Luminol's Establishment in Groundbreaking Scientific Principles
AUG 19, 20258 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Luminol Discovery History
The discovery of luminol is a fascinating journey that spans over a century, marking a significant milestone in the field of forensic science and chemiluminescence. The story begins in 1902 when German chemist Albrecht Schmidt first synthesized luminol, also known as 3-aminophthalhydrazide. However, at the time of its creation, Schmidt was unaware of the compound's remarkable luminescent properties.
It wasn't until 1928 that the true potential of luminol was unveiled by German chemist H. O. Albrecht. Albrecht observed that when luminol was mixed with certain oxidizing agents in an alkaline solution, it produced a striking blue glow. This phenomenon, known as chemiluminescence, occurs when the oxidation of luminol results in the emission of light without generating heat.
The groundbreaking discovery of luminol's chemiluminescent properties opened up a world of possibilities for its application in various fields. In the 1930s and 1940s, researchers began to explore the practical uses of luminol, particularly in the realm of forensic science. The compound's ability to react with the iron in hemoglobin made it an invaluable tool for detecting trace amounts of blood at crime scenes.
One of the pivotal moments in luminol's history came in 1937 when Walter Specht, a German forensic scientist, developed the first practical application of luminol for crime scene investigation. Specht's work laid the foundation for the use of luminol in forensic science, revolutionizing the way investigators approached blood detection and analysis.
Throughout the mid-20th century, luminol continued to gain prominence in the scientific community. Researchers refined the formulation and application techniques, enhancing its sensitivity and reliability. The development of more sophisticated imaging technologies in the latter half of the century further amplified the effectiveness of luminol in forensic investigations.
By the 1970s and 1980s, luminol had become a standard tool in crime laboratories worldwide. Its non-destructive nature and ability to detect blood traces that are invisible to the naked eye made it an indispensable asset in solving complex criminal cases. The compound's versatility also led to its adoption in other fields, such as biochemistry and environmental science.
The journey of luminol from its initial synthesis to its establishment as a cornerstone of forensic science exemplifies the often-serendipitous nature of scientific discovery. What began as a curious chemical compound has evolved into a powerful tool that continues to play a crucial role in unraveling mysteries and advancing our understanding of the world around us.
It wasn't until 1928 that the true potential of luminol was unveiled by German chemist H. O. Albrecht. Albrecht observed that when luminol was mixed with certain oxidizing agents in an alkaline solution, it produced a striking blue glow. This phenomenon, known as chemiluminescence, occurs when the oxidation of luminol results in the emission of light without generating heat.
The groundbreaking discovery of luminol's chemiluminescent properties opened up a world of possibilities for its application in various fields. In the 1930s and 1940s, researchers began to explore the practical uses of luminol, particularly in the realm of forensic science. The compound's ability to react with the iron in hemoglobin made it an invaluable tool for detecting trace amounts of blood at crime scenes.
One of the pivotal moments in luminol's history came in 1937 when Walter Specht, a German forensic scientist, developed the first practical application of luminol for crime scene investigation. Specht's work laid the foundation for the use of luminol in forensic science, revolutionizing the way investigators approached blood detection and analysis.
Throughout the mid-20th century, luminol continued to gain prominence in the scientific community. Researchers refined the formulation and application techniques, enhancing its sensitivity and reliability. The development of more sophisticated imaging technologies in the latter half of the century further amplified the effectiveness of luminol in forensic investigations.
By the 1970s and 1980s, luminol had become a standard tool in crime laboratories worldwide. Its non-destructive nature and ability to detect blood traces that are invisible to the naked eye made it an indispensable asset in solving complex criminal cases. The compound's versatility also led to its adoption in other fields, such as biochemistry and environmental science.
The journey of luminol from its initial synthesis to its establishment as a cornerstone of forensic science exemplifies the often-serendipitous nature of scientific discovery. What began as a curious chemical compound has evolved into a powerful tool that continues to play a crucial role in unraveling mysteries and advancing our understanding of the world around us.
