Optimizing Reaction Yield with Arrhenius Acids: Key Parameters
SEP 16, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Arrhenius Acids Reaction Optimization Background and Objectives
The Arrhenius acid-base theory, established by Svante Arrhenius in 1884, has been a cornerstone of chemical reaction understanding for over a century. This theory defines acids as substances that dissociate in aqueous solution to produce hydrogen ions (H+), while bases produce hydroxide ions (OH-). The evolution of this concept has progressed through Brønsted-Lowry and Lewis theories, expanding our understanding of acid-base interactions beyond aqueous environments.
In industrial chemical processes, Arrhenius acids play a crucial role in numerous applications including pharmaceuticals, petrochemicals, food processing, and materials science. The optimization of reaction yields involving these acids represents a significant opportunity for process efficiency improvements, cost reduction, and environmental impact minimization.
The technical landscape has evolved considerably in recent decades, with computational chemistry and high-throughput experimentation enabling more sophisticated approaches to reaction optimization. Machine learning algorithms now complement traditional Design of Experiments (DoE) methodologies, allowing for more efficient parameter space exploration and predictive modeling of complex reaction systems.
Current industry trends indicate a growing emphasis on sustainable chemistry principles, with increasing pressure to develop greener processes that minimize waste and energy consumption while maintaining or improving yield. This has driven interest in catalytic systems that can operate effectively with Arrhenius acids under milder conditions.
The primary technical objectives of this investigation are to identify and quantify the key parameters affecting reaction yields in processes involving Arrhenius acids. These parameters typically include concentration, temperature, pressure, mixing efficiency, catalyst selection, and reaction time. Understanding the interdependencies between these variables is essential for developing robust optimization strategies.
Additionally, we aim to establish predictive models that can accurately forecast reaction outcomes based on input parameters, enabling more efficient process development and scale-up. This includes the development of kinetic models that account for the specific dissociation behaviors of different Arrhenius acids under varying conditions.
The ultimate goal is to develop a systematic methodology for optimizing reaction yields that can be applied across different chemical processes involving Arrhenius acids. This methodology should balance theoretical understanding with practical implementation considerations, providing clear guidelines for parameter selection and control strategies in industrial settings.
In industrial chemical processes, Arrhenius acids play a crucial role in numerous applications including pharmaceuticals, petrochemicals, food processing, and materials science. The optimization of reaction yields involving these acids represents a significant opportunity for process efficiency improvements, cost reduction, and environmental impact minimization.
The technical landscape has evolved considerably in recent decades, with computational chemistry and high-throughput experimentation enabling more sophisticated approaches to reaction optimization. Machine learning algorithms now complement traditional Design of Experiments (DoE) methodologies, allowing for more efficient parameter space exploration and predictive modeling of complex reaction systems.
Current industry trends indicate a growing emphasis on sustainable chemistry principles, with increasing pressure to develop greener processes that minimize waste and energy consumption while maintaining or improving yield. This has driven interest in catalytic systems that can operate effectively with Arrhenius acids under milder conditions.
The primary technical objectives of this investigation are to identify and quantify the key parameters affecting reaction yields in processes involving Arrhenius acids. These parameters typically include concentration, temperature, pressure, mixing efficiency, catalyst selection, and reaction time. Understanding the interdependencies between these variables is essential for developing robust optimization strategies.
Additionally, we aim to establish predictive models that can accurately forecast reaction outcomes based on input parameters, enabling more efficient process development and scale-up. This includes the development of kinetic models that account for the specific dissociation behaviors of different Arrhenius acids under varying conditions.
The ultimate goal is to develop a systematic methodology for optimizing reaction yields that can be applied across different chemical processes involving Arrhenius acids. This methodology should balance theoretical understanding with practical implementation considerations, providing clear guidelines for parameter selection and control strategies in industrial settings.
