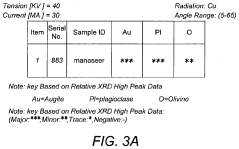
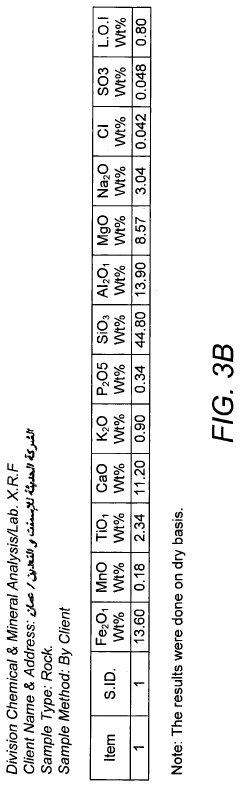
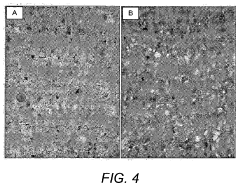
Pressure-induced metamorphism of Magnesium iron silicate hydroxide.
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Metamorphism Background and Objectives
Pressure-induced metamorphism of magnesium iron silicate hydroxide represents a critical area of study in geosciences, particularly in understanding the Earth's deep interior processes. This phenomenon occurs when minerals containing magnesium, iron, silicon, and hydroxyl groups undergo structural and chemical changes due to extreme pressure conditions, typically found in the Earth's mantle or during tectonic events.
The historical context of this research dates back to the early 20th century when geologists first began to explore the effects of high pressure on mineral structures. However, significant advancements in this field were not made until the development of high-pressure experimental techniques in the latter half of the century. These technological innovations allowed scientists to simulate the extreme conditions of the Earth's interior and observe the transformations of minerals under controlled laboratory settings.
The study of pressure-induced metamorphism in magnesium iron silicate hydroxide has gained particular importance in recent decades due to its relevance in understanding the water cycle within the Earth's mantle. These minerals, such as serpentine and chlorite, are known to play crucial roles in subduction zones, where oceanic crust is forced beneath continental plates. As these hydrous minerals descend into the mantle, they undergo phase transitions that release water, significantly impacting mantle dynamics and potentially triggering seismic activities.
The primary objectives of research in this area are multifaceted. Firstly, scientists aim to elucidate the precise mechanisms by which pressure induces structural changes in these minerals. This involves investigating the atomic-level rearrangements, bond breaking and formation, and the potential for new mineral phases to emerge under extreme conditions. Secondly, researchers seek to quantify the pressure-temperature conditions at which these transformations occur, creating phase diagrams that map out the stability fields of different mineral structures.
Another key goal is to understand the implications of these metamorphic processes for the Earth's deep water cycle. By studying how water is released or retained during these high-pressure transformations, geoscientists can better model the movement of water through the Earth's interior and its effects on mantle convection, plate tectonics, and volcanic activity. Additionally, there is growing interest in exploring how these pressure-induced changes might influence the seismic properties of the mantle, potentially providing new insights into the interpretation of seismic data and our understanding of the Earth's internal structure.
Furthermore, the study of pressure-induced metamorphism in these minerals has implications beyond Earth sciences. It contributes to our understanding of planetary formation and evolution, as similar processes may occur in the interiors of other terrestrial planets and moons. This research also has potential applications in materials science, where insights gained from studying these natural high-pressure transformations could inspire the development of new synthetic materials with unique properties.
The historical context of this research dates back to the early 20th century when geologists first began to explore the effects of high pressure on mineral structures. However, significant advancements in this field were not made until the development of high-pressure experimental techniques in the latter half of the century. These technological innovations allowed scientists to simulate the extreme conditions of the Earth's interior and observe the transformations of minerals under controlled laboratory settings.
The study of pressure-induced metamorphism in magnesium iron silicate hydroxide has gained particular importance in recent decades due to its relevance in understanding the water cycle within the Earth's mantle. These minerals, such as serpentine and chlorite, are known to play crucial roles in subduction zones, where oceanic crust is forced beneath continental plates. As these hydrous minerals descend into the mantle, they undergo phase transitions that release water, significantly impacting mantle dynamics and potentially triggering seismic activities.