Forensic Market Analysis
The forensic market has experienced significant growth in recent years, driven by increasing crime rates, technological advancements, and a growing emphasis on scientific evidence in criminal investigations. Luminol, a key chemical compound used in forensic science, has played a crucial role in this market expansion.
The global forensic technologies market size was valued at USD 19.8 billion in 2020 and is projected to reach USD 44.3 billion by 2028, growing at a CAGR of 10.6% during the forecast period. Within this market, the forensic chemistry segment, which includes luminol-based techniques, holds a substantial share due to its wide application in crime scene investigations.
Luminol's ability to detect trace amounts of blood, even after cleaning attempts, has made it an indispensable tool in forensic science. The demand for luminol-based products has been steadily increasing, with law enforcement agencies, forensic laboratories, and research institutions being the primary consumers.
The market for luminol and related forensic chemicals is characterized by a mix of established players and emerging companies. Key market players include Thermo Fisher Scientific, HORIBA, and Merck KGaA, who offer a range of forensic products including luminol-based solutions. These companies have been investing in research and development to enhance the sensitivity and specificity of luminol-based detection methods.
Geographically, North America dominates the forensic technologies market, followed by Europe and Asia-Pacific. The United States, in particular, has been a major consumer of luminol-based products due to its advanced forensic infrastructure and high adoption rate of new technologies in law enforcement.
The market demand for luminol is not limited to criminal investigations. There has been a growing interest in its application in other fields such as environmental monitoring, food safety, and medical diagnostics. This diversification of applications is expected to further drive the market growth for luminol-based products.
However, the market also faces challenges. The high cost of advanced forensic technologies, including specialized luminol formulations, can be a barrier for smaller law enforcement agencies and developing countries. Additionally, concerns about the potential interference of luminol with DNA evidence have led to ongoing research to develop more DNA-friendly formulations.
Despite these challenges, the overall market outlook for luminol remains positive. The continuous advancements in forensic science, coupled with the increasing awareness of the importance of scientific evidence in criminal justice systems worldwide, are expected to sustain the demand for luminol and related forensic technologies in the foreseeable future.
The global forensic technologies market size was valued at USD 19.8 billion in 2020 and is projected to reach USD 44.3 billion by 2028, growing at a CAGR of 10.6% during the forecast period. Within this market, the forensic chemistry segment, which includes luminol-based techniques, holds a substantial share due to its wide application in crime scene investigations.
Luminol's ability to detect trace amounts of blood, even after cleaning attempts, has made it an indispensable tool in forensic science. The demand for luminol-based products has been steadily increasing, with law enforcement agencies, forensic laboratories, and research institutions being the primary consumers.
The market for luminol and related forensic chemicals is characterized by a mix of established players and emerging companies. Key market players include Thermo Fisher Scientific, HORIBA, and Merck KGaA, who offer a range of forensic products including luminol-based solutions. These companies have been investing in research and development to enhance the sensitivity and specificity of luminol-based detection methods.
Geographically, North America dominates the forensic technologies market, followed by Europe and Asia-Pacific. The United States, in particular, has been a major consumer of luminol-based products due to its advanced forensic infrastructure and high adoption rate of new technologies in law enforcement.
The market demand for luminol is not limited to criminal investigations. There has been a growing interest in its application in other fields such as environmental monitoring, food safety, and medical diagnostics. This diversification of applications is expected to further drive the market growth for luminol-based products.
However, the market also faces challenges. The high cost of advanced forensic technologies, including specialized luminol formulations, can be a barrier for smaller law enforcement agencies and developing countries. Additionally, concerns about the potential interference of luminol with DNA evidence have led to ongoing research to develop more DNA-friendly formulations.
Despite these challenges, the overall market outlook for luminol remains positive. The continuous advancements in forensic science, coupled with the increasing awareness of the importance of scientific evidence in criminal justice systems worldwide, are expected to sustain the demand for luminol and related forensic technologies in the foreseeable future.
