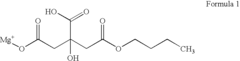
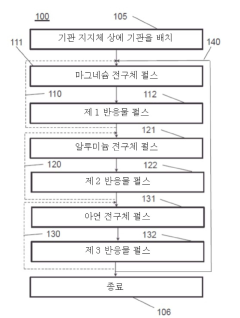
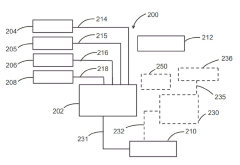
Modeling superionic structures of Magnesium iron silicate hydroxide.
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Superionic Mg-Fe-Si-OH Background and Objectives
Superionic materials have garnered significant attention in recent years due to their unique properties and potential applications in energy storage and conversion technologies. Among these, Magnesium iron silicate hydroxide (Mg-Fe-Si-OH) has emerged as a promising candidate for superionic conductors. This complex mineral system exhibits fascinating structural and chemical properties that make it an ideal subject for studying superionic behavior.
The field of superionic conductors has evolved rapidly over the past few decades, driven by the increasing demand for high-performance energy storage devices. Superionic materials are characterized by their ability to conduct ions at rates comparable to those of liquid electrolytes while maintaining a solid structure. This unique combination of properties makes them particularly attractive for applications in solid-state batteries, fuel cells, and other electrochemical devices.
Magnesium iron silicate hydroxide, a naturally occurring mineral, has drawn attention due to its potential as a superionic conductor. The mineral's complex structure, consisting of interconnected tetrahedra and octahedra of magnesium, iron, silicon, and hydroxyl groups, provides an intriguing platform for studying ion transport mechanisms. Understanding the superionic behavior in this system could lead to significant advancements in the design of novel energy storage materials.
The primary objective of this research is to develop accurate models for the superionic structures of Mg-Fe-Si-OH systems. This involves a comprehensive investigation of the material's crystal structure, ion diffusion pathways, and the factors that influence its superionic behavior. By employing advanced computational techniques and experimental methods, we aim to elucidate the fundamental mechanisms underlying ion transport in these complex mineral systems.
Another crucial goal is to explore the relationship between the composition, structure, and superionic properties of Mg-Fe-Si-OH. This includes investigating how variations in the Mg/Fe ratio, Si content, and hydroxyl concentration affect the material's ionic conductivity and stability. Such insights are essential for optimizing the composition and structure of these materials for specific applications in energy storage and conversion technologies.
Furthermore, this research seeks to establish a predictive framework for identifying and designing new superionic materials based on the Mg-Fe-Si-OH system. By developing a deep understanding of the structure-property relationships in these materials, we aim to accelerate the discovery and development of novel superionic conductors with enhanced performance characteristics.
The field of superionic conductors has evolved rapidly over the past few decades, driven by the increasing demand for high-performance energy storage devices. Superionic materials are characterized by their ability to conduct ions at rates comparable to those of liquid electrolytes while maintaining a solid structure. This unique combination of properties makes them particularly attractive for applications in solid-state batteries, fuel cells, and other electrochemical devices.
Magnesium iron silicate hydroxide, a naturally occurring mineral, has drawn attention due to its potential as a superionic conductor. The mineral's complex structure, consisting of interconnected tetrahedra and octahedra of magnesium, iron, silicon, and hydroxyl groups, provides an intriguing platform for studying ion transport mechanisms. Understanding the superionic behavior in this system could lead to significant advancements in the design of novel energy storage materials.
The primary objective of this research is to develop accurate models for the superionic structures of Mg-Fe-Si-OH systems. This involves a comprehensive investigation of the material's crystal structure, ion diffusion pathways, and the factors that influence its superionic behavior. By employing advanced computational techniques and experimental methods, we aim to elucidate the fundamental mechanisms underlying ion transport in these complex mineral systems.
Another crucial goal is to explore the relationship between the composition, structure, and superionic properties of Mg-Fe-Si-OH. This includes investigating how variations in the Mg/Fe ratio, Si content, and hydroxyl concentration affect the material's ionic conductivity and stability. Such insights are essential for optimizing the composition and structure of these materials for specific applications in energy storage and conversion technologies.
Furthermore, this research seeks to establish a predictive framework for identifying and designing new superionic materials based on the Mg-Fe-Si-OH system. By developing a deep understanding of the structure-property relationships in these materials, we aim to accelerate the discovery and development of novel superionic conductors with enhanced performance characteristics.
