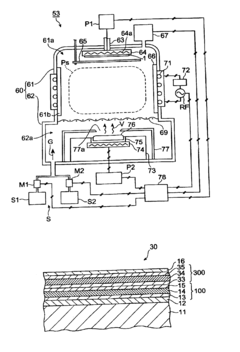
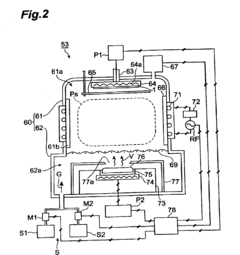
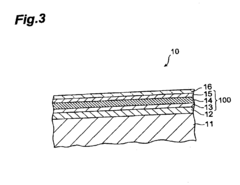
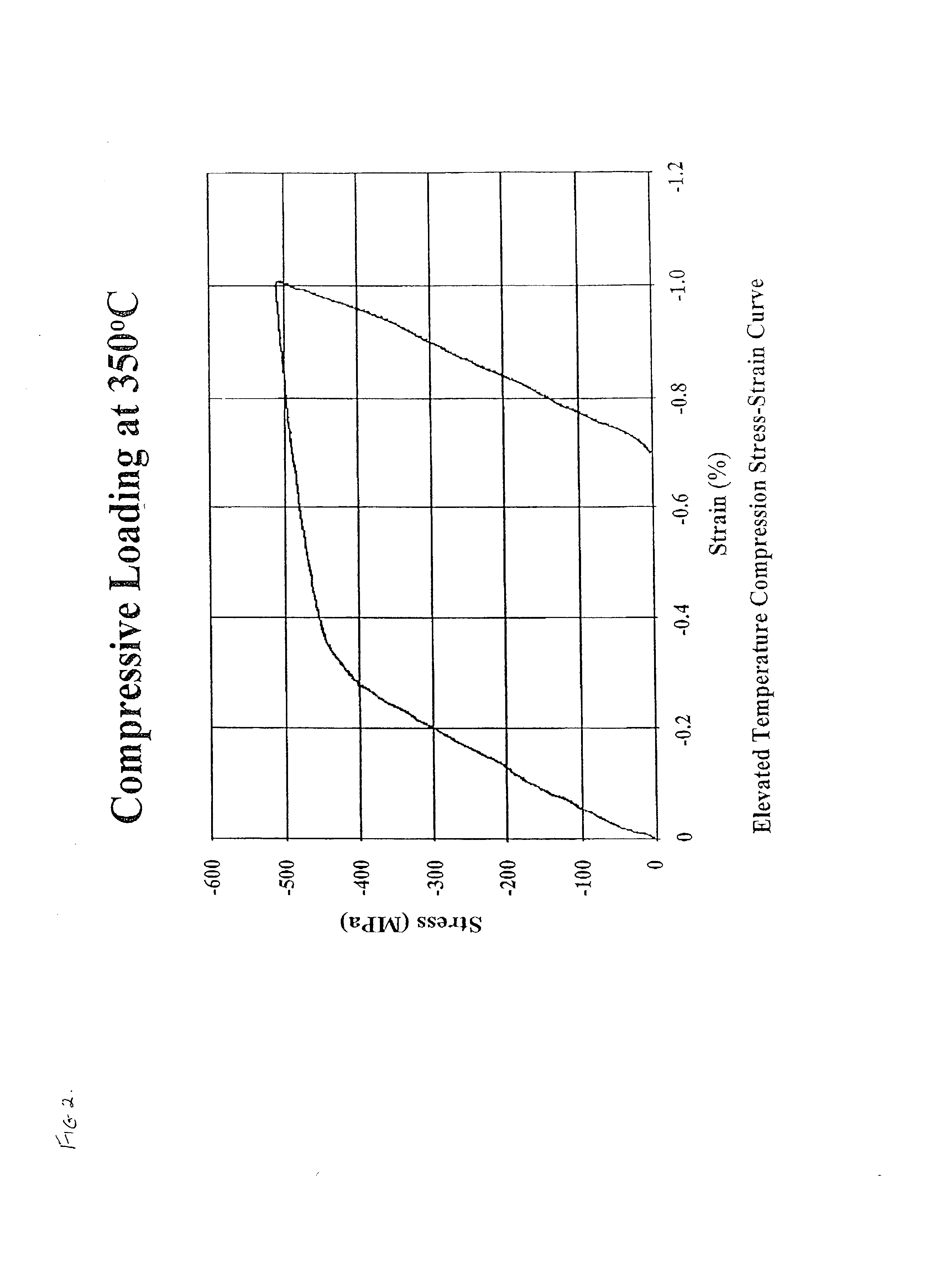