The primary objectives of research in this area are multifaceted. Firstly, scientists aim to elucidate the precise mechanisms by which pressure induces structural changes in these minerals. This involves investigating the atomic-level rearrangements, bond breaking and formation, and the potential for new mineral phases to emerge under extreme conditions. Secondly, researchers seek to quantify the pressure-temperature conditions at which these transformations occur, creating phase diagrams that map out the stability fields of different mineral structures.
Another key goal is to understand the implications of these metamorphic processes for the Earth's deep water cycle. By studying how water is released or retained during these high-pressure transformations, geoscientists can better model the movement of water through the Earth's interior and its effects on mantle convection, plate tectonics, and volcanic activity. Additionally, there is growing interest in exploring how these pressure-induced changes might influence the seismic properties of the mantle, potentially providing new insights into the interpretation of seismic data and our understanding of the Earth's internal structure.
Furthermore, the study of pressure-induced metamorphism in these minerals has implications beyond Earth sciences. It contributes to our understanding of planetary formation and evolution, as similar processes may occur in the interiors of other terrestrial planets and moons. This research also has potential applications in materials science, where insights gained from studying these natural high-pressure transformations could inspire the development of new synthetic materials with unique properties.
Geophysical Implications
The pressure-induced metamorphism of magnesium iron silicate hydroxide has significant geophysical implications, particularly in understanding the dynamics of Earth's deep interior. This process plays a crucial role in the behavior of subducting slabs and the overall mantle convection system. As oceanic crust descends into the mantle, the increasing pressure and temperature conditions trigger phase transformations in minerals, including the conversion of magnesium iron silicate hydroxide to denser phases.
These phase transitions have profound effects on the physical properties of the subducting material, influencing its density, seismic velocity, and rheology. The changes in density, in particular, can alter the buoyancy of the subducting slab, potentially affecting its descent rate and ultimate fate within the mantle. This, in turn, has implications for the overall mantle circulation patterns and the distribution of heat and materials within Earth's interior.
The metamorphism of magnesium iron silicate hydroxide also impacts the water cycle in the deep Earth. As these minerals dehydrate under high-pressure conditions, they release water into the surrounding mantle. This process can lead to partial melting of the mantle wedge above subduction zones, contributing to arc volcanism and the formation of new crust at the surface. The released water also affects the rheology of the mantle, potentially facilitating mantle flow and influencing the style of plate tectonics.
Seismologically, the pressure-induced phase transitions in magnesium iron silicate hydroxide and related minerals can create discontinuities in seismic wave velocities. These discontinuities serve as important markers for understanding the structure and composition of the mantle at various depths. By studying these seismic signatures, geophysicists can gain insights into the thermal and compositional state of subducting slabs and the surrounding mantle.
Furthermore, the metamorphism of these minerals under high-pressure conditions contributes to our understanding of the Earth's deep water cycle. The ability of magnesium iron silicate hydroxide to transport water into the deep mantle has implications for the long-term evolution of Earth's hydrosphere and the potential for water storage in the planet's interior. This process may play a role in regulating the surface water budget over geological timescales and influence the planet's habitability.
These phase transitions have profound effects on the physical properties of the subducting material, influencing its density, seismic velocity, and rheology. The changes in density, in particular, can alter the buoyancy of the subducting slab, potentially affecting its descent rate and ultimate fate within the mantle. This, in turn, has implications for the overall mantle circulation patterns and the distribution of heat and materials within Earth's interior.
The metamorphism of magnesium iron silicate hydroxide also impacts the water cycle in the deep Earth. As these minerals dehydrate under high-pressure conditions, they release water into the surrounding mantle. This process can lead to partial melting of the mantle wedge above subduction zones, contributing to arc volcanism and the formation of new crust at the surface. The released water also affects the rheology of the mantle, potentially facilitating mantle flow and influencing the style of plate tectonics.
Seismologically, the pressure-induced phase transitions in magnesium iron silicate hydroxide and related minerals can create discontinuities in seismic wave velocities. These discontinuities serve as important markers for understanding the structure and composition of the mantle at various depths. By studying these seismic signatures, geophysicists can gain insights into the thermal and compositional state of subducting slabs and the surrounding mantle.