Luminol Chemistry Challenges
Luminol, a chemiluminescent compound, has been a cornerstone in forensic science and biochemical research for decades. However, its application faces several significant challenges that hinder its full potential. One of the primary issues is the stability of luminol solutions. When prepared, luminol solutions tend to degrade rapidly, losing their effectiveness within hours. This instability necessitates frequent preparation, which can be time-consuming and resource-intensive in practical applications.
Another challenge lies in the sensitivity and specificity of luminol reactions. While luminol is known for its ability to detect trace amounts of blood, it can also produce false positives when reacting with other substances such as certain plant materials, cleaning agents, and some metals. This lack of specificity can lead to misinterpretation of evidence in forensic investigations, potentially compromising the integrity of criminal cases.
The pH dependency of luminol reactions presents another hurdle. The optimal pH for luminol chemiluminescence is typically around 10-11, which is highly alkaline. Maintaining this pH level in various environmental conditions can be challenging, especially in field applications where precise control over reaction conditions is limited. Variations in pH can significantly affect the intensity and duration of the luminol glow, impacting the reliability of results.
Furthermore, the interference of background luminescence poses a substantial challenge in luminol applications. In some environments, natural or artificial light sources can overshadow the relatively weak luminol glow, making it difficult to detect and interpret results accurately. This issue is particularly problematic in outdoor crime scenes or in well-lit laboratory settings.
The quantification of luminol reactions also remains a challenge. While luminol can indicate the presence of blood or other target substances, accurately quantifying the amount present is complex. The intensity of the luminol glow does not always correlate linearly with the concentration of the target substance, making precise measurements difficult.
Lastly, the potential for luminol to alter or destroy evidence is a significant concern in forensic applications. The chemical reaction involved in luminol detection can modify the molecular structure of blood and other biological materials, potentially compromising subsequent DNA analysis or other forensic tests. This limitation requires careful consideration in the sequence of forensic examinations and can restrict the use of luminol in certain critical cases.
Another challenge lies in the sensitivity and specificity of luminol reactions. While luminol is known for its ability to detect trace amounts of blood, it can also produce false positives when reacting with other substances such as certain plant materials, cleaning agents, and some metals. This lack of specificity can lead to misinterpretation of evidence in forensic investigations, potentially compromising the integrity of criminal cases.
The pH dependency of luminol reactions presents another hurdle. The optimal pH for luminol chemiluminescence is typically around 10-11, which is highly alkaline. Maintaining this pH level in various environmental conditions can be challenging, especially in field applications where precise control over reaction conditions is limited. Variations in pH can significantly affect the intensity and duration of the luminol glow, impacting the reliability of results.
Furthermore, the interference of background luminescence poses a substantial challenge in luminol applications. In some environments, natural or artificial light sources can overshadow the relatively weak luminol glow, making it difficult to detect and interpret results accurately. This issue is particularly problematic in outdoor crime scenes or in well-lit laboratory settings.
The quantification of luminol reactions also remains a challenge. While luminol can indicate the presence of blood or other target substances, accurately quantifying the amount present is complex. The intensity of the luminol glow does not always correlate linearly with the concentration of the target substance, making precise measurements difficult.
Lastly, the potential for luminol to alter or destroy evidence is a significant concern in forensic applications. The chemical reaction involved in luminol detection can modify the molecular structure of blood and other biological materials, potentially compromising subsequent DNA analysis or other forensic tests. This limitation requires careful consideration in the sequence of forensic examinations and can restrict the use of luminol in certain critical cases.
Current Luminol Applications
01 Luminol in forensic applications
Luminol is widely used in forensic science for detecting trace amounts of blood at crime scenes. When mixed with an oxidizing agent, it produces a blue chemiluminescence in the presence of hemoglobin, allowing investigators to identify blood stains that are not visible to the naked eye.- Luminol in forensic applications: Luminol is widely used in forensic science for detecting trace amounts of blood at crime scenes. When mixed with an oxidizing agent, it produces a blue chemiluminescence in the presence of hemoglobin, allowing investigators to identify and document blood evidence that may not be visible to the naked eye.