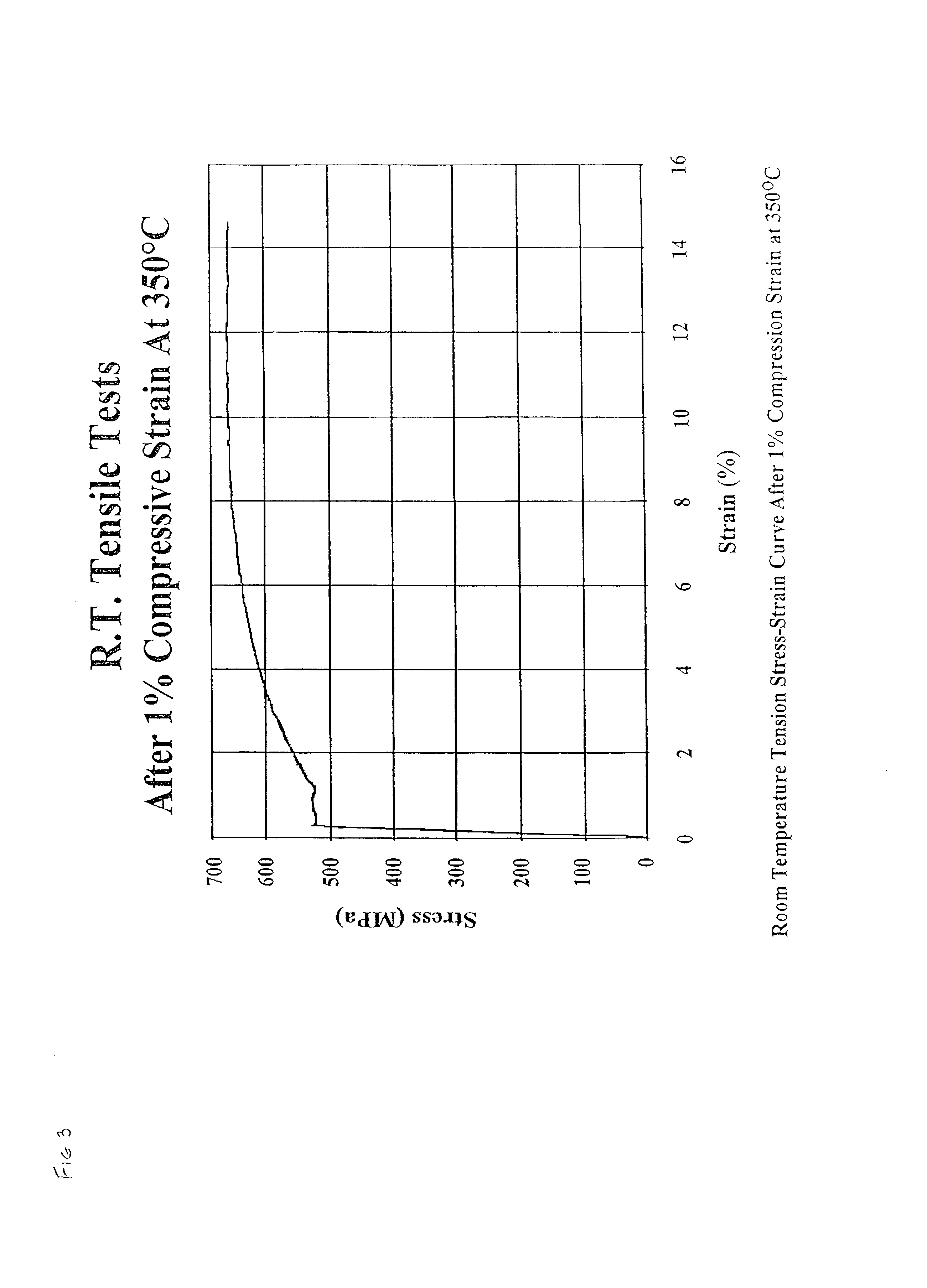
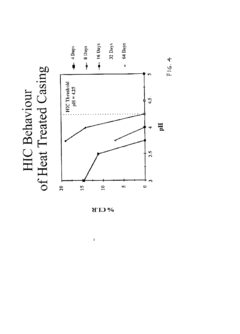
Molecular dynamics of Magnesium iron silicate hydroxide under stress.