Furthermore, the metamorphism of these minerals under high-pressure conditions contributes to our understanding of the Earth's deep water cycle. The ability of magnesium iron silicate hydroxide to transport water into the deep mantle has implications for the long-term evolution of Earth's hydrosphere and the potential for water storage in the planet's interior. This process may play a role in regulating the surface water budget over geological timescales and influence the planet's habitability.
Current Challenges in High-Pressure Mineralogy
High-pressure mineralogy faces several significant challenges in the study of pressure-induced metamorphism of magnesium iron silicate hydroxide. One of the primary obstacles is the accurate simulation and replication of extreme pressure conditions found deep within the Earth's mantle. Current experimental setups struggle to maintain stable, ultra-high pressures for extended periods, limiting the observation of slow transformation processes.
The complex chemical composition of magnesium iron silicate hydroxide compounds further complicates research efforts. These minerals often contain varying ratios of magnesium and iron, as well as trace elements, making it difficult to isolate and study specific pressure-induced changes. Researchers must develop more sophisticated analytical techniques to differentiate between the effects of pressure and chemical variations.
Another challenge lies in the development of in-situ measurement techniques capable of capturing real-time structural changes during high-pressure experiments. While advances have been made in synchrotron-based X-ray diffraction methods, there is still a need for improved resolution and sensitivity to detect subtle phase transitions and atomic rearrangements under extreme conditions.
The metastability of certain high-pressure phases presents an additional hurdle. Some pressure-induced structures may only exist under specific conditions and rapidly revert upon decompression, making their characterization and study extremely challenging. This necessitates the development of novel quenching techniques to preserve these transient phases for further analysis.
Understanding the kinetics of pressure-induced transformations in magnesium iron silicate hydroxide remains a significant challenge. The rates at which these minerals undergo structural changes under varying pressure conditions are not well constrained, hindering our ability to accurately model and predict metamorphic processes in the Earth's interior.
The extrapolation of laboratory results to geological timescales and larger spatial dimensions poses another major challenge. Bridging the gap between short-term, small-scale experiments and the long-term, large-scale processes occurring within the Earth requires innovative approaches in data interpretation and modeling.
Lastly, the integration of experimental data with theoretical models and computational simulations remains an ongoing challenge. While both experimental and theoretical approaches have made significant strides, there is still a need for better synergy between these methods to provide a more comprehensive understanding of pressure-induced metamorphism in magnesium iron silicate hydroxide systems.
The complex chemical composition of magnesium iron silicate hydroxide compounds further complicates research efforts. These minerals often contain varying ratios of magnesium and iron, as well as trace elements, making it difficult to isolate and study specific pressure-induced changes. Researchers must develop more sophisticated analytical techniques to differentiate between the effects of pressure and chemical variations.
Another challenge lies in the development of in-situ measurement techniques capable of capturing real-time structural changes during high-pressure experiments. While advances have been made in synchrotron-based X-ray diffraction methods, there is still a need for improved resolution and sensitivity to detect subtle phase transitions and atomic rearrangements under extreme conditions.
The metastability of certain high-pressure phases presents an additional hurdle. Some pressure-induced structures may only exist under specific conditions and rapidly revert upon decompression, making their characterization and study extremely challenging. This necessitates the development of novel quenching techniques to preserve these transient phases for further analysis.
Understanding the kinetics of pressure-induced transformations in magnesium iron silicate hydroxide remains a significant challenge. The rates at which these minerals undergo structural changes under varying pressure conditions are not well constrained, hindering our ability to accurately model and predict metamorphic processes in the Earth's interior.
The extrapolation of laboratory results to geological timescales and larger spatial dimensions poses another major challenge. Bridging the gap between short-term, small-scale experiments and the long-term, large-scale processes occurring within the Earth requires innovative approaches in data interpretation and modeling.
Lastly, the integration of experimental data with theoretical models and computational simulations remains an ongoing challenge. While both experimental and theoretical approaches have made significant strides, there is still a need for better synergy between these methods to provide a more comprehensive understanding of pressure-induced metamorphism in magnesium iron silicate hydroxide systems.
