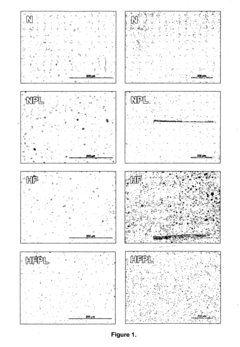
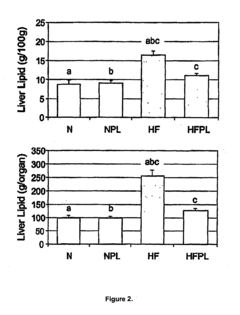
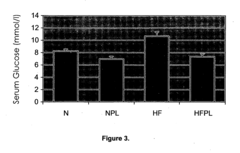
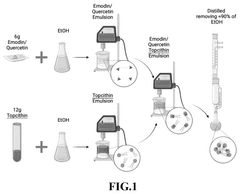
Phospholipid Strategies for Emerging Health Technologies
JUL 16, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Phospholipid Tech Evolution
Phospholipids have undergone significant technological evolution over the past decades, driven by advancements in biochemistry, nanotechnology, and medical sciences. Initially, these amphiphilic molecules were primarily studied for their structural role in cell membranes. However, as research progressed, their potential in drug delivery systems and biomedical applications became increasingly apparent.
The 1960s marked the beginning of liposome research, with the discovery that phospholipids could form bilayer vesicles. This breakthrough laid the foundation for using phospholipids as drug carriers. In the 1970s and 1980s, researchers focused on improving liposome stability and developing methods for controlled drug release. The introduction of PEGylated liposomes in the 1990s significantly enhanced their circulation time in the bloodstream, leading to the first FDA-approved liposomal drug formulation, Doxil, in 1995.
The early 2000s saw a shift towards more sophisticated phospholipid-based technologies. Researchers began exploring the use of phospholipids in targeted drug delivery systems, utilizing ligands and antibodies to enhance specificity. Concurrently, the development of solid lipid nanoparticles and nanostructured lipid carriers expanded the range of applications for phospholipids in drug delivery.
In recent years, the focus has shifted towards personalized medicine and theranostics. Phospholipid-based nanoparticles are being engineered to combine diagnostic and therapeutic functions, allowing for real-time monitoring of drug delivery and efficacy. Advanced manufacturing techniques, such as microfluidics and 3D printing, have enabled the production of more uniform and complex phospholipid structures.
The latest frontier in phospholipid technology involves their integration with other emerging fields. For instance, the combination of phospholipids with CRISPR-Cas9 technology has shown promise in gene therapy applications. Additionally, researchers are exploring the use of artificial intelligence and machine learning to optimize phospholipid formulations for specific therapeutic targets.
As we look to the future, the evolution of phospholipid technology is likely to continue in several directions. These include the development of stimuli-responsive phospholipid systems, the exploration of novel phospholipid sources (including synthetic and plant-based options), and the integration of phospholipids with other advanced materials such as graphene and quantum dots for enhanced functionality in both diagnostic and therapeutic applications.
The 1960s marked the beginning of liposome research, with the discovery that phospholipids could form bilayer vesicles. This breakthrough laid the foundation for using phospholipids as drug carriers. In the 1970s and 1980s, researchers focused on improving liposome stability and developing methods for controlled drug release. The introduction of PEGylated liposomes in the 1990s significantly enhanced their circulation time in the bloodstream, leading to the first FDA-approved liposomal drug formulation, Doxil, in 1995.
The early 2000s saw a shift towards more sophisticated phospholipid-based technologies. Researchers began exploring the use of phospholipids in targeted drug delivery systems, utilizing ligands and antibodies to enhance specificity. Concurrently, the development of solid lipid nanoparticles and nanostructured lipid carriers expanded the range of applications for phospholipids in drug delivery.
In recent years, the focus has shifted towards personalized medicine and theranostics. Phospholipid-based nanoparticles are being engineered to combine diagnostic and therapeutic functions, allowing for real-time monitoring of drug delivery and efficacy. Advanced manufacturing techniques, such as microfluidics and 3D printing, have enabled the production of more uniform and complex phospholipid structures.
The latest frontier in phospholipid technology involves their integration with other emerging fields. For instance, the combination of phospholipids with CRISPR-Cas9 technology has shown promise in gene therapy applications. Additionally, researchers are exploring the use of artificial intelligence and machine learning to optimize phospholipid formulations for specific therapeutic targets.
