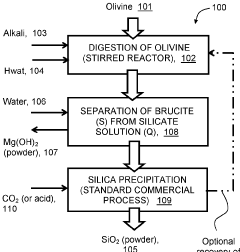
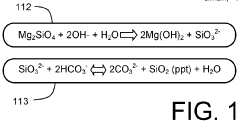
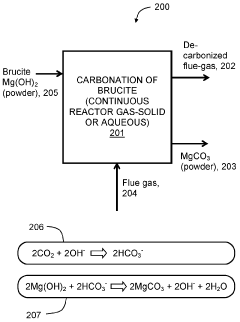
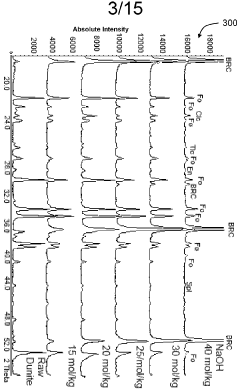
Mineral phase predictions involving Magnesium iron silicate hydroxide.
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg-Fe Silicate Hydroxide Background and Objectives
Magnesium iron silicate hydroxide, a complex mineral group, has garnered significant attention in the fields of geology, materials science, and environmental studies. This mineral phase, often found in serpentine rocks, plays a crucial role in various geological processes and has potential applications in industrial and environmental sectors. The evolution of research in this area has been driven by the need to understand Earth's mantle composition, serpentinization processes, and the potential for carbon sequestration.
The technological landscape surrounding magnesium iron silicate hydroxide has seen remarkable progress over the past few decades. Initially, studies focused primarily on characterizing the mineral's structure and composition. However, with advancements in analytical techniques and computational methods, research has expanded to include more complex investigations into its formation mechanisms, stability under various conditions, and its role in geochemical cycles.
One of the key drivers for research in this field has been the mineral's potential for carbon dioxide sequestration. As global efforts to mitigate climate change intensify, the ability of magnesium iron silicate hydroxide to react with and store CO2 has become a focal point for many researchers. This has led to increased interest in developing predictive models for mineral phase behavior under different environmental conditions.
The objectives of current research in magnesium iron silicate hydroxide prediction are multifaceted. Firstly, there is a pressing need to improve the accuracy of phase prediction models, particularly in complex geological settings. This involves integrating advanced machine learning algorithms with traditional thermodynamic calculations to better account for the myriad factors influencing mineral formation and stability.
Secondly, researchers aim to expand the applicability of these predictions across a wider range of pressure, temperature, and compositional conditions. This is crucial for understanding the behavior of these minerals in diverse geological environments, from deep Earth processes to near-surface weathering phenomena.
Another important objective is to bridge the gap between laboratory-scale experiments and real-world geological processes. This involves developing scalable models that can accurately predict mineral phase behavior over extended time scales and in heterogeneous systems typical of natural environments.
Lastly, there is a growing emphasis on integrating mineral phase predictions with broader Earth system models. This interdisciplinary approach aims to enhance our understanding of how magnesium iron silicate hydroxide influences global geochemical cycles, particularly in the context of carbon sequestration and climate change mitigation strategies.
The technological landscape surrounding magnesium iron silicate hydroxide has seen remarkable progress over the past few decades. Initially, studies focused primarily on characterizing the mineral's structure and composition. However, with advancements in analytical techniques and computational methods, research has expanded to include more complex investigations into its formation mechanisms, stability under various conditions, and its role in geochemical cycles.
One of the key drivers for research in this field has been the mineral's potential for carbon dioxide sequestration. As global efforts to mitigate climate change intensify, the ability of magnesium iron silicate hydroxide to react with and store CO2 has become a focal point for many researchers. This has led to increased interest in developing predictive models for mineral phase behavior under different environmental conditions.