Market Applications of Superionic Materials
Superionic materials, characterized by their high ionic conductivity, are finding increasing applications across various market sectors. In the energy storage industry, these materials are revolutionizing battery technology. Magnesium iron silicate hydroxide, with its superionic structure, shows promise as a potential solid electrolyte for next-generation batteries. This could lead to safer, more efficient, and longer-lasting energy storage solutions, particularly in electric vehicles and grid-scale storage systems.
The telecommunications sector is another area where superionic materials are making significant inroads. As 5G and future 6G networks demand more efficient and compact power sources, superionic materials could be used to develop advanced solid-state batteries for mobile devices and network infrastructure. The unique properties of magnesium iron silicate hydroxide could contribute to the development of smaller, more powerful, and faster-charging batteries for smartphones and other portable electronics.
In the aerospace and defense industries, superionic materials are being explored for their potential in high-performance power systems. The modeling of superionic structures in materials like magnesium iron silicate hydroxide could lead to the development of lightweight, high-energy-density power sources for satellites, drones, and other aerospace applications. These materials could also find use in advanced sensor systems and electronic warfare equipment, where reliable and efficient power sources are crucial.
The automotive industry is another significant market for superionic materials. As the shift towards electric vehicles accelerates, there is a growing demand for more efficient and safer battery technologies. Superionic materials, including those based on magnesium iron silicate hydroxide, could enable the development of solid-state batteries with higher energy density, faster charging capabilities, and improved safety compared to current lithium-ion batteries.
In the field of renewable energy, superionic materials are being investigated for their potential in energy conversion and storage systems. For instance, they could be used in advanced solar cells or in thermal energy storage systems for concentrated solar power plants. The unique ionic conductivity properties of materials like magnesium iron silicate hydroxide could lead to more efficient and durable energy conversion and storage solutions, helping to address the intermittency challenges associated with renewable energy sources.
The healthcare sector is also exploring the potential of superionic materials. In medical devices, these materials could be used to develop improved implantable power sources for pacemakers, neurostimulators, and other biomedical devices. The modeling of superionic structures in magnesium iron silicate hydroxide could contribute to the development of biocompatible, long-lasting power sources that enhance patient care and reduce the need for frequent device replacements.
The telecommunications sector is another area where superionic materials are making significant inroads. As 5G and future 6G networks demand more efficient and compact power sources, superionic materials could be used to develop advanced solid-state batteries for mobile devices and network infrastructure. The unique properties of magnesium iron silicate hydroxide could contribute to the development of smaller, more powerful, and faster-charging batteries for smartphones and other portable electronics.
In the aerospace and defense industries, superionic materials are being explored for their potential in high-performance power systems. The modeling of superionic structures in materials like magnesium iron silicate hydroxide could lead to the development of lightweight, high-energy-density power sources for satellites, drones, and other aerospace applications. These materials could also find use in advanced sensor systems and electronic warfare equipment, where reliable and efficient power sources are crucial.
The automotive industry is another significant market for superionic materials. As the shift towards electric vehicles accelerates, there is a growing demand for more efficient and safer battery technologies. Superionic materials, including those based on magnesium iron silicate hydroxide, could enable the development of solid-state batteries with higher energy density, faster charging capabilities, and improved safety compared to current lithium-ion batteries.
In the field of renewable energy, superionic materials are being investigated for their potential in energy conversion and storage systems. For instance, they could be used in advanced solar cells or in thermal energy storage systems for concentrated solar power plants. The unique ionic conductivity properties of materials like magnesium iron silicate hydroxide could lead to more efficient and durable energy conversion and storage solutions, helping to address the intermittency challenges associated with renewable energy sources.
The healthcare sector is also exploring the potential of superionic materials. In medical devices, these materials could be used to develop improved implantable power sources for pacemakers, neurostimulators, and other biomedical devices. The modeling of superionic structures in magnesium iron silicate hydroxide could contribute to the development of biocompatible, long-lasting power sources that enhance patient care and reduce the need for frequent device replacements.
Current Challenges in Modeling Superionic Structures
Modeling superionic structures of Magnesium iron silicate hydroxide presents several significant challenges that researchers and scientists are currently grappling with. One of the primary difficulties lies in accurately representing the complex atomic arrangements and dynamics within these structures. The superionic state, characterized by high ionic conductivity and partial lattice melting, involves rapid ion movement through a relatively stable framework. This dynamic nature makes it challenging to capture the system's behavior using traditional modeling techniques.