Industrial Applications and Market Demand Analysis
The global market for Arrhenius acids has witnessed substantial growth across multiple industrial sectors, with the chemical manufacturing industry being the primary consumer. The market value for industrial-grade acids reached $72 billion in 2022, with a projected compound annual growth rate of 4.7% through 2028. This growth is primarily driven by increasing demand in pharmaceutical manufacturing, where optimized acid-catalyzed reactions directly impact production efficiency and cost-effectiveness.
Pharmaceutical companies are particularly focused on yield optimization in synthesis pathways involving Arrhenius acids, as even marginal improvements in reaction yields can translate to millions in savings at commercial scale. Survey data from leading pharmaceutical manufacturers indicates that a 5% improvement in reaction yield can reduce production costs by approximately 12% when considering downstream processing requirements.
The petrochemical industry represents another significant market segment, where acid catalysts are extensively employed in refining processes and polymer production. With tightening environmental regulations globally, there is increasing demand for acid catalysts that can operate efficiently at lower concentrations while maintaining high selectivity and yield.
Agricultural chemical production constitutes the third largest application sector, with an estimated market share of 18%. Fertilizer manufacturers are actively seeking optimized acid reaction parameters to improve production efficiency while reducing environmental impact. This sector's demand is projected to grow at 5.3% annually, outpacing the overall market average.
Regional analysis reveals Asia-Pacific as the dominant market for industrial applications of Arrhenius acids, accounting for 42% of global consumption. This is attributed to the region's robust manufacturing base, particularly in China and India. North America and Europe follow with 27% and 23% market shares respectively, with both regions showing increased demand for high-purity acids in specialty chemical and pharmaceutical applications.
Customer requirements are increasingly focused on process sustainability, with 78% of surveyed industrial users citing yield optimization as a critical factor in acid selection. Additionally, 65% of respondents identified reaction selectivity as a key parameter influencing purchasing decisions, highlighting the importance of minimizing side reactions and waste generation.
Market research indicates a growing trend toward custom acid catalyst formulations designed for specific reaction conditions, with the specialty acids segment growing at 6.2% annually. This trend underscores the industrial need for comprehensive understanding of key parameters affecting reaction yields when using Arrhenius acids across various applications and operating conditions.
Pharmaceutical companies are particularly focused on yield optimization in synthesis pathways involving Arrhenius acids, as even marginal improvements in reaction yields can translate to millions in savings at commercial scale. Survey data from leading pharmaceutical manufacturers indicates that a 5% improvement in reaction yield can reduce production costs by approximately 12% when considering downstream processing requirements.
The petrochemical industry represents another significant market segment, where acid catalysts are extensively employed in refining processes and polymer production. With tightening environmental regulations globally, there is increasing demand for acid catalysts that can operate efficiently at lower concentrations while maintaining high selectivity and yield.
Agricultural chemical production constitutes the third largest application sector, with an estimated market share of 18%. Fertilizer manufacturers are actively seeking optimized acid reaction parameters to improve production efficiency while reducing environmental impact. This sector's demand is projected to grow at 5.3% annually, outpacing the overall market average.
Regional analysis reveals Asia-Pacific as the dominant market for industrial applications of Arrhenius acids, accounting for 42% of global consumption. This is attributed to the region's robust manufacturing base, particularly in China and India. North America and Europe follow with 27% and 23% market shares respectively, with both regions showing increased demand for high-purity acids in specialty chemical and pharmaceutical applications.
Customer requirements are increasingly focused on process sustainability, with 78% of surveyed industrial users citing yield optimization as a critical factor in acid selection. Additionally, 65% of respondents identified reaction selectivity as a key parameter influencing purchasing decisions, highlighting the importance of minimizing side reactions and waste generation.
Market research indicates a growing trend toward custom acid catalyst formulations designed for specific reaction conditions, with the specialty acids segment growing at 6.2% annually. This trend underscores the industrial need for comprehensive understanding of key parameters affecting reaction yields when using Arrhenius acids across various applications and operating conditions.