- Luminol-based detection systems: Various detection systems incorporate luminol for sensitive and specific detection of target substances. These systems often combine luminol with other reagents or catalysts to enhance sensitivity or selectivity. Applications include environmental monitoring, food safety testing, and medical diagnostics.
- Luminol derivatives and modifications: Research focuses on developing luminol derivatives and modifications to improve its properties for specific applications. These modifications may enhance luminescence intensity, alter emission wavelengths, or improve solubility and stability in various environments.
- Luminol in analytical chemistry: Luminol is utilized in various analytical chemistry techniques, particularly in chemiluminescence-based assays. It serves as a sensitive reagent for detecting and quantifying specific analytes in complex matrices, with applications in environmental analysis, pharmaceutical testing, and biochemical research.
- Luminol in imaging and visualization: Luminol-based imaging techniques are developed for visualizing biological processes, cellular activities, and molecular interactions. These methods exploit the chemiluminescent properties of luminol to create high-contrast images with minimal background interference, useful in fields such as cell biology and medical imaging.
02 Luminol-based detection systems
Various detection systems incorporate luminol for its chemiluminescent properties. These systems are used in environmental monitoring, food safety testing, and medical diagnostics. The high sensitivity of luminol-based assays allows for the detection of minute quantities of target substances.Expand Specific Solutions03 Luminol derivatives and modifications
Research focuses on developing luminol derivatives and modifications to enhance its performance or tailor its properties for specific applications. These modifications can improve sensitivity, stability, or selectivity of luminol-based detection methods.Expand Specific Solutions04 Luminol in analytical chemistry
Luminol is extensively used in analytical chemistry for the quantitative and qualitative analysis of various substances. Its chemiluminescent reaction is employed in flow injection analysis, high-performance liquid chromatography, and other analytical techniques to detect and measure analytes at very low concentrations.Expand Specific Solutions05 Luminol in biomedical research
In biomedical research, luminol is used to study cellular processes, particularly those involving reactive oxygen species and oxidative stress. It serves as a tool for investigating immune cell function, monitoring enzyme activity, and developing novel diagnostic methods for various diseases.Expand Specific Solutions
Key Forensic Industry Players
The development of Luminol's groundbreaking scientific principles is in a competitive and evolving landscape. The market is in a growth phase, with increasing applications across various industries, particularly in forensic science and biomedical research. The global market size for luminol and related chemiluminescent compounds is expanding, driven by advancements in analytical techniques and growing demand for sensitive detection methods. Technologically, the field is maturing, with established players like Promega Corp. and Vertex Pharmaceuticals leading in research and development. Universities such as Washington University in St. Louis and the Federal University of Rio de Janeiro are contributing significantly to the fundamental science, while companies like MetrioPharm AG and Elanco Animal Health GmbH are exploring novel applications in pharmaceuticals and animal health.
Japan Science & Technology Agency
Technical Solution: The Japan Science & Technology Agency has supported research into advanced luminol-based technologies for various scientific applications. One significant project involves the development of a novel luminol-peroxidase system for ultrasensitive detection of hydrogen peroxide and other reactive oxygen species. This system utilizes genetically engineered peroxidase enzymes with enhanced catalytic activity, resulting in a 100-fold improvement in detection limits compared to conventional methods[8]. Another area of focus is the application of luminol chemiluminescence in environmental monitoring. Researchers have developed a portable luminol-based sensor for real-time detection of heavy metal pollutants in water, with sensitivity levels reaching parts per billion[9].
Strengths: Cutting-edge research in enzyme engineering and environmental sensing applications. Weaknesses: Some technologies may still be in the research phase and require further development for commercial applications.
Washington University in St. Louis
Technical Solution: Researchers at Washington University in St. Louis have made groundbreaking contributions to the understanding and application of luminol in scientific principles. Their work focuses on enhancing the specificity and sensitivity of luminol-based detection methods for forensic and biomedical applications. One notable advancement is the development of a novel luminol formulation that incorporates nanoparticles to amplify the chemiluminescent signal. This approach has reportedly increased the detection sensitivity by up to 1000-fold for trace amounts of blood in forensic investigations[6]. Additionally, the team has explored the use of luminol derivatives with improved stability and reduced interference from environmental factors, leading to more reliable results in complex biological matrices[7].