As we look to the future, the evolution of phospholipid technology is likely to continue in several directions. These include the development of stimuli-responsive phospholipid systems, the exploration of novel phospholipid sources (including synthetic and plant-based options), and the integration of phospholipids with other advanced materials such as graphene and quantum dots for enhanced functionality in both diagnostic and therapeutic applications.
Health Market Demand
The global health market is experiencing a significant shift towards innovative technologies that leverage phospholipids for improved therapeutic outcomes. This trend is driven by the increasing demand for more effective and targeted drug delivery systems, as well as the growing prevalence of chronic diseases and the need for personalized medicine.
Phospholipid-based strategies are gaining traction in various health sectors, particularly in the pharmaceutical and nutraceutical industries. The market for liposomal drug delivery systems, which utilize phospholipids as key components, is projected to grow substantially in the coming years. This growth is fueled by the ability of liposomes to enhance drug bioavailability, reduce toxicity, and improve patient compliance.
In the field of regenerative medicine, there is a rising demand for phospholipid-based scaffolds and matrices for tissue engineering applications. These biomaterials offer improved biocompatibility and can mimic the natural cellular environment more effectively than traditional synthetic materials.
The nutraceutical sector is also witnessing increased interest in phospholipid-based supplements, particularly those containing omega-3 fatty acids. Consumers are becoming more aware of the potential health benefits of phospholipids in supporting brain function, cardiovascular health, and overall cellular integrity.
Emerging health technologies are exploring the use of phospholipids in advanced diagnostic tools. For instance, phospholipid-coated nanoparticles are being developed for enhanced medical imaging and early disease detection. This application is expected to drive market growth in the medical diagnostics sector.
The cosmeceutical industry is another area where phospholipid strategies are gaining momentum. Phospholipid-based formulations are being increasingly used in skincare products for their moisturizing properties and ability to improve the delivery of active ingredients.
As personalized medicine continues to advance, there is a growing demand for phospholipid-based technologies that can facilitate targeted drug delivery and gene therapy. This trend is particularly evident in the oncology field, where liposomal formulations of cancer drugs are showing promising results in clinical trials.
The aging population in many developed countries is driving the demand for phospholipid-based technologies that address age-related health issues, such as cognitive decline and joint health. This demographic shift is expected to create new market opportunities for phospholipid-enhanced products in the coming years.
Phospholipid-based strategies are gaining traction in various health sectors, particularly in the pharmaceutical and nutraceutical industries. The market for liposomal drug delivery systems, which utilize phospholipids as key components, is projected to grow substantially in the coming years. This growth is fueled by the ability of liposomes to enhance drug bioavailability, reduce toxicity, and improve patient compliance.
In the field of regenerative medicine, there is a rising demand for phospholipid-based scaffolds and matrices for tissue engineering applications. These biomaterials offer improved biocompatibility and can mimic the natural cellular environment more effectively than traditional synthetic materials.
The nutraceutical sector is also witnessing increased interest in phospholipid-based supplements, particularly those containing omega-3 fatty acids. Consumers are becoming more aware of the potential health benefits of phospholipids in supporting brain function, cardiovascular health, and overall cellular integrity.
Emerging health technologies are exploring the use of phospholipids in advanced diagnostic tools. For instance, phospholipid-coated nanoparticles are being developed for enhanced medical imaging and early disease detection. This application is expected to drive market growth in the medical diagnostics sector.
The cosmeceutical industry is another area where phospholipid strategies are gaining momentum. Phospholipid-based formulations are being increasingly used in skincare products for their moisturizing properties and ability to improve the delivery of active ingredients.
As personalized medicine continues to advance, there is a growing demand for phospholipid-based technologies that can facilitate targeted drug delivery and gene therapy. This trend is particularly evident in the oncology field, where liposomal formulations of cancer drugs are showing promising results in clinical trials.
The aging population in many developed countries is driving the demand for phospholipid-based technologies that address age-related health issues, such as cognitive decline and joint health. This demographic shift is expected to create new market opportunities for phospholipid-enhanced products in the coming years.
Current Challenges
Phospholipid-based strategies in emerging health technologies face several significant challenges that hinder their widespread adoption and effectiveness. One of the primary obstacles is the stability of phospholipid formulations. These structures are inherently sensitive to environmental factors such as temperature, pH, and oxidative stress, which can lead to degradation and loss of functionality. This instability poses difficulties in storage, transportation, and maintaining the efficacy of phospholipid-based therapeutics over extended periods.