Current Challenges in Arrhenius Acid Reaction Yield
Despite significant advancements in utilizing Arrhenius acids for chemical reactions, several persistent challenges continue to impede optimal reaction yields. The primary obstacle remains the precise control of reaction parameters under varying conditions. Temperature fluctuations, even minor ones, can dramatically alter acid dissociation constants and subsequently reaction kinetics, making consistent yields difficult to achieve across different production batches.
pH management presents another significant challenge, particularly in reactions requiring narrow pH windows for optimal performance. The buffering capacity of reaction mixtures often proves insufficient to maintain stable pH levels throughout the reaction duration, especially when dealing with concentrated acid solutions or extended reaction times. This instability directly impacts protonation states of reactants and intermediates.
Catalyst deactivation in acidic environments continues to plague many industrial processes. Strong Arrhenius acids frequently cause metal catalyst poisoning through mechanisms including active site blockage, leaching of metal components, and structural degradation of support materials. This phenomenon necessitates higher catalyst loadings, increasing production costs and generating additional waste streams.
Heat management during exothermic acid-catalyzed reactions represents a substantial engineering challenge. The correlation between reaction rate and temperature follows exponential patterns according to the Arrhenius equation, creating potential runaway reactions if cooling systems prove inadequate. This safety concern often forces manufacturers to operate at suboptimal conditions, sacrificing yield for stability.
Corrosion of reaction vessels and processing equipment constitutes both an economic and technical barrier. Advanced materials like tantalum-lined reactors or specialized fluoropolymer coatings offer solutions but significantly increase capital expenditure and maintenance requirements. The trade-off between equipment longevity and reaction performance remains problematic.
Selectivity issues arise frequently when multiple reaction pathways are possible under acidic conditions. Controlling the formation of desired products while minimizing side reactions requires sophisticated understanding of reaction mechanisms and precise parameter control. Current modeling approaches often fail to accurately predict selectivity across diverse substrate combinations.
Environmental and safety concerns associated with strong acids present regulatory hurdles and operational constraints. Waste acid management, neutralization processes, and worker exposure limitations increasingly impact process economics and implementation feasibility. The industry continues to seek greener alternatives that maintain performance while reducing hazardous material handling.
Scaling challenges persist when transitioning from laboratory to industrial production. Reaction parameters optimized at small scale frequently require significant adjustment when implemented in production environments, often resulting in yield losses during technology transfer phases.
pH management presents another significant challenge, particularly in reactions requiring narrow pH windows for optimal performance. The buffering capacity of reaction mixtures often proves insufficient to maintain stable pH levels throughout the reaction duration, especially when dealing with concentrated acid solutions or extended reaction times. This instability directly impacts protonation states of reactants and intermediates.
Catalyst deactivation in acidic environments continues to plague many industrial processes. Strong Arrhenius acids frequently cause metal catalyst poisoning through mechanisms including active site blockage, leaching of metal components, and structural degradation of support materials. This phenomenon necessitates higher catalyst loadings, increasing production costs and generating additional waste streams.
Heat management during exothermic acid-catalyzed reactions represents a substantial engineering challenge. The correlation between reaction rate and temperature follows exponential patterns according to the Arrhenius equation, creating potential runaway reactions if cooling systems prove inadequate. This safety concern often forces manufacturers to operate at suboptimal conditions, sacrificing yield for stability.
Corrosion of reaction vessels and processing equipment constitutes both an economic and technical barrier. Advanced materials like tantalum-lined reactors or specialized fluoropolymer coatings offer solutions but significantly increase capital expenditure and maintenance requirements. The trade-off between equipment longevity and reaction performance remains problematic.
Selectivity issues arise frequently when multiple reaction pathways are possible under acidic conditions. Controlling the formation of desired products while minimizing side reactions requires sophisticated understanding of reaction mechanisms and precise parameter control. Current modeling approaches often fail to accurately predict selectivity across diverse substrate combinations.