The objectives of current research in magnesium iron silicate hydroxide prediction are multifaceted. Firstly, there is a pressing need to improve the accuracy of phase prediction models, particularly in complex geological settings. This involves integrating advanced machine learning algorithms with traditional thermodynamic calculations to better account for the myriad factors influencing mineral formation and stability.
Secondly, researchers aim to expand the applicability of these predictions across a wider range of pressure, temperature, and compositional conditions. This is crucial for understanding the behavior of these minerals in diverse geological environments, from deep Earth processes to near-surface weathering phenomena.
Another important objective is to bridge the gap between laboratory-scale experiments and real-world geological processes. This involves developing scalable models that can accurately predict mineral phase behavior over extended time scales and in heterogeneous systems typical of natural environments.
Lastly, there is a growing emphasis on integrating mineral phase predictions with broader Earth system models. This interdisciplinary approach aims to enhance our understanding of how magnesium iron silicate hydroxide influences global geochemical cycles, particularly in the context of carbon sequestration and climate change mitigation strategies.
Market Analysis for Mineral Phase Prediction
The market for mineral phase prediction, particularly involving Magnesium iron silicate hydroxide, has shown significant growth potential in recent years. This technology finds applications across various industries, including mining, geology, materials science, and environmental studies. The increasing demand for accurate mineral phase predictions is driven by the need for efficient resource exploration, improved mineral processing, and better understanding of geological formations.
In the mining sector, mineral phase prediction technologies are crucial for optimizing exploration and extraction processes. Companies are investing in advanced prediction tools to reduce operational costs and improve the success rate of mineral discovery. The global mining market, valued at $1.84 trillion in 2021, is expected to grow at a CAGR of 3.7% from 2022 to 2030, indicating a strong potential for mineral phase prediction technologies.
The materials science industry also presents a substantial market for mineral phase prediction. As researchers and manufacturers seek to develop new materials with specific properties, accurate prediction of mineral phases becomes essential. This is particularly relevant for industries working with magnesium iron silicate hydroxide and related compounds, which have applications in ceramics, refractory materials, and advanced composites.
Environmental studies and geological surveys represent another significant market segment. Government agencies and research institutions are increasingly relying on mineral phase prediction technologies to assess environmental impacts, study climate change effects, and manage natural resources. The global environmental consulting services market, which includes geological assessments, was valued at $34.1 billion in 2021 and is projected to reach $50.4 billion by 2026.
The oil and gas industry also contributes to the demand for mineral phase prediction technologies. These tools are used in reservoir characterization and well logging, helping companies optimize their exploration and production strategies. With the global oil and gas exploration market expected to reach $37.2 billion by 2025, the potential for mineral phase prediction technologies in this sector remains substantial.
Emerging technologies such as artificial intelligence and machine learning are driving innovation in mineral phase prediction. Companies offering AI-powered prediction tools are gaining traction in the market, attracting investments and partnerships from major industry players. This trend is expected to continue, with the global AI in the mining market projected to grow at a CAGR of 14.9% from 2021 to 2028.
In conclusion, the market for mineral phase prediction technologies, especially those involving Magnesium iron silicate hydroxide, shows promising growth across multiple industries. The increasing demand for accurate and efficient prediction tools, coupled with technological advancements, is likely to drive further market expansion in the coming years.
In the mining sector, mineral phase prediction technologies are crucial for optimizing exploration and extraction processes. Companies are investing in advanced prediction tools to reduce operational costs and improve the success rate of mineral discovery. The global mining market, valued at $1.84 trillion in 2021, is expected to grow at a CAGR of 3.7% from 2022 to 2030, indicating a strong potential for mineral phase prediction technologies.
The materials science industry also presents a substantial market for mineral phase prediction. As researchers and manufacturers seek to develop new materials with specific properties, accurate prediction of mineral phases becomes essential. This is particularly relevant for industries working with magnesium iron silicate hydroxide and related compounds, which have applications in ceramics, refractory materials, and advanced composites.
