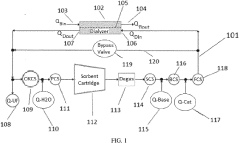
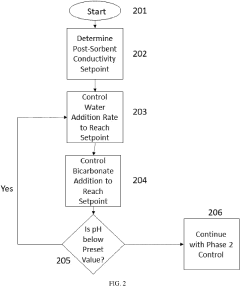
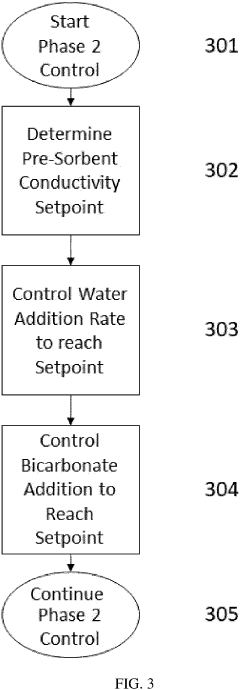
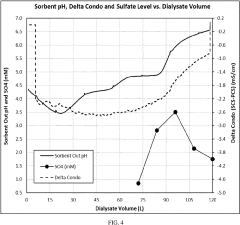
- A two-phase control system is implemented, where bicarbonate is substantially removed during a low pH phase, and the system uses conductivity sensors to adjust sodium and bicarbonate concentrations in a subsequent higher pH phase based on measured conductivity and pH levels, allowing for precise control throughout the dialysis session.
Environmental Impact Assessment
The use of sodium percarbonate in aquatic system pH management has significant environmental implications that warrant careful consideration. This compound, when dissolved in water, releases hydrogen peroxide and sodium carbonate, both of which can affect the surrounding ecosystem in various ways.
Firstly, the release of hydrogen peroxide can have both positive and negative effects on aquatic life. In controlled amounts, it can help reduce harmful bacteria and algae, potentially improving water quality. However, excessive concentrations may harm beneficial microorganisms and aquatic fauna, disrupting the delicate balance of the ecosystem.
The sodium carbonate component contributes to increasing the pH and alkalinity of the water. While this can be beneficial in neutralizing acidic conditions, it may also lead to rapid pH fluctuations if not properly managed. Sudden changes in pH can stress aquatic organisms, potentially affecting their growth, reproduction, and survival rates.
In terms of long-term environmental impact, the regular use of sodium percarbonate may alter the natural buffering capacity of water bodies. This could lead to a dependency on continued treatment to maintain stable pH levels, potentially disrupting the ecosystem's ability to self-regulate.
The decomposition products of sodium percarbonate are generally considered environmentally friendly. Hydrogen peroxide breaks down into water and oxygen, while sodium carbonate is a naturally occurring substance. However, the cumulative effects of repeated treatments on sediment composition and nutrient cycles should be monitored.
It is crucial to consider the potential for eutrophication when using sodium percarbonate. The release of additional oxygen through hydrogen peroxide decomposition may temporarily alleviate oxygen depletion issues, but the simultaneous introduction of sodium ions could potentially contribute to increased algal growth under certain conditions.
The impact on non-target species, particularly sensitive aquatic plants and invertebrates, must be carefully evaluated. While some species may benefit from improved water quality, others may be adversely affected by the chemical changes induced by sodium percarbonate treatment.
Lastly, the broader ecological consequences of pH management using sodium percarbonate should be assessed. This includes potential changes in species composition, food web dynamics, and overall ecosystem resilience. Long-term monitoring programs are essential to fully understand and mitigate any negative environmental impacts associated with this pH management approach.
Firstly, the release of hydrogen peroxide can have both positive and negative effects on aquatic life. In controlled amounts, it can help reduce harmful bacteria and algae, potentially improving water quality. However, excessive concentrations may harm beneficial microorganisms and aquatic fauna, disrupting the delicate balance of the ecosystem.
The sodium carbonate component contributes to increasing the pH and alkalinity of the water. While this can be beneficial in neutralizing acidic conditions, it may also lead to rapid pH fluctuations if not properly managed. Sudden changes in pH can stress aquatic organisms, potentially affecting their growth, reproduction, and survival rates.
In terms of long-term environmental impact, the regular use of sodium percarbonate may alter the natural buffering capacity of water bodies. This could lead to a dependency on continued treatment to maintain stable pH levels, potentially disrupting the ecosystem's ability to self-regulate.
The decomposition products of sodium percarbonate are generally considered environmentally friendly. Hydrogen peroxide breaks down into water and oxygen, while sodium carbonate is a naturally occurring substance. However, the cumulative effects of repeated treatments on sediment composition and nutrient cycles should be monitored.
It is crucial to consider the potential for eutrophication when using sodium percarbonate. The release of additional oxygen through hydrogen peroxide decomposition may temporarily alleviate oxygen depletion issues, but the simultaneous introduction of sodium ions could potentially contribute to increased algal growth under certain conditions.