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Magnesium Iron Silicate Hydroxide Background
Magnesium iron silicate hydroxide, also known as (Mg,Fe)SiO3(OH), is a mineral phase of significant importance in Earth sciences and materials research. This compound belongs to the family of hydrous silicates and plays a crucial role in various geological processes, particularly in the Earth's mantle and subduction zones.
The study of magnesium iron silicate hydroxide under stress conditions has gained considerable attention in recent years due to its relevance in understanding deep Earth dynamics and material behavior under extreme conditions. This mineral phase is believed to be a major component of the transition zone and lower mantle, where it experiences high pressures and temperatures.
The molecular dynamics of this compound under stress is of particular interest as it provides insights into the deformation mechanisms, phase transitions, and rheological properties of Earth's interior. Understanding these dynamics is essential for developing accurate models of mantle convection, plate tectonics, and the overall evolution of our planet.
Historically, research on magnesium iron silicate hydroxide has been challenging due to the extreme conditions required to simulate its natural environment. However, advancements in high-pressure experimental techniques and computational methods have enabled scientists to investigate its behavior more comprehensively.
The composition of magnesium iron silicate hydroxide can vary, with the ratio of magnesium to iron influencing its properties and behavior under stress. This variability adds complexity to the study of its molecular dynamics but also provides an opportunity to understand how compositional changes affect material properties at the atomic scale.
Recent studies have focused on the role of water in the structure of this mineral, as the hydroxyl group significantly impacts its stability and deformation mechanisms. The presence of water in the crystal structure can lead to weakening effects, potentially influencing the rheology of the mantle and the dynamics of subducting slabs.
The investigation of magnesium iron silicate hydroxide under stress conditions has implications beyond Earth sciences. It contributes to our understanding of material behavior under extreme conditions, which is valuable for developing new materials for industrial applications and advancing our knowledge in high-pressure physics and chemistry.
The study of magnesium iron silicate hydroxide under stress conditions has gained considerable attention in recent years due to its relevance in understanding deep Earth dynamics and material behavior under extreme conditions. This mineral phase is believed to be a major component of the transition zone and lower mantle, where it experiences high pressures and temperatures.
The molecular dynamics of this compound under stress is of particular interest as it provides insights into the deformation mechanisms, phase transitions, and rheological properties of Earth's interior. Understanding these dynamics is essential for developing accurate models of mantle convection, plate tectonics, and the overall evolution of our planet.
Historically, research on magnesium iron silicate hydroxide has been challenging due to the extreme conditions required to simulate its natural environment. However, advancements in high-pressure experimental techniques and computational methods have enabled scientists to investigate its behavior more comprehensively.
The composition of magnesium iron silicate hydroxide can vary, with the ratio of magnesium to iron influencing its properties and behavior under stress. This variability adds complexity to the study of its molecular dynamics but also provides an opportunity to understand how compositional changes affect material properties at the atomic scale.
Recent studies have focused on the role of water in the structure of this mineral, as the hydroxyl group significantly impacts its stability and deformation mechanisms. The presence of water in the crystal structure can lead to weakening effects, potentially influencing the rheology of the mantle and the dynamics of subducting slabs.
The investigation of magnesium iron silicate hydroxide under stress conditions has implications beyond Earth sciences. It contributes to our understanding of material behavior under extreme conditions, which is valuable for developing new materials for industrial applications and advancing our knowledge in high-pressure physics and chemistry.
Market Applications of MISH
Magnesium iron silicate hydroxide (MISH) has emerged as a material of significant interest in various industrial and technological applications. The unique properties of MISH, particularly its behavior under stress, have opened up new possibilities in several market sectors. In the construction industry, MISH has shown promise as an additive in cement and concrete formulations. Its ability to enhance the mechanical strength and durability of building materials has attracted attention from manufacturers seeking to develop high-performance construction products. The incorporation of MISH in concrete mixtures has demonstrated potential for improving resistance to cracking and increasing overall structural integrity, especially in high-stress environments such as bridges and skyscrapers.