Environmental studies and geological surveys represent another significant market segment. Government agencies and research institutions are increasingly relying on mineral phase prediction technologies to assess environmental impacts, study climate change effects, and manage natural resources. The global environmental consulting services market, which includes geological assessments, was valued at $34.1 billion in 2021 and is projected to reach $50.4 billion by 2026.
The oil and gas industry also contributes to the demand for mineral phase prediction technologies. These tools are used in reservoir characterization and well logging, helping companies optimize their exploration and production strategies. With the global oil and gas exploration market expected to reach $37.2 billion by 2025, the potential for mineral phase prediction technologies in this sector remains substantial.
Emerging technologies such as artificial intelligence and machine learning are driving innovation in mineral phase prediction. Companies offering AI-powered prediction tools are gaining traction in the market, attracting investments and partnerships from major industry players. This trend is expected to continue, with the global AI in the mining market projected to grow at a CAGR of 14.9% from 2021 to 2028.
In conclusion, the market for mineral phase prediction technologies, especially those involving Magnesium iron silicate hydroxide, shows promising growth across multiple industries. The increasing demand for accurate and efficient prediction tools, coupled with technological advancements, is likely to drive further market expansion in the coming years.
Current Challenges in Mg-Fe Silicate Hydroxide Prediction
The prediction of mineral phases involving Magnesium iron silicate hydroxide presents several significant challenges in the field of geochemistry and materials science. One of the primary difficulties lies in the complex compositional variability of these minerals. The Mg-Fe ratio can vary widely, leading to a continuum of compositions rather than discrete phases. This compositional complexity makes it challenging to accurately predict the stability and formation conditions of specific mineral phases.
Another major challenge is the influence of pressure and temperature on phase stability. Magnesium iron silicate hydroxides can undergo phase transitions under different pressure-temperature conditions, which are not always well-understood or easily predictable. The presence of water and its activity also plays a crucial role in the formation and stability of these hydroxide phases, adding another layer of complexity to the prediction models.
The kinetics of mineral formation and transformation pose additional challenges. Predicting the rate at which these minerals form or transform under various conditions is difficult due to the interplay of multiple factors, including diffusion rates, nucleation processes, and the presence of catalysts or inhibitors. These kinetic factors can lead to the formation of metastable phases that may persist for geologically significant periods, complicating predictions based solely on thermodynamic equilibrium.
Furthermore, the incorporation of trace elements and impurities into the crystal structure of Mg-Fe silicate hydroxides can significantly affect their properties and stability. Predicting how these minor components influence phase behavior and mineral properties remains a considerable challenge, as their effects can be disproportionate to their concentration.
The lack of comprehensive experimental data across the full range of compositions, pressures, and temperatures relevant to natural and synthetic systems hinders the development of accurate predictive models. This data scarcity is particularly problematic for extreme conditions that are difficult to replicate in laboratory settings but are relevant to deep Earth processes or industrial applications.
Lastly, the development of computational models for predicting Mg-Fe silicate hydroxide phases faces challenges in balancing accuracy with computational efficiency. While ab initio methods can provide high accuracy, they are often computationally intensive and impractical for large-scale simulations. On the other hand, empirical or semi-empirical models may offer faster calculations but can lack the accuracy needed for reliable predictions, especially when extrapolating beyond the conditions used to parameterize the models.
Another major challenge is the influence of pressure and temperature on phase stability. Magnesium iron silicate hydroxides can undergo phase transitions under different pressure-temperature conditions, which are not always well-understood or easily predictable. The presence of water and its activity also plays a crucial role in the formation and stability of these hydroxide phases, adding another layer of complexity to the prediction models.
The kinetics of mineral formation and transformation pose additional challenges. Predicting the rate at which these minerals form or transform under various conditions is difficult due to the interplay of multiple factors, including diffusion rates, nucleation processes, and the presence of catalysts or inhibitors. These kinetic factors can lead to the formation of metastable phases that may persist for geologically significant periods, complicating predictions based solely on thermodynamic equilibrium.