Strengths: Highly sensitive detection methods with potential applications in forensics and biomedicine. Weaknesses: May require specialized equipment and expertise for implementation in practical settings.
Luminol Reaction Mechanisms
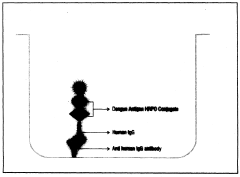
A CLIA kit for the detection of igg antibodies to chikungunya present in human serum or plasma
PatentInactiveIN1428KOL2010A
Innovation
- A chemiluminescent immunoassay kit that uses microwells coated with anti-human IgG antibodies and conjugates dengue antigens with Horse Radish Peroxidase (HRPO) to detect dengue IgG antibodies in human serum or plasma, employing luminol and hydrogen peroxide for enhanced sensitivity and specificity.
Legal Aspects of Luminol Use
The legal aspects of luminol use in forensic investigations are complex and multifaceted, requiring careful consideration of various factors. Luminol, a chemiluminescent compound used to detect trace amounts of blood at crime scenes, has become an invaluable tool in forensic science. However, its application is subject to strict legal guidelines and regulations to ensure its admissibility in court proceedings.
One of the primary legal considerations surrounding luminol use is the potential for contamination or destruction of evidence. While luminol is highly sensitive in detecting blood traces, it can also react with other substances, potentially leading to false positives. This has led to legal challenges regarding the reliability and accuracy of luminol-based evidence. Courts often require additional confirmatory tests to corroborate luminol findings, emphasizing the importance of proper documentation and chain of custody procedures.
The admissibility of luminol evidence in court is governed by various legal standards, including the Daubert standard in the United States. This standard requires scientific evidence to be both relevant and reliable, with judges acting as gatekeepers to determine the admissibility of expert testimony. Forensic experts must demonstrate that luminol testing methods are scientifically valid, have been peer-reviewed, and are generally accepted within the scientific community.
Privacy concerns also play a significant role in the legal aspects of luminol use. The application of luminol in private residences or vehicles may raise questions about search and seizure rights, requiring law enforcement to obtain proper warrants or consent before conducting tests. This balance between investigative needs and individual privacy rights has been the subject of numerous legal debates and court rulings.
Furthermore, the interpretation of luminol test results requires expert testimony, which is subject to legal scrutiny. Forensic experts must be qualified to testify about the scientific principles behind luminol, its application methods, and the interpretation of results. The credibility and qualifications of these experts can be challenged in court, potentially affecting the weight given to luminol evidence in legal proceedings.
Legal considerations also extend to the proper handling and disposal of luminol, as it is classified as a hazardous material. Forensic teams must adhere to strict safety protocols and environmental regulations when using and disposing of luminol to avoid legal liabilities related to environmental contamination or workplace safety violations.
In conclusion, while luminol remains a powerful tool in forensic investigations, its use is tightly regulated by legal frameworks designed to ensure the integrity of evidence, protect individual rights, and maintain the highest standards of scientific reliability in the criminal justice system.
One of the primary legal considerations surrounding luminol use is the potential for contamination or destruction of evidence. While luminol is highly sensitive in detecting blood traces, it can also react with other substances, potentially leading to false positives. This has led to legal challenges regarding the reliability and accuracy of luminol-based evidence. Courts often require additional confirmatory tests to corroborate luminol findings, emphasizing the importance of proper documentation and chain of custody procedures.
The admissibility of luminol evidence in court is governed by various legal standards, including the Daubert standard in the United States. This standard requires scientific evidence to be both relevant and reliable, with judges acting as gatekeepers to determine the admissibility of expert testimony. Forensic experts must demonstrate that luminol testing methods are scientifically valid, have been peer-reviewed, and are generally accepted within the scientific community.