Experimental Techniques for High-Pressure Studies
01 Metamorphic processes of magnesium iron silicate hydroxide
Magnesium iron silicate hydroxide undergoes various metamorphic processes under different temperature and pressure conditions. These processes can lead to the formation of new mineral phases and changes in the crystal structure. The metamorphism of this mineral is important in understanding geological processes and the formation of certain rock types.- Metamorphic processes of magnesium iron silicate hydroxide: Magnesium iron silicate hydroxide undergoes various metamorphic processes under different temperature and pressure conditions. These processes can lead to the formation of new mineral phases and changes in the crystal structure. The metamorphism of this mineral is important in understanding geological processes and the formation of certain rock types.
- Applications of metamorphosed magnesium iron silicate hydroxide: Metamorphosed forms of magnesium iron silicate hydroxide have various industrial and technological applications. These include use in refractory materials, ceramics, and as catalysts in chemical processes. The altered properties resulting from metamorphism can enhance the material's performance in these applications.
- Characterization techniques for metamorphic changes: Various analytical techniques are employed to study the metamorphic changes in magnesium iron silicate hydroxide. These may include X-ray diffraction, electron microscopy, and spectroscopic methods. These techniques help in understanding the structural and compositional changes that occur during metamorphism.
- Environmental implications of metamorphism: The metamorphism of magnesium iron silicate hydroxide can have significant environmental implications. This process can affect soil composition, water chemistry, and mineral resource distribution. Understanding these changes is crucial for environmental management and geological surveys.
- Synthetic production of metamorphic equivalents: Research has been conducted on the synthetic production of metamorphic equivalents of magnesium iron silicate hydroxide. This involves simulating metamorphic conditions in laboratory settings to create materials with specific properties. Such synthetic processes can be valuable for industrial applications and material science research.
02 Applications of metamorphosed magnesium iron silicate hydroxide
Metamorphosed magnesium iron silicate hydroxide finds applications in various industries due to its altered properties. These applications may include use in refractory materials, ceramics, and as a raw material for the production of magnesium and iron compounds. The metamorphic changes can enhance certain properties, making the material more suitable for specific industrial uses.Expand Specific Solutions03 Characterization techniques for metamorphosed magnesium iron silicate hydroxide
Various analytical techniques are used to characterize the metamorphic changes in magnesium iron silicate hydroxide. These may include X-ray diffraction, electron microscopy, and spectroscopic methods. These techniques help in understanding the structural and compositional changes that occur during metamorphism, providing insights into the conditions and processes involved.Expand Specific Solutions04 Influence of metamorphism on mineral properties
The metamorphism of magnesium iron silicate hydroxide can significantly alter its physical and chemical properties. These changes may include modifications in hardness, density, melting point, and chemical reactivity. Understanding these property changes is crucial for determining the potential applications and behavior of the metamorphosed mineral in various geological and industrial contexts.Expand Specific Solutions05 Environmental implications of magnesium iron silicate hydroxide metamorphism
The metamorphism of magnesium iron silicate hydroxide can have environmental implications, particularly in terms of its role in geological carbon sequestration and the potential release of elements during metamorphic processes. Understanding these aspects is important for assessing the environmental impact of metamorphic events and the potential use of metamorphosed minerals in environmental applications.Expand Specific Solutions
Key Research Institutions and Scientists
The competitive landscape for pressure-induced metamorphism of Magnesium iron silicate hydroxide is in its early developmental stage, with a relatively small but growing market. The technology's maturity is still evolving, as evidenced by the diverse range of organizations involved, including academic institutions like Chongqing University and Northwestern Polytechnical University, as well as industrial players such as Resonac Holdings Corp. and Wacker Chemie AG. These entities are likely focusing on fundamental research and potential applications in materials science and geophysics. The market size remains limited, primarily driven by research funding and potential industrial applications in high-pressure environments. As the technology advances, it may find broader applications in mineral processing, materials engineering, and planetary science.