Furthermore, the incorporation of trace elements and impurities into the crystal structure of Mg-Fe silicate hydroxides can significantly affect their properties and stability. Predicting how these minor components influence phase behavior and mineral properties remains a considerable challenge, as their effects can be disproportionate to their concentration.
The lack of comprehensive experimental data across the full range of compositions, pressures, and temperatures relevant to natural and synthetic systems hinders the development of accurate predictive models. This data scarcity is particularly problematic for extreme conditions that are difficult to replicate in laboratory settings but are relevant to deep Earth processes or industrial applications.
Lastly, the development of computational models for predicting Mg-Fe silicate hydroxide phases faces challenges in balancing accuracy with computational efficiency. While ab initio methods can provide high accuracy, they are often computationally intensive and impractical for large-scale simulations. On the other hand, empirical or semi-empirical models may offer faster calculations but can lack the accuracy needed for reliable predictions, especially when extrapolating beyond the conditions used to parameterize the models.
Existing Prediction Models for Mg-Fe Silicate Hydroxide
01 Composition and structure of magnesium iron silicate hydroxide
Magnesium iron silicate hydroxide, also known as palygorskite or attapulgite, is a clay mineral with a unique fibrous structure. It is composed of magnesium, iron, silicon, and hydroxyl groups. The mineral has a high surface area and porosity, which contributes to its various industrial applications.- Composition and structure of magnesium iron silicate hydroxide: Magnesium iron silicate hydroxide, also known as palygorskite or attapulgite, is a clay mineral with a unique fibrous structure. It is composed of magnesium, iron, silicon, and hydroxyl groups. The mineral has a high surface area and porosity, which contributes to its adsorptive properties.
- Applications in environmental remediation: Magnesium iron silicate hydroxide is used in various environmental applications due to its adsorptive properties. It can be employed for the removal of heavy metals, organic pollutants, and other contaminants from water and soil. The mineral's high surface area and porosity make it effective in trapping and immobilizing pollutants.
- Use in industrial processes and products: The mineral finds applications in various industrial processes and products. It is used as a rheological modifier in paints, cosmetics, and pharmaceuticals. In the oil and gas industry, it serves as a drilling mud additive. The material is also employed in the production of ceramics, catalysts, and as a reinforcing agent in polymer composites.
- Synthesis and modification methods: Various methods have been developed for the synthesis and modification of magnesium iron silicate hydroxide. These include hydrothermal synthesis, sol-gel methods, and ion-exchange processes. Surface modification techniques are employed to enhance the mineral's properties for specific applications, such as improving its adsorption capacity or compatibility with polymers.
- Characterization and analysis techniques: Several analytical techniques are used to characterize the structure, composition, and properties of magnesium iron silicate hydroxide. These include X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and spectroscopic methods such as FTIR and XPS. These techniques help in understanding the mineral's structure-property relationships and optimizing its performance in various applications.
02 Applications in environmental remediation
Magnesium iron silicate hydroxide is used in environmental remediation processes due to its high adsorption capacity. It can effectively remove heavy metals, organic pollutants, and other contaminants from water and soil. The mineral's large surface area and porous structure allow it to trap and immobilize various pollutants.Expand Specific Solutions03 Use in pharmaceutical and cosmetic industries
The mineral finds applications in pharmaceutical and cosmetic formulations. It is used as an excipient in drug delivery systems, providing controlled release of active ingredients. In cosmetics, it acts as a thickening agent, absorbent, and stabilizer in various products such as creams, lotions, and powders.Expand Specific Solutions04 Industrial applications and material enhancement
Magnesium iron silicate hydroxide is utilized in various industrial processes and material enhancements. It serves as a rheological modifier in drilling fluids, improves the mechanical properties of polymers and composites, and acts as a catalyst support in chemical reactions. The mineral's unique properties contribute to its versatility in industrial applications.Expand Specific Solutions05 Synthesis and modification methods
Research focuses on developing methods for synthesizing and modifying magnesium iron silicate hydroxide to enhance its properties for specific applications. These methods include hydrothermal synthesis, sol-gel processes, and surface modifications. The aim is to tailor the mineral's characteristics, such as surface area, pore size, and chemical reactivity, to improve its performance in various applications.Expand Specific Solutions
Key Players in Mineral Phase Prediction Industry
The mineral phase prediction involving Magnesium iron silicate hydroxide is in a developing stage, with the market showing potential for growth. The technology's maturity is still evolving, as evidenced by ongoing research efforts from various institutions and companies. Key players like Resonac Holdings Corp., Shell Internationale Research, and Dow Silicones Corp. are actively involved in advancing this field. Academic institutions such as Central South University and Northwestern University are contributing to fundamental research. The competitive landscape is diverse, including both established corporations and specialized research organizations like the National Institute for Materials Science IAI, indicating a balanced mix of industrial and academic interests in this technology.