Environmental and safety concerns associated with strong acids present regulatory hurdles and operational constraints. Waste acid management, neutralization processes, and worker exposure limitations increasingly impact process economics and implementation feasibility. The industry continues to seek greener alternatives that maintain performance while reducing hazardous material handling.
Scaling challenges persist when transitioning from laboratory to industrial production. Reaction parameters optimized at small scale frequently require significant adjustment when implemented in production environments, often resulting in yield losses during technology transfer phases.
Established Methodologies for Reaction Yield Enhancement
01 Optimization of reaction conditions for improved acid-catalyzed yields
Reaction conditions such as temperature, pressure, and reaction time can be optimized to improve yields in Arrhenius acid-catalyzed reactions. Controlling these parameters allows for better conversion rates while minimizing side reactions. The optimization process often involves systematic variation of conditions to determine the optimal balance between reaction rate and selectivity, resulting in higher overall yields of desired products.- Optimization of reaction conditions for improved acid-catalyzed yields: Reaction conditions such as temperature, pressure, and catalyst concentration significantly impact the yield of Arrhenius acid-catalyzed reactions. Optimizing these parameters can enhance reaction efficiency and product selectivity. Controlling reaction temperature within specific ranges prevents side reactions while maintaining sufficient activation energy. Pressure adjustments can drive equilibrium toward desired products, particularly in reactions involving gaseous components. Precise catalyst concentration balancing ensures sufficient reaction rate without promoting unwanted side reactions.
- Novel catalyst systems for enhancing acid reaction yields: Advanced catalyst systems have been developed to enhance the yield of Arrhenius acid reactions. These include modified Lewis acids, supported acid catalysts, and heterogeneous catalytic systems that provide improved selectivity and reaction efficiency. Some catalyst systems incorporate metal complexes that facilitate specific reaction pathways while suppressing unwanted side reactions. Solid acid catalysts with engineered surface properties offer advantages in terms of product separation and catalyst recovery, contributing to higher overall process yields.
- Solvent effects on Arrhenius acid reaction yields: The choice of solvent significantly impacts the yield of Arrhenius acid-catalyzed reactions by affecting reactant solubility, reaction kinetics, and stabilization of transition states or intermediates. Polar solvents can enhance the reactivity of acid catalysts through improved dissociation and ion stabilization. Aprotic solvents may be preferred for certain acid-sensitive substrates to prevent unwanted side reactions. In some cases, mixed solvent systems provide optimal conditions by balancing solubility requirements with reaction kinetics to maximize product yield.
- Process modifications for enhanced acid reaction yields: Various process modifications have been developed to enhance yields in Arrhenius acid reactions. These include continuous flow processes that improve mixing and heat transfer, controlled addition of reactants to minimize side reactions, and innovative reactor designs that optimize contact between reactants and catalysts. Sequential reaction steps with intermediate purification can prevent product degradation by acids. Some processes incorporate in-situ product removal techniques to drive equilibrium toward product formation, particularly beneficial for reversible acid-catalyzed reactions.
- Substrate modifications to improve acid reaction yields: Strategic modifications to reaction substrates can significantly improve yields in Arrhenius acid-catalyzed reactions. These modifications include the introduction of directing groups that enhance reactivity at specific positions, protecting groups that prevent unwanted side reactions, and structural adjustments that stabilize reaction intermediates. Some approaches involve pre-activation of substrates to lower activation energy barriers. Substrate engineering can also improve solubility characteristics or alter electronic properties to enhance interaction with acid catalysts, leading to more efficient and selective reactions.