The automotive and aerospace industries have also recognized the potential of MISH in lightweight material development. As these sectors continuously strive for fuel efficiency and reduced emissions, the use of MISH in composite materials offers a pathway to create stronger, lighter components. This application is particularly relevant in the design of vehicle bodies and aircraft fuselages, where weight reduction without compromising structural integrity is crucial.
In the field of energy storage, MISH has shown promise in the development of advanced battery technologies. Research into the use of MISH as a cathode material in lithium-ion batteries has indicated potential improvements in energy density and cycle life. This application could have far-reaching implications for the electric vehicle market and renewable energy storage systems, addressing key challenges in these rapidly growing sectors.
The environmental remediation sector has identified MISH as a potential agent for soil and water treatment. Its ability to adsorb heavy metals and organic pollutants makes it an attractive option for cleaning contaminated sites. This application aligns with the increasing global focus on environmental sustainability and the remediation of industrial waste sites.
In the field of nanotechnology, MISH nanoparticles are being explored for various applications, including drug delivery systems and advanced coatings. The unique surface properties of MISH at the nanoscale offer opportunities for developing novel materials with enhanced functionalities, such as improved drug targeting in pharmaceutical applications or self-cleaning surfaces in consumer products.
The textile industry has also begun to investigate the potential of MISH in developing smart fabrics. By incorporating MISH into textile fibers, researchers aim to create clothing with enhanced thermal regulation properties, potentially revolutionizing protective wear for extreme environments or improving comfort in everyday garments.
As research into the molecular dynamics of MISH under stress continues to advance, it is likely that new applications will emerge across various industries. The versatility of this material, coupled with its unique behavior under different stress conditions, positions MISH as a key component in the development of next-generation materials and technologies across multiple market sectors.
The automotive and aerospace industries have also recognized the potential of MISH in lightweight material development. As these sectors continuously strive for fuel efficiency and reduced emissions, the use of MISH in composite materials offers a pathway to create stronger, lighter components. This application is particularly relevant in the design of vehicle bodies and aircraft fuselages, where weight reduction without compromising structural integrity is crucial.
In the field of energy storage, MISH has shown promise in the development of advanced battery technologies. Research into the use of MISH as a cathode material in lithium-ion batteries has indicated potential improvements in energy density and cycle life. This application could have far-reaching implications for the electric vehicle market and renewable energy storage systems, addressing key challenges in these rapidly growing sectors.
The environmental remediation sector has identified MISH as a potential agent for soil and water treatment. Its ability to adsorb heavy metals and organic pollutants makes it an attractive option for cleaning contaminated sites. This application aligns with the increasing global focus on environmental sustainability and the remediation of industrial waste sites.
In the field of nanotechnology, MISH nanoparticles are being explored for various applications, including drug delivery systems and advanced coatings. The unique surface properties of MISH at the nanoscale offer opportunities for developing novel materials with enhanced functionalities, such as improved drug targeting in pharmaceutical applications or self-cleaning surfaces in consumer products.
The textile industry has also begun to investigate the potential of MISH in developing smart fabrics. By incorporating MISH into textile fibers, researchers aim to create clothing with enhanced thermal regulation properties, potentially revolutionizing protective wear for extreme environments or improving comfort in everyday garments.
As research into the molecular dynamics of MISH under stress continues to advance, it is likely that new applications will emerge across various industries. The versatility of this material, coupled with its unique behavior under different stress conditions, positions MISH as a key component in the development of next-generation materials and technologies across multiple market sectors.
Current Challenges in MISH Simulation
Molecular dynamics (MD) simulations of Magnesium iron silicate hydroxide (MISH) under stress face several significant challenges that hinder accurate and comprehensive analysis. One of the primary obstacles is the complexity of the MISH structure, which contains multiple elements and intricate bonding arrangements. This complexity makes it difficult to develop accurate interatomic potentials that can faithfully represent the interactions between all components under various stress conditions.
Another major challenge lies in the computational demands of simulating large-scale MISH systems. As the size of the simulated system increases, the computational resources required grow exponentially. This limitation often forces researchers to compromise between system size and simulation duration, potentially affecting the reliability of results, especially when studying long-term stress effects or large-scale structural changes.