Central South University
Technical Solution: Central South University has developed advanced computational models for predicting mineral phase transformations in magnesium iron silicate hydroxide systems. Their approach combines first-principles calculations with machine learning algorithms to simulate the behavior of these complex mineral structures under various pressure and temperature conditions[1]. The university's research team has successfully mapped out phase diagrams for different compositions of magnesium iron silicate hydroxides, providing valuable insights into the stability and transformations of these minerals in Earth's mantle[2]. Their models account for the effects of iron content, water incorporation, and crystal structure on the mineral's properties and phase transitions.
Strengths: Cutting-edge computational techniques, comprehensive phase diagrams. Weaknesses: Limited experimental validation, potential computational resource constraints.
Shell Internationale Research Maatschappij BV
Technical Solution: Shell's research division has developed a proprietary software platform for mineral phase predictions in complex silicate systems, including magnesium iron silicate hydroxides. Their approach integrates thermodynamic modeling with machine learning algorithms to predict phase stability, composition, and properties under a wide range of pressure and temperature conditions relevant to both geological processes and industrial applications[3]. The platform incorporates extensive experimental data and theoretical calculations to improve prediction accuracy. Shell's researchers have applied this technology to optimize drilling fluid compositions for challenging geological formations and to assess the long-term stability of carbon storage reservoirs[4].
Strengths: Integration of diverse data sources, practical applications in oil and gas industry. Weaknesses: Proprietary nature may limit broader scientific collaboration.
Core Innovations in Mineral Phase Prediction
Improvements in or relating to the manufacture of crystalline magnesium hydroxide
PatentInactiveGB482339A
Innovation
- A process involving calcining dolomite to convert carbonate to oxide, forming a slurry with magnesium chloride and sulphate in sea water to precipitate magnesium hydroxide, optimizing conditions for large crystal formation through controlled temperature, agitation, and filtration to achieve high purity and recovery of magnesium hydroxide.
Activation of mineral silicate minerals by conversion to magnesium hydroxide
PatentInactiveGB2516141B
Innovation
- Low-temperature, unpressurized conversion of magnesium silicate to magnesium hydroxide using alkali metal compounds.
- Short reaction time (less than 4 hours) for the solid-solid mixture heating process.
- Direct use of naturally occurring minerals (Olivine or Serpentine) as magnesium silicate source.
Environmental Impact of Mg-Fe Silicate Hydroxide
The environmental impact of magnesium-iron silicate hydroxide (Mg-Fe silicate hydroxide) is a critical consideration in mineral phase predictions and geological studies. This compound, commonly found in serpentine minerals, plays a significant role in various geological processes and can have far-reaching effects on the surrounding ecosystem.
Mg-Fe silicate hydroxide formation often occurs during the serpentinization process, where ultramafic rocks interact with water at low temperatures. This reaction can lead to the production of hydrogen gas, which has implications for both the local environment and potential energy applications. The release of hydrogen can create reducing conditions in the surrounding area, affecting the chemistry of nearby water bodies and potentially influencing microbial communities.