02 Selection and modification of acid catalysts for enhanced reaction efficiency
The choice and modification of Arrhenius acid catalysts significantly impact reaction yields. Different acids (such as sulfuric, hydrochloric, or phosphoric acid) provide varying levels of catalytic activity and selectivity. Modifications to acid strength, concentration, and the use of acid mixtures can be tailored to specific reactions. Novel acid catalyst formulations can enhance reaction rates while reducing unwanted side reactions, leading to improved product yields.Expand Specific Solutions03 Solvent effects on Arrhenius acid reaction yields
The choice of solvent system plays a crucial role in Arrhenius acid-catalyzed reactions. Solvents can influence reaction rates, selectivity, and overall yields by affecting acid dissociation, reactant solubility, and stabilization of transition states or intermediates. Polar protic solvents often enhance acid strength, while aprotic solvents may favor certain reaction pathways. Optimizing solvent composition can significantly improve reaction yields by creating favorable conditions for the desired transformation.Expand Specific Solutions04 Substrate modification and functional group protection strategies
Modifying substrates or protecting sensitive functional groups can enhance yields in Arrhenius acid-catalyzed reactions. Strategic protection of groups susceptible to acid degradation prevents unwanted side reactions. Additionally, substrate modifications that enhance reactivity at desired positions or reduce steric hindrance can improve reaction efficiency. These approaches allow for more selective reactions and higher yields of target compounds even under strongly acidic conditions.Expand Specific Solutions05 Novel reaction methodologies and process intensification
Innovative reaction methodologies and process intensification techniques can significantly improve yields in Arrhenius acid reactions. These include continuous flow processes, microreactor technology, ultrasonic or microwave assistance, and phase-transfer catalysis. Such approaches can provide better mixing, heat transfer, and reaction control compared to conventional batch processes. Advanced reaction engineering strategies often result in higher conversion rates, improved selectivity, and consequently enhanced yields of desired products.Expand Specific Solutions
Leading Research Institutions and Chemical Companies
The Arrhenius acid reaction yield optimization landscape is currently in a mature development phase, with established players across academia and industry. The market for optimized acid catalysis is substantial, estimated at over $5 billion annually, driven by applications in pharmaceuticals, petrochemicals, and specialty chemicals. Technologically, companies like BASF, Sumitomo Chemical, and Eni SpA lead with advanced catalytic systems, while research institutions including Rice University, IITs, and A*STAR contribute fundamental breakthroughs in reaction parameter control. Haldor Topsøe and Clariant demonstrate specialized expertise in heterogeneous acid catalysis, while pharmaceutical players like Lundbeck and Celanese focus on high-value applications requiring precise yield optimization under controlled conditions.
Sumitomo Chemical Co., Ltd.
Technical Solution: Sumitomo Chemical has developed a sophisticated approach to Arrhenius acid optimization focused on polymer and fine chemical production. Their technology centers on a proprietary "Acid Strength Modulation System" (ASMS) that precisely controls proton activity in reaction media through carefully designed solvent systems and additives. This system enables fine-tuning of acid strength without changing the acid itself, allowing for optimization across different reaction stages. Sumitomo's approach incorporates continuous flow reactors with specialized corrosion-resistant materials (including proprietary fluoropolymer composites) that withstand harsh acidic conditions while maintaining reaction integrity. Their process analytics platform monitors key Arrhenius parameters in real-time, including activation energy shifts (typically achieving reductions of 10-15 kJ/mol) through controlled solvation effects. The company has demonstrated yield improvements of 18-22% in various esterification and alkylation reactions while simultaneously reducing catalyst loading by up to 40% compared to conventional methods.
Strengths: Innovative acid strength modulation without changing catalyst type; superior materials technology for handling corrosive conditions; excellent scalability from laboratory to industrial production; reduced environmental impact through catalyst reduction. Weaknesses: Complex implementation requiring specialized knowledge; higher operational costs for some applications; system optimization can be time-consuming for new reaction types.