The multiscale nature of stress-induced phenomena in MISH presents an additional hurdle. Stress can induce changes at the atomic level that propagate to macroscopic scales, requiring simulations to bridge vastly different time and length scales. Current MD techniques struggle to efficiently capture these multiscale effects, often necessitating the use of hybrid approaches that combine MD with other computational methods.
Furthermore, the treatment of long-range interactions, particularly in systems with charged species, poses a significant challenge. Accurate representation of electrostatic interactions is crucial for MISH simulations, but traditional methods for handling long-range forces can be computationally expensive and may introduce artifacts in stress-related calculations.
The dynamic nature of stress application and its effects on MISH structure also present difficulties in simulation design. Determining appropriate stress application rates and methods that mimic real-world conditions without introducing artificial dynamics is a delicate balance. This challenge is compounded by the need to maintain proper thermodynamic ensembles during stress application, which can be particularly tricky in non-equilibrium simulations.
Lastly, the validation of MD simulation results for MISH under stress remains a significant challenge. The scarcity of experimental data for direct comparison, especially under extreme stress conditions, makes it difficult to assess the accuracy of simulation predictions. This lack of validation data hampers the refinement of simulation models and the development of more accurate interatomic potentials for MISH systems under stress.
Another major challenge lies in the computational demands of simulating large-scale MISH systems. As the size of the simulated system increases, the computational resources required grow exponentially. This limitation often forces researchers to compromise between system size and simulation duration, potentially affecting the reliability of results, especially when studying long-term stress effects or large-scale structural changes.
The multiscale nature of stress-induced phenomena in MISH presents an additional hurdle. Stress can induce changes at the atomic level that propagate to macroscopic scales, requiring simulations to bridge vastly different time and length scales. Current MD techniques struggle to efficiently capture these multiscale effects, often necessitating the use of hybrid approaches that combine MD with other computational methods.
Furthermore, the treatment of long-range interactions, particularly in systems with charged species, poses a significant challenge. Accurate representation of electrostatic interactions is crucial for MISH simulations, but traditional methods for handling long-range forces can be computationally expensive and may introduce artifacts in stress-related calculations.
The dynamic nature of stress application and its effects on MISH structure also present difficulties in simulation design. Determining appropriate stress application rates and methods that mimic real-world conditions without introducing artificial dynamics is a delicate balance. This challenge is compounded by the need to maintain proper thermodynamic ensembles during stress application, which can be particularly tricky in non-equilibrium simulations.
Lastly, the validation of MD simulation results for MISH under stress remains a significant challenge. The scarcity of experimental data for direct comparison, especially under extreme stress conditions, makes it difficult to assess the accuracy of simulation predictions. This lack of validation data hampers the refinement of simulation models and the development of more accurate interatomic potentials for MISH systems under stress.
Existing MD Models for MISH
01 Molecular dynamics simulations of magnesium iron silicate hydroxide
Molecular dynamics simulations are used to study the structural and dynamic properties of magnesium iron silicate hydroxide at the atomic level. These simulations help in understanding the behavior of the material under various conditions, such as temperature and pressure, and provide insights into its physical and chemical properties.- Molecular dynamics simulations of magnesium iron silicate hydroxide: Molecular dynamics simulations are used to study the structural and dynamic properties of magnesium iron silicate hydroxide at the atomic level. These simulations help in understanding the behavior of the material under various conditions, such as temperature and pressure changes, and provide insights into its physical and chemical properties.
- Synthesis and characterization of magnesium iron silicate hydroxide: Various methods for synthesizing magnesium iron silicate hydroxide are explored, including hydrothermal synthesis and sol-gel processes. The resulting materials are characterized using techniques such as X-ray diffraction, electron microscopy, and spectroscopic methods to determine their composition, structure, and properties.
- Applications of magnesium iron silicate hydroxide in catalysis: Magnesium iron silicate hydroxide is investigated for its potential use as a catalyst or catalyst support in various chemical reactions. Its unique structure and properties make it suitable for applications in areas such as environmental remediation, organic synthesis, and energy conversion processes.