The presence of Mg-Fe silicate hydroxide in soils can significantly alter their physical and chemical properties. These minerals can increase the soil's capacity to retain water and nutrients, potentially benefiting plant growth in certain environments. However, they may also contribute to the immobilization of certain heavy metals, which can have both positive and negative consequences for soil quality and plant uptake.
In aquatic environments, the dissolution of Mg-Fe silicate hydroxide can lead to changes in water chemistry. This process may result in increased alkalinity and the release of various ions, including magnesium and iron. While these changes can sometimes improve water quality by reducing acidity, they may also disrupt the delicate balance of aquatic ecosystems if occurring in excess.
The weathering of Mg-Fe silicate hydroxide-rich rocks can contribute to carbon sequestration through the formation of carbonate minerals. This natural process of CO2 removal from the atmosphere has garnered interest as a potential mechanism for mitigating climate change. However, the rate and efficiency of this process in natural settings are still subjects of ongoing research.
Mining activities targeting serpentine minerals or other deposits containing Mg-Fe silicate hydroxide can have significant environmental impacts. These include habitat destruction, soil erosion, and the potential release of asbestos-like fibers if proper precautions are not taken. Careful management and rehabilitation practices are essential to minimize these negative effects.
The presence of Mg-Fe silicate hydroxide in construction materials, particularly in cement alternatives, has been explored as a means to reduce the carbon footprint of the construction industry. While this application shows promise for environmental sustainability, it requires careful consideration of the long-term stability and performance of these materials in various environmental conditions.
Mg-Fe silicate hydroxide formation often occurs during the serpentinization process, where ultramafic rocks interact with water at low temperatures. This reaction can lead to the production of hydrogen gas, which has implications for both the local environment and potential energy applications. The release of hydrogen can create reducing conditions in the surrounding area, affecting the chemistry of nearby water bodies and potentially influencing microbial communities.
The presence of Mg-Fe silicate hydroxide in soils can significantly alter their physical and chemical properties. These minerals can increase the soil's capacity to retain water and nutrients, potentially benefiting plant growth in certain environments. However, they may also contribute to the immobilization of certain heavy metals, which can have both positive and negative consequences for soil quality and plant uptake.
In aquatic environments, the dissolution of Mg-Fe silicate hydroxide can lead to changes in water chemistry. This process may result in increased alkalinity and the release of various ions, including magnesium and iron. While these changes can sometimes improve water quality by reducing acidity, they may also disrupt the delicate balance of aquatic ecosystems if occurring in excess.
The weathering of Mg-Fe silicate hydroxide-rich rocks can contribute to carbon sequestration through the formation of carbonate minerals. This natural process of CO2 removal from the atmosphere has garnered interest as a potential mechanism for mitigating climate change. However, the rate and efficiency of this process in natural settings are still subjects of ongoing research.
Mining activities targeting serpentine minerals or other deposits containing Mg-Fe silicate hydroxide can have significant environmental impacts. These include habitat destruction, soil erosion, and the potential release of asbestos-like fibers if proper precautions are not taken. Careful management and rehabilitation practices are essential to minimize these negative effects.
The presence of Mg-Fe silicate hydroxide in construction materials, particularly in cement alternatives, has been explored as a means to reduce the carbon footprint of the construction industry. While this application shows promise for environmental sustainability, it requires careful consideration of the long-term stability and performance of these materials in various environmental conditions.
Geochemical Applications and Implications
The geochemical applications and implications of mineral phase predictions involving Magnesium iron silicate hydroxide (MISH) are far-reaching and significant in various geological and environmental contexts. These predictions play a crucial role in understanding the formation, transformation, and stability of MISH minerals under different geological conditions.