Another critical challenge lies in the controlled release and targeted delivery of phospholipid-based drug carriers. While phospholipids offer excellent biocompatibility and the ability to encapsulate various therapeutic agents, achieving precise control over the release kinetics and ensuring targeted delivery to specific tissues or cells remains complex. This challenge is particularly pronounced in the treatment of diseases that require sustained drug release or site-specific action.
The scalability of phospholipid-based technologies presents a significant hurdle in their transition from laboratory research to industrial production. The processes involved in manufacturing phospholipid formulations at a large scale while maintaining consistency and quality are often complex and costly. This scalability issue affects not only the production of therapeutics but also the development of diagnostic tools and other health technologies that rely on phospholipid strategies.
Regulatory challenges also play a crucial role in the development and commercialization of phospholipid-based health technologies. The novel nature of many phospholipid formulations means that regulatory frameworks may not be fully equipped to assess their safety and efficacy. This can lead to prolonged approval processes and increased development costs, potentially deterring investment in these innovative technologies.
Furthermore, the biological variability among individuals poses a challenge in developing universally effective phospholipid-based therapies. Differences in metabolism, immune responses, and physiological conditions can significantly impact the performance of phospholipid formulations, necessitating personalized approaches that are often difficult to implement on a large scale.
Lastly, the integration of phospholipid strategies with other emerging technologies, such as nanotechnology and gene therapy, presents both opportunities and challenges. While these combinations offer immense potential for innovative treatments, they also introduce additional layers of complexity in terms of design, characterization, and safety assessment. Overcoming these multifaceted challenges requires interdisciplinary collaboration and continued research efforts to unlock the full potential of phospholipid strategies in emerging health technologies.
Another critical challenge lies in the controlled release and targeted delivery of phospholipid-based drug carriers. While phospholipids offer excellent biocompatibility and the ability to encapsulate various therapeutic agents, achieving precise control over the release kinetics and ensuring targeted delivery to specific tissues or cells remains complex. This challenge is particularly pronounced in the treatment of diseases that require sustained drug release or site-specific action.
The scalability of phospholipid-based technologies presents a significant hurdle in their transition from laboratory research to industrial production. The processes involved in manufacturing phospholipid formulations at a large scale while maintaining consistency and quality are often complex and costly. This scalability issue affects not only the production of therapeutics but also the development of diagnostic tools and other health technologies that rely on phospholipid strategies.
Regulatory challenges also play a crucial role in the development and commercialization of phospholipid-based health technologies. The novel nature of many phospholipid formulations means that regulatory frameworks may not be fully equipped to assess their safety and efficacy. This can lead to prolonged approval processes and increased development costs, potentially deterring investment in these innovative technologies.
Furthermore, the biological variability among individuals poses a challenge in developing universally effective phospholipid-based therapies. Differences in metabolism, immune responses, and physiological conditions can significantly impact the performance of phospholipid formulations, necessitating personalized approaches that are often difficult to implement on a large scale.
Lastly, the integration of phospholipid strategies with other emerging technologies, such as nanotechnology and gene therapy, presents both opportunities and challenges. While these combinations offer immense potential for innovative treatments, they also introduce additional layers of complexity in terms of design, characterization, and safety assessment. Overcoming these multifaceted challenges requires interdisciplinary collaboration and continued research efforts to unlock the full potential of phospholipid strategies in emerging health technologies.
Existing Applications
01 Phospholipid synthesis and extraction methods
Various methods for synthesizing and extracting phospholipids from natural sources or through chemical processes. These techniques aim to produce high-quality phospholipids for use in pharmaceuticals, cosmetics, and food industries.- Phospholipid synthesis and modification: Various methods for synthesizing and modifying phospholipids are described. These include enzymatic processes, chemical reactions, and novel synthetic pathways to produce phospholipids with specific properties or structures. The techniques aim to create phospholipids for use in pharmaceuticals, cosmetics, and food industries.
- Phospholipid-based drug delivery systems: Phospholipids are utilized in the development of advanced drug delivery systems. These include liposomes, nanoparticles, and other lipid-based carriers that can encapsulate and transport therapeutic agents. The phospholipid-based systems enhance drug solubility, stability, and targeted delivery to specific tissues or cells.
- Analytical methods for phospholipid characterization: Various analytical techniques are employed to characterize phospholipids and their properties. These methods include mass spectrometry, chromatography, and spectroscopic approaches. The analytical tools are used to identify, quantify, and study the structure and behavior of phospholipids in different environments.