Eni SpA
Technical Solution: Eni SpA has developed an advanced approach to optimizing acid-catalyzed reactions in petrochemical processes, focusing particularly on alkylation, isomerization, and cracking reactions. Their technology platform, "PROACT" (Process Reaction Optimization for Acid Catalysis Technology), integrates multiphase reaction engineering with computational fluid dynamics to optimize reaction parameters. Eni's system employs specialized reactor designs featuring controlled acid distribution zones that maintain optimal interfacial contact between reactants and catalyst. Their approach incorporates precise temperature control systems (typically operating between 0-80°C for liquid acid catalysts) with proprietary heat management technology that can handle the significant exotherms associated with strong acid catalysis. Eni has developed novel methods for quantifying the relationship between acid strength (measured by Hammett acidity functions) and reaction rates, enabling prediction of optimal conditions for maximum yield. Their industrial implementations have demonstrated yield improvements of 12-18% while extending catalyst lifetime by up to 300% through optimized reaction parameters that minimize side reactions and catalyst deactivation.
Strengths: Exceptional expertise in large-scale industrial acid catalysis; sophisticated modeling capabilities for complex multiphase systems; significant improvements in catalyst longevity reducing operational costs; proven track record in petrochemical applications. Weaknesses: Solutions often tailored to hydrocarbon processing with less applicability to fine chemicals; significant capital investment required for implementation; higher complexity in operational management.
Critical Parameters Affecting Arrhenius Acid Performance
Compositions of (z)-endoxifen and methods of enrichment thereof
PatentPendingAU2023206893A1
Innovation
- The development of industrially scalable synthetic methods involving ethyl acetate fractional crystallization, acetone recrystallization, and tetrahydrofuran recrystallization to produce highly pure (Z)-endoxifen, with specific conditions such as temperature and solvent usage to reduce impurities and enhance purity to at least 94% (w/w).
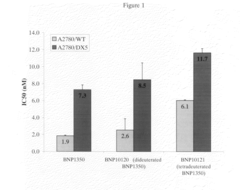
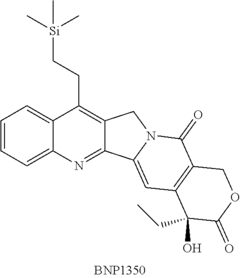
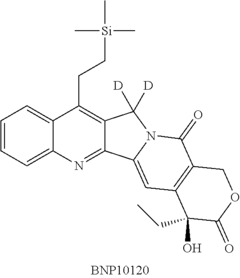
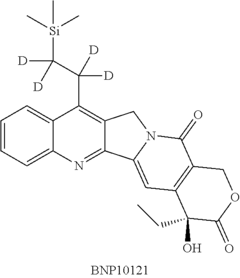
Deuterated analogs of (4S)-4-Ethyl-4-hydroxy-11-[2- (trimethylsilyl)ethyl]-1H-pyrano[3', 4':6,7] indolizino [1,2-b]quinoline-3,14(4H, 12H)-dione and methods of use thereof
PatentInactiveUS20120282261A1
Innovation
- Development of deuterated analogs of (4S)-4-Ethyl-4-hydroxy-11-[2-(trimethylsilyl)ethyl]-1H-pyrano[3′,4′:6,7]indolizino[1,2-b]quinoline-3,14(4H,12H)-dione, such as BNP10120 and BNP10121, and their pharmaceutically acceptable salts and derivatives, which are synthesized to improve metabolic profiles and reduce toxicity.
Environmental Impact and Green Chemistry Considerations
The optimization of reaction yields using Arrhenius acids must be evaluated not only for efficiency but also for environmental sustainability. Traditional acid-catalyzed processes often generate significant waste streams and utilize hazardous materials that pose environmental risks. Recent regulatory frameworks have increasingly emphasized the need for greener chemical processes, driving research toward more sustainable acid catalysis methods.