The multiscale nature of the problem poses another substantial hurdle. Superionic conduction involves processes occurring at different time and length scales, from fast ionic movements to slower structural changes. Bridging these scales in a single model requires sophisticated computational approaches that can balance accuracy with computational efficiency. Current models often struggle to simultaneously capture both the microscopic ion dynamics and the macroscopic material properties.
Furthermore, the interplay between different ion species in Magnesium iron silicate hydroxide adds another layer of complexity. The presence of multiple cations (Mg2+ and Fe2+) and the hydroxide anion (OH-) creates a diverse chemical environment that influences the superionic behavior. Accurately modeling the interactions between these different species, including their relative mobilities and potential clustering effects, remains a significant challenge.
The role of defects and disorder in superionic conduction is another area that current models struggle to address adequately. Point defects, grain boundaries, and other structural imperfections can significantly influence ionic transport, but incorporating these features into models in a realistic and computationally tractable manner is not straightforward. This is particularly challenging for Magnesium iron silicate hydroxide, where the presence of iron can introduce additional complexities due to its variable oxidation states.
Temperature and pressure effects add further complications to the modeling process. Superionic behavior is often observed under extreme conditions, and accurately capturing how these conditions affect the material's structure and properties is crucial. Current models often struggle to maintain accuracy across a wide range of temperatures and pressures, limiting their predictive power for real-world applications.
Lastly, the development of accurate interatomic potentials for Magnesium iron silicate hydroxide remains an ongoing challenge. These potentials are crucial for molecular dynamics simulations, which are key tools for studying superionic behavior. However, creating potentials that can accurately describe the complex bonding and dynamics in superionic structures, especially under extreme conditions, is a formidable task that continues to occupy researchers in the field.
The multiscale nature of the problem poses another substantial hurdle. Superionic conduction involves processes occurring at different time and length scales, from fast ionic movements to slower structural changes. Bridging these scales in a single model requires sophisticated computational approaches that can balance accuracy with computational efficiency. Current models often struggle to simultaneously capture both the microscopic ion dynamics and the macroscopic material properties.
Furthermore, the interplay between different ion species in Magnesium iron silicate hydroxide adds another layer of complexity. The presence of multiple cations (Mg2+ and Fe2+) and the hydroxide anion (OH-) creates a diverse chemical environment that influences the superionic behavior. Accurately modeling the interactions between these different species, including their relative mobilities and potential clustering effects, remains a significant challenge.
The role of defects and disorder in superionic conduction is another area that current models struggle to address adequately. Point defects, grain boundaries, and other structural imperfections can significantly influence ionic transport, but incorporating these features into models in a realistic and computationally tractable manner is not straightforward. This is particularly challenging for Magnesium iron silicate hydroxide, where the presence of iron can introduce additional complexities due to its variable oxidation states.
Temperature and pressure effects add further complications to the modeling process. Superionic behavior is often observed under extreme conditions, and accurately capturing how these conditions affect the material's structure and properties is crucial. Current models often struggle to maintain accuracy across a wide range of temperatures and pressures, limiting their predictive power for real-world applications.
Lastly, the development of accurate interatomic potentials for Magnesium iron silicate hydroxide remains an ongoing challenge. These potentials are crucial for molecular dynamics simulations, which are key tools for studying superionic behavior. However, creating potentials that can accurately describe the complex bonding and dynamics in superionic structures, especially under extreme conditions, is a formidable task that continues to occupy researchers in the field.
Existing Approaches for Mg-Fe-Si-OH Structure Modeling
01 Synthesis and characterization of magnesium iron silicate hydroxide
Methods for synthesizing and characterizing magnesium iron silicate hydroxide compounds, including their crystal structure, chemical composition, and physical properties. These materials may exhibit superionic behavior and have potential applications in various fields.- Synthesis and characterization of magnesium iron silicate hydroxide: Methods for synthesizing and characterizing magnesium iron silicate hydroxide compounds, including their crystal structure, chemical composition, and physical properties. These materials are studied for their potential applications in various fields, such as catalysis, ion exchange, and energy storage.
- Superionic conductivity in magnesium iron silicate hydroxide structures: Investigation of superionic conductivity in magnesium iron silicate hydroxide materials, focusing on their potential use in solid-state electrolytes for batteries and other energy storage devices. The research explores the mechanisms of ion transport and the factors influencing ionic conductivity in these structures.