- Modification and functionalization of magnesium iron silicate hydroxide: Research focuses on modifying the surface and structure of magnesium iron silicate hydroxide to enhance its properties and expand its applications. This includes doping with other elements, surface functionalization, and creating composite materials with improved performance characteristics.
- Environmental and geological studies of magnesium iron silicate hydroxide: Investigations into the role of magnesium iron silicate hydroxide in geological processes and its environmental impact are conducted. This includes studies on its formation in natural settings, its interaction with other minerals, and its potential for carbon sequestration and environmental remediation applications.
02 Synthesis and characterization of magnesium iron silicate hydroxide
Various methods are employed to synthesize magnesium iron silicate hydroxide, including hydrothermal synthesis and sol-gel processes. The resulting material is characterized using techniques such as X-ray diffraction, electron microscopy, and spectroscopic methods to determine its composition, structure, and properties.Expand Specific Solutions03 Applications of magnesium iron silicate hydroxide in catalysis
Magnesium iron silicate hydroxide is investigated for its potential use as a catalyst or catalyst support in various chemical reactions. Its unique structure and properties make it suitable for applications in areas such as environmental remediation, organic synthesis, and energy conversion processes.Expand Specific Solutions04 Modification and functionalization of magnesium iron silicate hydroxide
Research focuses on modifying and functionalizing magnesium iron silicate hydroxide to enhance its properties and expand its potential applications. This includes surface modification, doping with other elements, and creating composite materials to tailor the material for specific uses.Expand Specific Solutions05 Computational studies of magnesium iron silicate hydroxide properties
Computational methods, including density functional theory calculations and ab initio simulations, are used to investigate the electronic structure, mechanical properties, and thermodynamic stability of magnesium iron silicate hydroxide. These studies provide valuable insights into the material's behavior at the atomic and molecular levels.Expand Specific Solutions
Key Players in MD Simulation
The molecular dynamics of Magnesium iron silicate hydroxide under stress represents an emerging field of research with significant implications for materials science and geophysics. The competitive landscape is characterized by a nascent market in its early development stages, with potential applications in various industries. The global market size for this specific research area is still relatively small but growing, driven by increasing interest in understanding the behavior of complex mineral structures under extreme conditions. Technologically, the field is in its early maturity phase, with ongoing research efforts from academic institutions like Massachusetts Institute of Technology and Monash University, as well as industrial players such as BASF SE and Samsung Electronics Co., Ltd., who are exploring potential applications in advanced materials and electronic components.
BASF SE
Technical Solution: BASF SE has developed a comprehensive approach to studying the molecular dynamics of magnesium iron silicate hydroxide under stress, focusing on its applications in industrial catalysis and materials science. Their method combines advanced spectroscopic techniques with molecular dynamics simulations to investigate the structural and chemical changes in these minerals under various stress conditions. The research team has implemented a multi-technique characterization approach, including in situ X-ray absorption spectroscopy (XAS) and nuclear magnetic resonance (NMR) spectroscopy, to probe the local atomic environment and electronic structure of magnesium and iron atoms[9]. These experimental results are then used to validate and refine their molecular dynamics models, which are based on reactive force fields optimized for heterogeneous catalysis applications[10].
Strengths: Integration of advanced spectroscopic techniques with molecular simulations, focus on industrial applications. Weaknesses: Potential limitations in studying extreme pressure conditions relevant to deep Earth processes, focus may be more on surface properties rather than bulk behavior.
Massachusetts Institute of Technology
Technical Solution: MIT has developed advanced computational models for studying the molecular dynamics of magnesium iron silicate hydroxide under stress. Their approach combines ab initio molecular dynamics simulations with machine learning techniques to predict the behavior of these complex mineral structures under various pressure and temperature conditions. The research team has implemented a novel force field that accurately captures the interactions between magnesium, iron, silicon, and hydroxyl groups, allowing for more precise simulations of atomic-scale processes[1]. Additionally, they have utilized high-performance computing clusters to run large-scale simulations, enabling the study of systems containing millions of atoms over extended time scales[3].
Strengths: Cutting-edge computational techniques, access to high-performance computing resources, and interdisciplinary expertise in materials science and geophysics. Weaknesses: High computational costs and potential limitations in experimental validation of simulated results.