- Phospholipid applications in food and nutrition: Phospholipids are used in food and nutritional products for their emulsifying, stabilizing, and health-promoting properties. They are incorporated into functional foods, dietary supplements, and infant formulas. The applications focus on improving product texture, stability, and nutritional value.
- Phospholipid-based membrane models and biosensors: Phospholipids are used to create artificial membrane models and biosensors for studying cellular processes and detecting biomolecules. These systems mimic natural cell membranes and can be used to investigate membrane protein functions, drug interactions, and develop novel sensing platforms for various analytes.
02 Phospholipid-based drug delivery systems
Development of phospholipid-based formulations for improved drug delivery. These systems enhance drug solubility, stability, and bioavailability, leading to more effective therapeutic outcomes in various medical applications.Expand Specific Solutions03 Phospholipid analysis and characterization techniques
Advanced analytical methods for identifying, quantifying, and characterizing phospholipids in complex biological samples. These techniques include mass spectrometry, chromatography, and spectroscopic methods to elucidate phospholipid structures and compositions.Expand Specific Solutions04 Phospholipid applications in food and nutrition
Utilization of phospholipids in food products and nutritional supplements. These applications focus on improving food texture, stability, and nutritional value, as well as developing functional foods with health-promoting properties.Expand Specific Solutions05 Phospholipid-based cosmetic and personal care formulations
Incorporation of phospholipids in cosmetic and personal care products to enhance skin hydration, barrier function, and overall appearance. These formulations leverage the unique properties of phospholipids to improve product efficacy and stability.Expand Specific Solutions
Key Industry Players
The phospholipid strategies for emerging health technologies market is in a growth phase, driven by increasing applications in drug delivery, nutraceuticals, and personalized medicine. The global market size is estimated to reach several billion dollars by 2025, with a compound annual growth rate of over 10%. Technologically, the field is advancing rapidly, with companies like Vascular Biogenics, NOF Corp., and Aker Biomarine Antarctic AS leading innovation in areas such as targeted drug delivery and biocompatible materials. Academic institutions like The University of Sydney and Max Planck Society are contributing significant research. While some applications are mature, novel areas like nanomedicine and liposomal formulations are still evolving, indicating potential for further growth and technological breakthroughs in this competitive landscape.
Vascular Biogenics Ltd.
Technical Solution: Vascular Biogenics Ltd. has developed a proprietary Vascular Targeting System (VTS) platform that utilizes phospholipid-based nanoparticles to selectively target and destroy diseased blood vessels. Their lead candidate, VB-111, combines a non-replicating adenovirus with a proprietary modified murine pre-proendothelin promoter (PPE-1-3x) for the targeted expression of a fas-chimera transgene specifically in angiogenic blood vessels[1]. This approach allows for highly specific targeting of tumor vasculature while minimizing off-target effects. The phospholipid nanoparticles enable efficient delivery of the genetic payload to endothelial cells, enhancing the therapeutic potential for various cancers and other angiogenesis-dependent diseases[2].
Strengths: Highly specific targeting of angiogenic blood vessels, minimizing off-target effects. Versatile platform applicable to multiple diseases. Weaknesses: Complex manufacturing process, potential immunogenicity of viral vector.
NOF Corp.
Technical Solution: NOF Corporation has developed advanced phospholipid technologies for drug delivery and emerging health applications. Their COATSOME® liposome technology utilizes synthetic phospholipids to create stable, uniform liposomes for encapsulating various therapeutic agents[3]. These liposomes can be tailored with different surface modifications, such as PEGylation, to enhance circulation time and targeting efficiency. NOF's phospholipid-based drug delivery systems have been applied in various fields, including cancer therapy, gene delivery, and vaccine development. The company has also developed novel phospholipid conjugates, such as their SUNBRIGHT® series, which can be used to create stealth liposomes or for protein modification[4]. These technologies enable the development of more effective and targeted therapies with improved pharmacokinetics and reduced side effects.
Strengths: Versatile phospholipid technologies applicable to multiple therapeutic areas. Established track record in drug delivery. Weaknesses: Potential competition from other advanced drug delivery technologies. Regulatory challenges for novel formulations.
Innovative Formulations
Treatment of metabolic disease
PatentInactiveUS20100267611A1
Innovation
- A method involving the use of a composition containing phospholipids effective for lowering triglyceride and/or cholesterol levels, which can be administered as a medicament, dietary supplement, or functional food, with a specific ratio of saturated, monounsaturated, and polyunsaturated phospholipids, along with triglycerides and non-lipid components, to minimize the accumulation of these lipids in individuals at risk or with metabolic diseases.