When employing Arrhenius acids in industrial processes, waste management represents a critical environmental concern. Strong mineral acids like sulfuric and hydrochloric acids generate substantial quantities of neutralization waste, often containing metal salts that require specialized disposal procedures. Advanced recovery and recycling systems for these acids can significantly reduce waste volumes, with closed-loop systems demonstrating up to 95% reduction in acid disposal requirements in certain applications.
Energy consumption associated with Arrhenius acid reactions presents another environmental challenge. Many acid-catalyzed processes require elevated temperatures or pressure conditions, contributing to carbon emissions. Optimization strategies that focus on lowering activation energies through careful acid selection can reduce these energy demands. For instance, replacing traditional mineral acids with solid acid catalysts has shown energy requirement reductions of 30-40% in selected petrochemical processes.
Green chemistry principles offer valuable frameworks for improving the environmental profile of Arrhenius acid applications. The substitution of conventional mineral acids with bio-derived organic acids represents a promising approach. Citric and lactic acids, derived from fermentation processes, have demonstrated comparable catalytic activity to traditional acids in certain reactions while significantly reducing environmental impact. These bio-derived alternatives produce biodegradable waste streams and require less energy-intensive neutralization procedures.
Solvent considerations also play a crucial role in the environmental footprint of acid-catalyzed reactions. Water-based systems or solvent-free approaches can dramatically reduce the environmental impact compared to traditional organic solvent systems. Recent developments in ionic liquid technology have enabled the creation of designer acidic media that combine high catalytic activity with minimal volatility and toxicity, addressing multiple green chemistry principles simultaneously.
Process intensification techniques further contribute to environmental optimization. Continuous flow reactors utilizing Arrhenius acids have demonstrated significant reductions in reagent usage and waste generation compared to batch processes. These systems enable precise control of reaction parameters, often allowing for lower acid concentrations while maintaining or improving yields, thereby reducing environmental impact while enhancing economic performance.
When employing Arrhenius acids in industrial processes, waste management represents a critical environmental concern. Strong mineral acids like sulfuric and hydrochloric acids generate substantial quantities of neutralization waste, often containing metal salts that require specialized disposal procedures. Advanced recovery and recycling systems for these acids can significantly reduce waste volumes, with closed-loop systems demonstrating up to 95% reduction in acid disposal requirements in certain applications.
Energy consumption associated with Arrhenius acid reactions presents another environmental challenge. Many acid-catalyzed processes require elevated temperatures or pressure conditions, contributing to carbon emissions. Optimization strategies that focus on lowering activation energies through careful acid selection can reduce these energy demands. For instance, replacing traditional mineral acids with solid acid catalysts has shown energy requirement reductions of 30-40% in selected petrochemical processes.
Green chemistry principles offer valuable frameworks for improving the environmental profile of Arrhenius acid applications. The substitution of conventional mineral acids with bio-derived organic acids represents a promising approach. Citric and lactic acids, derived from fermentation processes, have demonstrated comparable catalytic activity to traditional acids in certain reactions while significantly reducing environmental impact. These bio-derived alternatives produce biodegradable waste streams and require less energy-intensive neutralization procedures.
Solvent considerations also play a crucial role in the environmental footprint of acid-catalyzed reactions. Water-based systems or solvent-free approaches can dramatically reduce the environmental impact compared to traditional organic solvent systems. Recent developments in ionic liquid technology have enabled the creation of designer acidic media that combine high catalytic activity with minimal volatility and toxicity, addressing multiple green chemistry principles simultaneously.
Process intensification techniques further contribute to environmental optimization. Continuous flow reactors utilizing Arrhenius acids have demonstrated significant reductions in reagent usage and waste generation compared to batch processes. These systems enable precise control of reaction parameters, often allowing for lower acid concentrations while maintaining or improving yields, thereby reducing environmental impact while enhancing economic performance.
Scale-up Challenges and Industrial Implementation Strategies
Transitioning from laboratory-scale optimization to industrial production presents significant challenges when working with Arrhenius acids. The scale-up process introduces variables that are often not apparent in controlled laboratory environments, requiring careful engineering considerations and strategic implementation approaches.