Core Innovations in MISH MD
Method of making iron silicide and method of making photoelectric transducer
PatentInactiveUS6949463B2
Innovation
- A method involving a substrate with fixed pressure, heating, and plasma formation using a silicon-atom-containing gas and hydrogen gas, along with iron atom supply, to form an iron silicide film with hydrogen atoms, which terminates dangling bonds and enhances semiconductor properties at lower temperatures.
Hydrogen-induced-cracking resistant and sulphide-stress-cracking resistant steel alloy
PatentInactiveUS20030136476A1
Innovation
- A quench-and-temper steel alloy with specific chemical composition, including a carbon range of 0.15% to 0.35%, molybdenum of at least 0.15%, and sulfur less than 0.002%, which forms precipitated spheroidal Mo carbides in Mn and C-rich bands, reducing MnS inclusion content and boron and titanium presence to enhance HIC and SSC resistance, corrosion resistance, and stability at elevated temperatures.
Environmental Impact of MISH
The environmental impact of Magnesium Iron Silicate Hydroxide (MISH) under stress conditions is a critical aspect to consider in various geological and industrial applications. MISH, commonly known as serpentine minerals, plays a significant role in carbon sequestration processes and has potential implications for climate change mitigation strategies.
When subjected to stress, MISH undergoes molecular dynamics that can lead to changes in its physical and chemical properties. These alterations may affect its interaction with the surrounding environment, particularly in terms of carbon dioxide absorption and storage capabilities. The stress-induced changes in MISH can potentially enhance or diminish its effectiveness as a natural carbon sink, thereby influencing global carbon cycles.
The environmental impact of MISH under stress extends to soil and water systems. As the mineral structure changes due to stress, it may release or absorb different elements, affecting soil composition and water chemistry. This can have cascading effects on local ecosystems, potentially altering nutrient availability for plants and microorganisms. Furthermore, the stress-induced transformations of MISH may influence its ability to act as a natural filter for contaminants in soil and water, impacting environmental remediation efforts.
In industrial applications, such as mining and construction, the behavior of MISH under stress conditions is of particular concern. The mineral's response to mechanical and thermal stresses can affect the stability of geological formations, potentially leading to increased erosion or landslide risks. Additionally, the altered properties of stressed MISH may impact its use in engineered applications, such as building materials or industrial processes, necessitating careful consideration of environmental consequences.
The molecular dynamics of MISH under stress also have implications for geothermal energy production. As MISH is often present in geothermal reservoirs, understanding its behavior under high-pressure and high-temperature conditions is crucial for assessing the environmental impact of geothermal energy extraction. The stress-induced changes in MISH could affect the permeability and heat transfer properties of reservoir rocks, potentially influencing the efficiency and sustainability of geothermal energy systems.
Lastly, the environmental impact of stressed MISH extends to its role in the Earth's deep carbon cycle. The mineral's behavior under high-pressure conditions in subduction zones can influence the release or retention of carbon dioxide, affecting long-term climate regulation processes. Understanding these dynamics is essential for accurately modeling global carbon fluxes and predicting future climate scenarios.
When subjected to stress, MISH undergoes molecular dynamics that can lead to changes in its physical and chemical properties. These alterations may affect its interaction with the surrounding environment, particularly in terms of carbon dioxide absorption and storage capabilities. The stress-induced changes in MISH can potentially enhance or diminish its effectiveness as a natural carbon sink, thereby influencing global carbon cycles.
The environmental impact of MISH under stress extends to soil and water systems. As the mineral structure changes due to stress, it may release or absorb different elements, affecting soil composition and water chemistry. This can have cascading effects on local ecosystems, potentially altering nutrient availability for plants and microorganisms. Furthermore, the stress-induced transformations of MISH may influence its ability to act as a natural filter for contaminants in soil and water, impacting environmental remediation efforts.
In industrial applications, such as mining and construction, the behavior of MISH under stress conditions is of particular concern. The mineral's response to mechanical and thermal stresses can affect the stability of geological formations, potentially leading to increased erosion or landslide risks. Additionally, the altered properties of stressed MISH may impact its use in engineered applications, such as building materials or industrial processes, necessitating careful consideration of environmental consequences.