In the field of petrology, MISH phase predictions contribute to the interpretation of metamorphic processes and the evolution of rock assemblages. By accurately predicting the stability fields of MISH minerals, geologists can better constrain the pressure-temperature conditions experienced by rocks during metamorphism. This information is vital for reconstructing the tectonic history of geological terrains and understanding the deep Earth processes that shape our planet's crust.
Environmental geochemistry benefits greatly from MISH phase predictions, particularly in the context of carbon sequestration and greenhouse gas mitigation. MISH minerals, such as serpentine and talc, have shown potential for CO2 capture and storage through mineral carbonation processes. Accurate predictions of MISH stability and reactivity under various environmental conditions are essential for optimizing these carbon sequestration strategies and assessing their long-term effectiveness.
In the realm of ore deposit geology, MISH phase predictions aid in the exploration and assessment of mineral resources. Many economically important ore deposits, including nickel laterites and some types of gold mineralization, are associated with MISH minerals. By predicting the occurrence and distribution of these minerals, geologists can develop more effective exploration models and improve the efficiency of mineral resource evaluation.
The study of planetary geology also benefits from MISH phase predictions. The presence of MISH minerals on other celestial bodies, such as Mars, provides valuable insights into their geological history and potential for habitability. Accurate predictions of MISH stability under extraterrestrial conditions help interpret remote sensing data and guide future exploration missions.
In the field of geotechnical engineering, MISH phase predictions contribute to the assessment of slope stability and the behavior of clay-rich soils. Understanding the transformation of MISH minerals under varying environmental conditions is crucial for predicting the mechanical properties of soils and rocks, which is essential for the design and construction of infrastructure in challenging geological settings.
Lastly, MISH phase predictions have important implications for the study of global geochemical cycles. The weathering of MISH minerals plays a significant role in the long-term carbon cycle and the regulation of atmospheric CO2 levels. Accurate predictions of MISH stability and reactivity under different climatic conditions are essential for modeling these geochemical cycles and understanding their impact on Earth's climate system.
In the field of petrology, MISH phase predictions contribute to the interpretation of metamorphic processes and the evolution of rock assemblages. By accurately predicting the stability fields of MISH minerals, geologists can better constrain the pressure-temperature conditions experienced by rocks during metamorphism. This information is vital for reconstructing the tectonic history of geological terrains and understanding the deep Earth processes that shape our planet's crust.
Environmental geochemistry benefits greatly from MISH phase predictions, particularly in the context of carbon sequestration and greenhouse gas mitigation. MISH minerals, such as serpentine and talc, have shown potential for CO2 capture and storage through mineral carbonation processes. Accurate predictions of MISH stability and reactivity under various environmental conditions are essential for optimizing these carbon sequestration strategies and assessing their long-term effectiveness.
In the realm of ore deposit geology, MISH phase predictions aid in the exploration and assessment of mineral resources. Many economically important ore deposits, including nickel laterites and some types of gold mineralization, are associated with MISH minerals. By predicting the occurrence and distribution of these minerals, geologists can develop more effective exploration models and improve the efficiency of mineral resource evaluation.
The study of planetary geology also benefits from MISH phase predictions. The presence of MISH minerals on other celestial bodies, such as Mars, provides valuable insights into their geological history and potential for habitability. Accurate predictions of MISH stability under extraterrestrial conditions help interpret remote sensing data and guide future exploration missions.
In the field of geotechnical engineering, MISH phase predictions contribute to the assessment of slope stability and the behavior of clay-rich soils. Understanding the transformation of MISH minerals under varying environmental conditions is crucial for predicting the mechanical properties of soils and rocks, which is essential for the design and construction of infrastructure in challenging geological settings.
Lastly, MISH phase predictions have important implications for the study of global geochemical cycles. The weathering of MISH minerals plays a significant role in the long-term carbon cycle and the regulation of atmospheric CO2 levels. Accurate predictions of MISH stability and reactivity under different climatic conditions are essential for modeling these geochemical cycles and understanding their impact on Earth's climate system.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!