Temperature control becomes increasingly critical at industrial scales, as larger reaction volumes create heat transfer limitations. Exothermic reactions involving strong Arrhenius acids can lead to dangerous hotspots or runaway reactions if cooling systems are inadequately designed. Industrial implementations typically require sophisticated heat exchangers and temperature monitoring systems distributed throughout reaction vessels.
Material compatibility issues are magnified during scale-up, as extended contact with corrosive acids demands specialized construction materials. While laboratory glassware may suffice for small-scale experiments, industrial reactors often require expensive alloys like Hastelloy or specialized coatings to withstand prolonged acid exposure while maintaining product purity.
Mixing efficiency represents another significant challenge, as achieving uniform acid distribution becomes more difficult in larger vessels. Industrial implementations frequently employ computational fluid dynamics to optimize impeller design and placement, ensuring homogeneous conditions throughout the reaction medium while minimizing energy consumption.
Safety considerations become paramount at industrial scale, necessitating robust containment systems, neutralization capabilities, and emergency protocols. Automated process control systems with redundant safety features are essential for managing the risks associated with large quantities of Arrhenius acids.
Continuous flow processing has emerged as a preferred implementation strategy for many acid-catalyzed reactions, offering better heat transfer, improved mixing, and enhanced safety profiles compared to batch processes. Microreactor and flow chemistry technologies enable precise residence time control and can significantly reduce the inventory of hazardous materials present at any given time.
Economic viability must be carefully assessed during scale-up, balancing capital expenditures against operational costs. Recovery and recycling systems for acids often become economically justified at industrial scales, reducing both raw material costs and waste treatment expenses.
Regulatory compliance adds another layer of complexity, with industrial implementations requiring thorough documentation of safety measures, environmental impact assessments, and worker protection protocols. Developing standardized operating procedures that address these requirements while maintaining optimal reaction yields is essential for successful commercialization.
Temperature control becomes increasingly critical at industrial scales, as larger reaction volumes create heat transfer limitations. Exothermic reactions involving strong Arrhenius acids can lead to dangerous hotspots or runaway reactions if cooling systems are inadequately designed. Industrial implementations typically require sophisticated heat exchangers and temperature monitoring systems distributed throughout reaction vessels.
Material compatibility issues are magnified during scale-up, as extended contact with corrosive acids demands specialized construction materials. While laboratory glassware may suffice for small-scale experiments, industrial reactors often require expensive alloys like Hastelloy or specialized coatings to withstand prolonged acid exposure while maintaining product purity.
Mixing efficiency represents another significant challenge, as achieving uniform acid distribution becomes more difficult in larger vessels. Industrial implementations frequently employ computational fluid dynamics to optimize impeller design and placement, ensuring homogeneous conditions throughout the reaction medium while minimizing energy consumption.
Safety considerations become paramount at industrial scale, necessitating robust containment systems, neutralization capabilities, and emergency protocols. Automated process control systems with redundant safety features are essential for managing the risks associated with large quantities of Arrhenius acids.
Continuous flow processing has emerged as a preferred implementation strategy for many acid-catalyzed reactions, offering better heat transfer, improved mixing, and enhanced safety profiles compared to batch processes. Microreactor and flow chemistry technologies enable precise residence time control and can significantly reduce the inventory of hazardous materials present at any given time.
Economic viability must be carefully assessed during scale-up, balancing capital expenditures against operational costs. Recovery and recycling systems for acids often become economically justified at industrial scales, reducing both raw material costs and waste treatment expenses.
Regulatory compliance adds another layer of complexity, with industrial implementations requiring thorough documentation of safety measures, environmental impact assessments, and worker protection protocols. Developing standardized operating procedures that address these requirements while maintaining optimal reaction yields is essential for successful commercialization.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!