Privacy concerns also play a significant role in the legal aspects of luminol use. The application of luminol in private residences or vehicles may raise questions about search and seizure rights, requiring law enforcement to obtain proper warrants or consent before conducting tests. This balance between investigative needs and individual privacy rights has been the subject of numerous legal debates and court rulings.
Furthermore, the interpretation of luminol test results requires expert testimony, which is subject to legal scrutiny. Forensic experts must be qualified to testify about the scientific principles behind luminol, its application methods, and the interpretation of results. The credibility and qualifications of these experts can be challenged in court, potentially affecting the weight given to luminol evidence in legal proceedings.
Legal considerations also extend to the proper handling and disposal of luminol, as it is classified as a hazardous material. Forensic teams must adhere to strict safety protocols and environmental regulations when using and disposing of luminol to avoid legal liabilities related to environmental contamination or workplace safety violations.
In conclusion, while luminol remains a powerful tool in forensic investigations, its use is tightly regulated by legal frameworks designed to ensure the integrity of evidence, protect individual rights, and maintain the highest standards of scientific reliability in the criminal justice system.
Environmental Impact Assessment
The environmental impact assessment of luminol's establishment in groundbreaking scientific principles reveals both positive and negative implications. On the positive side, luminol's chemiluminescent properties have enabled significant advancements in forensic science and crime scene investigation, leading to more efficient and accurate detection of blood traces. This has contributed to improved law enforcement capabilities and enhanced public safety without introducing harmful chemicals into the environment.
However, the production and disposal of luminol and its associated reagents raise some environmental concerns. The synthesis of luminol involves several chemical processes that may generate waste products and emissions. While these are typically managed within controlled laboratory environments, improper handling or disposal could potentially lead to localized contamination of soil or water resources.
The use of luminol in field applications, particularly in outdoor crime scenes, introduces the chemical directly into the environment. Although luminol is generally considered to have low toxicity, its long-term effects on ecosystems and biodiversity are not fully understood. There is a need for further research to assess the potential bioaccumulation of luminol and its breakdown products in various environmental compartments.
From a sustainability perspective, the widespread adoption of luminol-based techniques has increased demand for its production. This may lead to increased resource consumption and energy use in manufacturing processes. However, the small quantities typically required for forensic applications mitigate these impacts to some extent.
It is worth noting that the development of luminol and related chemiluminescent compounds has spurred research into more environmentally friendly alternatives. This has led to the exploration of bio-based luminescent materials and greener synthesis methods, which could potentially reduce the environmental footprint of forensic technologies in the future.
In conclusion, while luminol's establishment in scientific principles has brought significant benefits to forensic science and criminal investigations, it is crucial to continue monitoring and mitigating its potential environmental impacts. Balancing the societal benefits with environmental considerations will be key to ensuring the sustainable use of luminol and related technologies in the long term.
However, the production and disposal of luminol and its associated reagents raise some environmental concerns. The synthesis of luminol involves several chemical processes that may generate waste products and emissions. While these are typically managed within controlled laboratory environments, improper handling or disposal could potentially lead to localized contamination of soil or water resources.
The use of luminol in field applications, particularly in outdoor crime scenes, introduces the chemical directly into the environment. Although luminol is generally considered to have low toxicity, its long-term effects on ecosystems and biodiversity are not fully understood. There is a need for further research to assess the potential bioaccumulation of luminol and its breakdown products in various environmental compartments.
From a sustainability perspective, the widespread adoption of luminol-based techniques has increased demand for its production. This may lead to increased resource consumption and energy use in manufacturing processes. However, the small quantities typically required for forensic applications mitigate these impacts to some extent.
It is worth noting that the development of luminol and related chemiluminescent compounds has spurred research into more environmentally friendly alternatives. This has led to the exploration of bio-based luminescent materials and greener synthesis methods, which could potentially reduce the environmental footprint of forensic technologies in the future.
In conclusion, while luminol's establishment in scientific principles has brought significant benefits to forensic science and criminal investigations, it is crucial to continue monitoring and mitigating its potential environmental impacts. Balancing the societal benefits with environmental considerations will be key to ensuring the sustainable use of luminol and related technologies in the long term.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!