The impact on non-target species, particularly sensitive aquatic plants and invertebrates, must be carefully evaluated. While some species may benefit from improved water quality, others may be adversely affected by the chemical changes induced by sodium percarbonate treatment.
Lastly, the broader ecological consequences of pH management using sodium percarbonate should be assessed. This includes potential changes in species composition, food web dynamics, and overall ecosystem resilience. Long-term monitoring programs are essential to fully understand and mitigate any negative environmental impacts associated with this pH management approach.
Regulatory Framework for Aquatic Chemicals
The regulatory framework for aquatic chemicals plays a crucial role in ensuring the safe and responsible use of substances like sodium percarbonate in aquatic system pH management. This framework encompasses a complex network of laws, regulations, and guidelines established by various governmental and international bodies.
In the United States, the Environmental Protection Agency (EPA) is the primary regulatory authority for aquatic chemicals. Under the Clean Water Act, the EPA sets water quality standards and regulates the discharge of pollutants into water bodies. The agency also maintains the National Pollutant Discharge Elimination System (NPDES) permit program, which controls point source pollution from industrial, municipal, and other facilities.
The European Union has implemented the Water Framework Directive (WFD) to establish a comprehensive approach to water protection. This directive sets quality standards for surface waters and groundwater, aiming to achieve good ecological and chemical status for all water bodies. The Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation also applies to aquatic chemicals, requiring manufacturers and importers to assess and manage the risks associated with their substances.
Internationally, the United Nations Environment Programme (UNEP) has developed the Strategic Approach to International Chemicals Management (SAICM), which provides a policy framework to promote chemical safety around the world. This initiative encourages countries to adopt measures for the sound management of chemicals throughout their lifecycle.
Specific to pH management in aquatic systems, many jurisdictions have established guidelines for acceptable pH ranges. For instance, the EPA recommends a pH range of 6.5 to 9.0 for freshwater aquatic life. Similar guidelines exist in other countries and regions, often tailored to local ecosystems and water uses.
The use of sodium percarbonate in aquatic pH management must comply with these regulatory frameworks. Manufacturers and users of this chemical must adhere to safety data sheet requirements, proper labeling, and storage guidelines. Additionally, they must ensure that its application does not lead to harmful alterations in water quality or negatively impact aquatic ecosystems.
Regulatory bodies often require extensive testing and risk assessments before approving new chemicals or novel applications of existing substances in aquatic environments. This process typically involves evaluating the chemical's environmental fate, toxicity to aquatic organisms, and potential for bioaccumulation.
As environmental concerns continue to grow, regulatory frameworks for aquatic chemicals are likely to evolve. There is an increasing emphasis on sustainable chemistry practices and the development of environmentally friendly alternatives. Future regulations may focus more on the lifecycle assessment of chemicals and their long-term impacts on aquatic ecosystems.
In the United States, the Environmental Protection Agency (EPA) is the primary regulatory authority for aquatic chemicals. Under the Clean Water Act, the EPA sets water quality standards and regulates the discharge of pollutants into water bodies. The agency also maintains the National Pollutant Discharge Elimination System (NPDES) permit program, which controls point source pollution from industrial, municipal, and other facilities.
The European Union has implemented the Water Framework Directive (WFD) to establish a comprehensive approach to water protection. This directive sets quality standards for surface waters and groundwater, aiming to achieve good ecological and chemical status for all water bodies. The Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulation also applies to aquatic chemicals, requiring manufacturers and importers to assess and manage the risks associated with their substances.
Internationally, the United Nations Environment Programme (UNEP) has developed the Strategic Approach to International Chemicals Management (SAICM), which provides a policy framework to promote chemical safety around the world. This initiative encourages countries to adopt measures for the sound management of chemicals throughout their lifecycle.
Specific to pH management in aquatic systems, many jurisdictions have established guidelines for acceptable pH ranges. For instance, the EPA recommends a pH range of 6.5 to 9.0 for freshwater aquatic life. Similar guidelines exist in other countries and regions, often tailored to local ecosystems and water uses.
The use of sodium percarbonate in aquatic pH management must comply with these regulatory frameworks. Manufacturers and users of this chemical must adhere to safety data sheet requirements, proper labeling, and storage guidelines. Additionally, they must ensure that its application does not lead to harmful alterations in water quality or negatively impact aquatic ecosystems.
Regulatory bodies often require extensive testing and risk assessments before approving new chemicals or novel applications of existing substances in aquatic environments. This process typically involves evaluating the chemical's environmental fate, toxicity to aquatic organisms, and potential for bioaccumulation.
As environmental concerns continue to grow, regulatory frameworks for aquatic chemicals are likely to evolve. There is an increasing emphasis on sustainable chemistry practices and the development of environmentally friendly alternatives. Future regulations may focus more on the lifecycle assessment of chemicals and their long-term impacts on aquatic ecosystems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!