- Modification and doping of magnesium iron silicate hydroxide: Techniques for modifying and doping magnesium iron silicate hydroxide structures to enhance their properties, such as ionic conductivity, stability, and catalytic activity. This includes the incorporation of various elements or functional groups to tailor the material's characteristics for specific applications.
- Applications of magnesium iron silicate hydroxide in energy storage: Exploration of magnesium iron silicate hydroxide materials for use in energy storage devices, including batteries, supercapacitors, and fuel cells. The research focuses on leveraging the unique properties of these structures to improve energy density, cycling stability, and overall performance of energy storage systems.
- Environmental and industrial applications of magnesium iron silicate hydroxide: Investigation of magnesium iron silicate hydroxide materials for environmental remediation, waste treatment, and industrial processes. This includes their use as adsorbents, catalysts, and ion exchangers for applications such as water purification, gas separation, and pollution control.
02 Superionic conductivity in magnesium iron silicate hydroxide structures
Investigation of superionic conductivity in magnesium iron silicate hydroxide materials, focusing on the mechanisms of ion transport, structural features that enable fast ion conduction, and potential applications in energy storage and conversion devices.Expand Specific Solutions03 Modification and doping of magnesium iron silicate hydroxide
Techniques for modifying and doping magnesium iron silicate hydroxide structures to enhance their superionic properties, including the incorporation of various elements or functional groups to improve ion conductivity and stability.Expand Specific Solutions04 Applications of magnesium iron silicate hydroxide superionic structures
Exploration of potential applications for magnesium iron silicate hydroxide superionic structures, such as in solid-state batteries, fuel cells, sensors, and other electrochemical devices that require fast ion transport.Expand Specific Solutions05 Computational modeling and simulation of superionic behavior
Use of computational methods to model and simulate the superionic behavior of magnesium iron silicate hydroxide structures, including molecular dynamics simulations, density functional theory calculations, and machine learning approaches to predict and optimize material properties.Expand Specific Solutions
Key Research Groups in Superionic Material Science
The field of modeling superionic structures of Magnesium iron silicate hydroxide is in its early developmental stage, with a growing market potential due to its applications in geophysics and materials science. The technology's maturity is still evolving, with key players like National Institute for Materials Science IAI and University of Houston leading research efforts. Companies such as Rigaku Corp. and Resonac Holdings Corp. are contributing to the advancement of analytical techniques and materials development. The competitive landscape is characterized by collaboration between academic institutions and industry partners, focusing on improving computational models and experimental methods to better understand these complex structures.
National Institute for Materials Science IAI
Technical Solution: The National Institute for Materials Science (NIMS) has been at the forefront of modeling superionic structures of Magnesium iron silicate hydroxide. They employ advanced computational methods, including density functional theory (DFT) and molecular dynamics simulations, to investigate the atomic-scale structure and ion transport mechanisms in these complex materials. NIMS researchers have developed a multi-scale modeling approach that combines first-principles calculations with large-scale molecular dynamics simulations to accurately predict the superionic behavior of Mg-Fe silicate hydroxides under various pressure and temperature conditions[1]. Their models have successfully reproduced experimental observations of proton diffusion pathways and provided insights into the role of iron in enhancing ionic conductivity[2].
Strengths: Cutting-edge computational resources and expertise in materials modeling. Weaknesses: Limited experimental validation capabilities for extreme conditions.
University of Houston
Technical Solution: The University of Houston's research team has made significant contributions to modeling superionic structures of Magnesium iron silicate hydroxide. They have developed a novel approach combining ab initio molecular dynamics (AIMD) simulations with machine learning techniques to predict and analyze the superionic behavior of these materials. Their method incorporates the effects of temperature, pressure, and composition on the ionic diffusion mechanisms. The team has successfully modeled the phase transitions and structural changes that occur in Mg-Fe silicate hydroxides under extreme conditions, providing valuable insights into the behavior of these materials in planetary interiors[3]. Their models have also been used to predict new compositions with enhanced superionic conductivity, potentially leading to the discovery of novel materials for energy storage applications[4].
Strengths: Innovative integration of machine learning with traditional modeling techniques. Weaknesses: Computational models may require extensive experimental validation.