Methods and formulations for improving the pharmacokinetic properties of nutraceutical compounds
PatentPendingUS20250064729A1
Innovation
- The development of a hydrogel composition that includes a phospholipid complex with solubilized phospholipids and nutraceutical compounds, enhancing their solubility and bioavailability for oral administration.
Regulatory Framework
The regulatory framework surrounding phospholipid-based health technologies is complex and evolving, reflecting the innovative nature of these emerging applications. Regulatory bodies worldwide are adapting their approaches to address the unique challenges posed by phospholipid-based products, which often blur the lines between traditional drug, device, and biological classifications.
In the United States, the Food and Drug Administration (FDA) has taken steps to develop specific guidance for phospholipid-based technologies. The agency's approach typically involves a case-by-case evaluation, considering the intended use, mechanism of action, and risk profile of each product. For instance, liposomal drug delivery systems are generally regulated as combination products, requiring coordination between the Center for Drug Evaluation and Research (CDER) and the Center for Devices and Radiological Health (CDRH).
The European Medicines Agency (EMA) has also recognized the need for tailored regulatory pathways for phospholipid-based technologies. The EMA's Committee for Advanced Therapies (CAT) plays a crucial role in assessing these innovative products, particularly when they involve gene therapies or tissue engineering. The agency has issued several reflection papers and guidelines addressing specific aspects of phospholipid-based technologies, such as quality considerations for liposomes and nanoparticles.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has implemented a "Sakigake" designation system to expedite the review process for breakthrough technologies, including some phospholipid-based innovations. This system aims to promote early practical application of cutting-edge medical products, potentially benefiting developers of novel phospholipid strategies.
Globally, regulatory harmonization efforts are underway to address the challenges posed by these emerging technologies. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has initiated discussions on developing guidelines specific to nanomedicines, which often incorporate phospholipid-based strategies.
As the field advances, regulatory frameworks are likely to continue evolving. Key areas of focus include establishing standardized characterization methods for phospholipid-based products, developing appropriate safety assessment protocols, and addressing long-term stability and biocompatibility concerns. Regulatory agencies are also grappling with the challenge of regulating personalized medicine applications that utilize phospholipid technologies, such as tailored liposomal formulations.
Manufacturers and researchers in this field must maintain close communication with regulatory authorities throughout the development process. Early engagement and frequent consultations can help navigate the complex regulatory landscape and ensure compliance with evolving requirements. As phospholipid strategies continue to push the boundaries of health technologies, a flexible and adaptive regulatory approach will be crucial to balance innovation with patient safety and product efficacy.
In the United States, the Food and Drug Administration (FDA) has taken steps to develop specific guidance for phospholipid-based technologies. The agency's approach typically involves a case-by-case evaluation, considering the intended use, mechanism of action, and risk profile of each product. For instance, liposomal drug delivery systems are generally regulated as combination products, requiring coordination between the Center for Drug Evaluation and Research (CDER) and the Center for Devices and Radiological Health (CDRH).
The European Medicines Agency (EMA) has also recognized the need for tailored regulatory pathways for phospholipid-based technologies. The EMA's Committee for Advanced Therapies (CAT) plays a crucial role in assessing these innovative products, particularly when they involve gene therapies or tissue engineering. The agency has issued several reflection papers and guidelines addressing specific aspects of phospholipid-based technologies, such as quality considerations for liposomes and nanoparticles.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has implemented a "Sakigake" designation system to expedite the review process for breakthrough technologies, including some phospholipid-based innovations. This system aims to promote early practical application of cutting-edge medical products, potentially benefiting developers of novel phospholipid strategies.
Globally, regulatory harmonization efforts are underway to address the challenges posed by these emerging technologies. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has initiated discussions on developing guidelines specific to nanomedicines, which often incorporate phospholipid-based strategies.
As the field advances, regulatory frameworks are likely to continue evolving. Key areas of focus include establishing standardized characterization methods for phospholipid-based products, developing appropriate safety assessment protocols, and addressing long-term stability and biocompatibility concerns. Regulatory agencies are also grappling with the challenge of regulating personalized medicine applications that utilize phospholipid technologies, such as tailored liposomal formulations.