The molecular dynamics of MISH under stress also have implications for geothermal energy production. As MISH is often present in geothermal reservoirs, understanding its behavior under high-pressure and high-temperature conditions is crucial for assessing the environmental impact of geothermal energy extraction. The stress-induced changes in MISH could affect the permeability and heat transfer properties of reservoir rocks, potentially influencing the efficiency and sustainability of geothermal energy systems.
Lastly, the environmental impact of stressed MISH extends to its role in the Earth's deep carbon cycle. The mineral's behavior under high-pressure conditions in subduction zones can influence the release or retention of carbon dioxide, affecting long-term climate regulation processes. Understanding these dynamics is essential for accurately modeling global carbon fluxes and predicting future climate scenarios.
High-Performance Computing for MD
High-performance computing (HPC) has become an indispensable tool for conducting molecular dynamics (MD) simulations of complex systems such as Magnesium iron silicate hydroxide under stress. The computational demands of these simulations, which involve tracking the interactions of millions of atoms over extended time scales, necessitate the use of powerful supercomputers and specialized software optimized for parallel processing.
Modern HPC systems for MD simulations typically employ a combination of CPU and GPU architectures to maximize computational efficiency. CPUs handle the overall simulation control and complex calculations, while GPUs excel at the massively parallel computations required for force calculations and neighbor list updates. This heterogeneous computing approach has significantly accelerated MD simulations, allowing researchers to study larger systems for longer timescales.
Parallel algorithms play a crucial role in distributing the computational workload across multiple processors. Domain decomposition methods, such as spatial decomposition or force decomposition, are commonly used to divide the simulation box into smaller subdomains. Each subdomain is then assigned to a different processor, enabling simultaneous calculations of atomic interactions within and between subdomains.
Communication between processors is a critical aspect of HPC for MD simulations. Efficient message-passing protocols, such as MPI (Message Passing Interface), are employed to minimize data transfer overhead and ensure proper synchronization between different parts of the simulation. Load balancing techniques are also implemented to evenly distribute the computational workload among processors, accounting for potential inhomogeneities in the system being simulated.
Optimizing MD codes for HPC environments involves careful consideration of data structures and memory access patterns. Techniques such as vectorization, cache optimization, and memory coalescing are employed to maximize the utilization of available hardware resources. Additionally, advanced algorithms like multiple time-step methods and enhanced sampling techniques are implemented to further improve simulation efficiency and explore longer time scales.
The use of HPC in MD simulations of Magnesium iron silicate hydroxide under stress enables researchers to investigate complex phenomena such as phase transitions, defect formation, and mechanical properties at atomic resolution. These simulations provide valuable insights into the behavior of Earth's mantle materials under extreme conditions, contributing to our understanding of geodynamics and mineral physics.
Modern HPC systems for MD simulations typically employ a combination of CPU and GPU architectures to maximize computational efficiency. CPUs handle the overall simulation control and complex calculations, while GPUs excel at the massively parallel computations required for force calculations and neighbor list updates. This heterogeneous computing approach has significantly accelerated MD simulations, allowing researchers to study larger systems for longer timescales.
Parallel algorithms play a crucial role in distributing the computational workload across multiple processors. Domain decomposition methods, such as spatial decomposition or force decomposition, are commonly used to divide the simulation box into smaller subdomains. Each subdomain is then assigned to a different processor, enabling simultaneous calculations of atomic interactions within and between subdomains.
Communication between processors is a critical aspect of HPC for MD simulations. Efficient message-passing protocols, such as MPI (Message Passing Interface), are employed to minimize data transfer overhead and ensure proper synchronization between different parts of the simulation. Load balancing techniques are also implemented to evenly distribute the computational workload among processors, accounting for potential inhomogeneities in the system being simulated.
Optimizing MD codes for HPC environments involves careful consideration of data structures and memory access patterns. Techniques such as vectorization, cache optimization, and memory coalescing are employed to maximize the utilization of available hardware resources. Additionally, advanced algorithms like multiple time-step methods and enhanced sampling techniques are implemented to further improve simulation efficiency and explore longer time scales.
The use of HPC in MD simulations of Magnesium iron silicate hydroxide under stress enables researchers to investigate complex phenomena such as phase transitions, defect formation, and mechanical properties at atomic resolution. These simulations provide valuable insights into the behavior of Earth's mantle materials under extreme conditions, contributing to our understanding of geodynamics and mineral physics.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!