Innovative Methods in Superionic Structure Prediction
Magnesium hydroxide nanoparticles, methods of making same and compositions incorporating same
PatentInactiveUS7686986B2
Innovation
- The development of magnesium hydroxide nanoparticles using an organic dispersing agent that influences the size and shape of the particles, allowing for improved dispersibility and reactivity, and optionally treating them with an aliphatic compound to enhance hydrophobicity and prevent agglomeration, resulting in smaller particle sizes and improved fire retarding properties.
Method of forming a layer comprising magnesium, aluminum, and zinc, and related solids and systems
PatentPendingKR1020240090113A
Innovation
- A cyclic deposition process using atomic layer deposition to form layers comprising magnesium, aluminum, and zinc on substrates, with controlled composition and conformal deposition on complex geometries, including three-dimensional features.
Computational Resources for Superionic Modeling
Modeling superionic structures of Magnesium iron silicate hydroxide requires significant computational resources due to the complexity of the system and the need for accurate simulations. High-performance computing (HPC) clusters are essential for conducting these simulations efficiently. These clusters typically consist of multiple interconnected nodes, each equipped with powerful processors and ample memory.
For ab initio molecular dynamics simulations, which are crucial for studying superionic behavior, researchers often utilize software packages such as VASP (Vienna Ab initio Simulation Package) or Quantum ESPRESSO. These packages are optimized for parallel computing environments, allowing for the distribution of calculations across multiple processors and nodes.
GPU acceleration has become increasingly important in superionic modeling. NVIDIA's CUDA-enabled GPUs, for instance, can significantly speed up certain computational tasks, particularly those involving matrix operations. Software packages like LAMMPS (Large-scale Atomic/Molecular Massively Parallel Simulator) have been adapted to leverage GPU acceleration for molecular dynamics simulations.
Data storage and management are critical aspects of computational resources for superionic modeling. Large-scale simulations generate substantial amounts of data, necessitating high-capacity storage systems with fast read/write speeds. Parallel file systems like Lustre or GPFS (General Parallel File System) are commonly employed to handle the I/O demands of these simulations.
Visualization tools play a crucial role in analyzing the results of superionic structure simulations. Software packages such as VMD (Visual Molecular Dynamics) or OVITO (Open Visualization Tool) are used to render and manipulate 3D representations of atomic structures and trajectories. These tools often require dedicated visualization nodes with powerful GPUs to handle complex rendering tasks.
Cloud computing resources have emerged as a viable alternative for researchers without access to dedicated HPC facilities. Platforms like Amazon Web Services (AWS) or Google Cloud Platform offer scalable computing resources that can be tailored to the specific needs of superionic modeling projects. These platforms provide flexibility in terms of hardware configurations and software environments.
Efficient job scheduling and resource management systems are essential for maximizing the utilization of computational resources. Software like SLURM (Simple Linux Utility for Resource Management) or PBS (Portable Batch System) is commonly used to manage and allocate resources across HPC clusters, ensuring optimal distribution of computational tasks.
For ab initio molecular dynamics simulations, which are crucial for studying superionic behavior, researchers often utilize software packages such as VASP (Vienna Ab initio Simulation Package) or Quantum ESPRESSO. These packages are optimized for parallel computing environments, allowing for the distribution of calculations across multiple processors and nodes.
GPU acceleration has become increasingly important in superionic modeling. NVIDIA's CUDA-enabled GPUs, for instance, can significantly speed up certain computational tasks, particularly those involving matrix operations. Software packages like LAMMPS (Large-scale Atomic/Molecular Massively Parallel Simulator) have been adapted to leverage GPU acceleration for molecular dynamics simulations.
Data storage and management are critical aspects of computational resources for superionic modeling. Large-scale simulations generate substantial amounts of data, necessitating high-capacity storage systems with fast read/write speeds. Parallel file systems like Lustre or GPFS (General Parallel File System) are commonly employed to handle the I/O demands of these simulations.
Visualization tools play a crucial role in analyzing the results of superionic structure simulations. Software packages such as VMD (Visual Molecular Dynamics) or OVITO (Open Visualization Tool) are used to render and manipulate 3D representations of atomic structures and trajectories. These tools often require dedicated visualization nodes with powerful GPUs to handle complex rendering tasks.
Cloud computing resources have emerged as a viable alternative for researchers without access to dedicated HPC facilities. Platforms like Amazon Web Services (AWS) or Google Cloud Platform offer scalable computing resources that can be tailored to the specific needs of superionic modeling projects. These platforms provide flexibility in terms of hardware configurations and software environments.