Manufacturers and researchers in this field must maintain close communication with regulatory authorities throughout the development process. Early engagement and frequent consultations can help navigate the complex regulatory landscape and ensure compliance with evolving requirements. As phospholipid strategies continue to push the boundaries of health technologies, a flexible and adaptive regulatory approach will be crucial to balance innovation with patient safety and product efficacy.
Biocompatibility Aspects
Phospholipids play a crucial role in the biocompatibility of emerging health technologies. Their structural similarity to cell membranes makes them ideal candidates for enhancing the integration of medical devices and drug delivery systems with biological tissues. The amphiphilic nature of phospholipids allows them to form stable bilayers and liposomes, which can encapsulate drugs or act as protective coatings for implants.
One of the primary biocompatibility aspects of phospholipid-based strategies is their ability to reduce foreign body responses. When used as coatings on medical implants, phospholipids can mimic the surface of native cells, effectively masking the device from the immune system. This reduction in immunogenicity leads to decreased inflammation and improved long-term compatibility of implanted devices.
In drug delivery applications, phospholipid-based nanocarriers demonstrate enhanced biocompatibility compared to synthetic polymers. Their natural origin and biodegradability contribute to reduced toxicity and improved cellular uptake. Liposomal formulations, in particular, have shown remarkable success in delivering therapeutic agents with minimal side effects, as evidenced by FDA-approved treatments like Doxil for cancer therapy.
The interaction between phospholipids and proteins is another critical aspect of biocompatibility. Phospholipid membranes can incorporate functional proteins, creating biomimetic interfaces that facilitate cell adhesion and tissue integration. This property is particularly valuable in tissue engineering applications, where phospholipid-based scaffolds can promote cell growth and differentiation.
Phospholipids also contribute to the hemocompatibility of blood-contacting devices. By mimicking the surface of red blood cells, phospholipid coatings can reduce platelet adhesion and activation, thereby minimizing the risk of thrombosis. This property is especially beneficial for cardiovascular implants and extracorporeal circulation devices.
Recent advancements in phospholipid engineering have led to the development of "stealth" liposomes, which incorporate polyethylene glycol (PEG) into their surface. These PEGylated phospholipid structures exhibit prolonged circulation times in the bloodstream, further enhancing their biocompatibility and therapeutic efficacy.
The biocompatibility of phospholipid-based technologies extends to their potential in crossing biological barriers. Lipid nanoparticles have shown promise in facilitating the delivery of drugs and genetic material across the blood-brain barrier, opening new avenues for treating neurological disorders. This ability to navigate complex biological interfaces while maintaining compatibility underscores the versatility of phospholipid strategies in emerging health technologies.
One of the primary biocompatibility aspects of phospholipid-based strategies is their ability to reduce foreign body responses. When used as coatings on medical implants, phospholipids can mimic the surface of native cells, effectively masking the device from the immune system. This reduction in immunogenicity leads to decreased inflammation and improved long-term compatibility of implanted devices.
In drug delivery applications, phospholipid-based nanocarriers demonstrate enhanced biocompatibility compared to synthetic polymers. Their natural origin and biodegradability contribute to reduced toxicity and improved cellular uptake. Liposomal formulations, in particular, have shown remarkable success in delivering therapeutic agents with minimal side effects, as evidenced by FDA-approved treatments like Doxil for cancer therapy.
The interaction between phospholipids and proteins is another critical aspect of biocompatibility. Phospholipid membranes can incorporate functional proteins, creating biomimetic interfaces that facilitate cell adhesion and tissue integration. This property is particularly valuable in tissue engineering applications, where phospholipid-based scaffolds can promote cell growth and differentiation.
Phospholipids also contribute to the hemocompatibility of blood-contacting devices. By mimicking the surface of red blood cells, phospholipid coatings can reduce platelet adhesion and activation, thereby minimizing the risk of thrombosis. This property is especially beneficial for cardiovascular implants and extracorporeal circulation devices.
Recent advancements in phospholipid engineering have led to the development of "stealth" liposomes, which incorporate polyethylene glycol (PEG) into their surface. These PEGylated phospholipid structures exhibit prolonged circulation times in the bloodstream, further enhancing their biocompatibility and therapeutic efficacy.
The biocompatibility of phospholipid-based technologies extends to their potential in crossing biological barriers. Lipid nanoparticles have shown promise in facilitating the delivery of drugs and genetic material across the blood-brain barrier, opening new avenues for treating neurological disorders. This ability to navigate complex biological interfaces while maintaining compatibility underscores the versatility of phospholipid strategies in emerging health technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!