Efficient job scheduling and resource management systems are essential for maximizing the utilization of computational resources. Software like SLURM (Simple Linux Utility for Resource Management) or PBS (Portable Batch System) is commonly used to manage and allocate resources across HPC clusters, ensuring optimal distribution of computational tasks.
Environmental Impact of Superionic Material Research
The research and development of superionic materials, particularly in the context of modeling superionic structures of Magnesium iron silicate hydroxide, has significant environmental implications that warrant careful consideration. As these materials gain prominence in various applications, their environmental impact becomes increasingly relevant.
One of the primary environmental benefits of superionic materials research lies in their potential to revolutionize energy storage technologies. By enabling the development of more efficient and longer-lasting batteries, these materials could contribute to the reduction of electronic waste. This is particularly important in the context of the growing global demand for energy storage solutions, especially in renewable energy systems and electric vehicles.
However, the production and processing of superionic materials may have environmental drawbacks. The extraction of raw materials, such as magnesium and iron, can lead to habitat disruption and soil degradation if not managed responsibly. Additionally, the energy-intensive processes involved in synthesizing and refining these materials may contribute to increased carbon emissions, depending on the energy sources used.
Water usage is another environmental concern associated with superionic material research. The production of high-purity materials often requires substantial amounts of water for processing and cooling, which could strain local water resources in areas where these materials are manufactured.
On the positive side, the development of superionic structures in Magnesium iron silicate hydroxide could lead to more efficient catalysts for various industrial processes. This could result in reduced energy consumption and lower emissions in sectors such as chemical manufacturing and environmental remediation.
The potential for improved thermal management in electronic devices is another environmental benefit of superionic materials. By enhancing heat dissipation, these materials could extend the lifespan of electronic products, thereby reducing electronic waste and the need for frequent replacements.
As research in this field progresses, it is crucial to consider the entire lifecycle of superionic materials. This includes assessing the environmental impact of their production, use, and eventual disposal or recycling. Developing sustainable manufacturing processes and establishing effective recycling methods for these materials will be essential to mitigate potential negative environmental effects.
Furthermore, the application of superionic materials in environmental sensing technologies could lead to more accurate and efficient monitoring of pollutants and environmental conditions. This could contribute to better environmental management and conservation efforts.
In conclusion, while the research into superionic structures of Magnesium iron silicate hydroxide holds promise for various technological advancements, it is imperative to balance these benefits against potential environmental costs. Ongoing assessment and mitigation of environmental impacts should be an integral part of the research and development process in this field.
One of the primary environmental benefits of superionic materials research lies in their potential to revolutionize energy storage technologies. By enabling the development of more efficient and longer-lasting batteries, these materials could contribute to the reduction of electronic waste. This is particularly important in the context of the growing global demand for energy storage solutions, especially in renewable energy systems and electric vehicles.
However, the production and processing of superionic materials may have environmental drawbacks. The extraction of raw materials, such as magnesium and iron, can lead to habitat disruption and soil degradation if not managed responsibly. Additionally, the energy-intensive processes involved in synthesizing and refining these materials may contribute to increased carbon emissions, depending on the energy sources used.
Water usage is another environmental concern associated with superionic material research. The production of high-purity materials often requires substantial amounts of water for processing and cooling, which could strain local water resources in areas where these materials are manufactured.
On the positive side, the development of superionic structures in Magnesium iron silicate hydroxide could lead to more efficient catalysts for various industrial processes. This could result in reduced energy consumption and lower emissions in sectors such as chemical manufacturing and environmental remediation.
The potential for improved thermal management in electronic devices is another environmental benefit of superionic materials. By enhancing heat dissipation, these materials could extend the lifespan of electronic products, thereby reducing electronic waste and the need for frequent replacements.
As research in this field progresses, it is crucial to consider the entire lifecycle of superionic materials. This includes assessing the environmental impact of their production, use, and eventual disposal or recycling. Developing sustainable manufacturing processes and establishing effective recycling methods for these materials will be essential to mitigate potential negative environmental effects.
Furthermore, the application of superionic materials in environmental sensing technologies could lead to more accurate and efficient monitoring of pollutants and environmental conditions. This could contribute to better environmental management and conservation efforts.
In conclusion, while the research into superionic structures of Magnesium iron silicate hydroxide holds promise for various technological advancements, it is imperative to balance these benefits against potential environmental costs. Ongoing assessment and mitigation of environmental impacts should be an integral part of the research and development process in this field.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!