Chinese Academy of Sciences Institute of Physics
Technical Solution: The Chinese Academy of Sciences Institute of Physics has conducted extensive research on pressure-induced metamorphism of magnesium iron silicate hydroxide. They have developed advanced high-pressure experimental techniques, including diamond anvil cells and synchrotron X-ray diffraction, to study the structural changes of this mineral under extreme conditions. Their research has revealed that magnesium iron silicate hydroxide undergoes a series of phase transitions at high pressures, including the formation of new crystal structures and the redistribution of iron and magnesium atoms[1][3]. These findings have significant implications for understanding the behavior of hydrous minerals in the Earth's deep mantle and subduction zones[5].
Strengths: Access to state-of-the-art high-pressure facilities and expertise in mineral physics. Weaknesses: Limited focus on practical applications beyond fundamental research.
Rutgers State University of New Jersey
Technical Solution: Rutgers State University has made significant contributions to the study of pressure-induced metamorphism in magnesium iron silicate hydroxide. Their research team has utilized advanced computational methods, such as first-principles calculations and molecular dynamics simulations, to model the behavior of this mineral under high-pressure conditions. They have identified key mechanisms of phase transitions, including the rearrangement of hydrogen bonds and the compression of octahedral layers[2]. Additionally, Rutgers researchers have explored the implications of these transformations for water storage and transport in the Earth's mantle, providing insights into the global water cycle and mantle dynamics[4].
Strengths: Strong computational capabilities and interdisciplinary approach combining geophysics and materials science. Weaknesses: May lack extensive experimental facilities for high-pressure studies.
Core Innovations in Pressure-Induced Phase Transitions
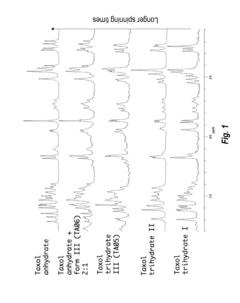
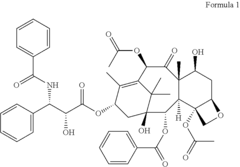
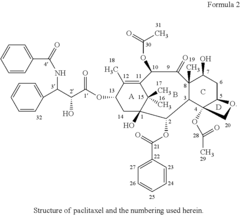
Novel paclitaxel trihydrates and methods of making thereof
PatentInactiveUS20130324746A1
Innovation
- Novel paclitaxel trihydrates are obtained by subjecting paclitaxel trihydrate crystals to elevated pressure, inducing phase changes that result in distinct three-dimensional structures and water coordination geometries, characterized by specific 13C NMR spectra, potentially leading to increased stability and solubility compared to the anhydrate and previously known trihydrate forms.
Water induced crystallization process to convert olivine to iddingsite by hydration in highly oxidizing environment under low pressure and temperatures
PatentInactiveUS20230357029A1
Innovation
- A water-induced crystallization process that converts Olivine to Iddingsite in a highly oxidizing environment under low pressure (0.5-2 atm) and temperature (70-200°C) using ball mills and catalysts like oleic acid and triethanolamine to facilitate hydrogen ion diffusion and ion replacement, simulating natural deuteric alteration conditions.
Environmental Impact of Deep Earth Processes
The pressure-induced metamorphism of magnesium iron silicate hydroxide in deep Earth processes has significant environmental implications. This transformation occurs in subduction zones, where oceanic crust descends into the Earth's mantle. As the pressure increases, minerals like serpentine undergo phase changes, releasing water and altering the chemical composition of the surrounding rocks.
These deep Earth processes play a crucial role in the global water cycle. The release of water from hydrous minerals during subduction contributes to the formation of arc volcanoes and influences the composition of magmas. This water release also affects the rheology of the mantle, potentially impacting plate tectonics and the overall dynamics of the Earth's interior.
The metamorphism of magnesium iron silicate hydroxide can lead to the formation of denser minerals, such as garnet and pyroxene. This densification process influences the buoyancy of subducting slabs, affecting their descent rate and the overall subduction dynamics. Consequently, these changes can impact the distribution of earthquakes and volcanic activity along subduction zones.
Furthermore, the pressure-induced transformations in the deep Earth have implications for the carbon cycle. Carbonate minerals in subducting slabs can break down under high pressure, releasing carbon dioxide. This process contributes to the long-term carbon cycle and can influence atmospheric CO2 levels over geological timescales.
The metamorphic reactions in the deep Earth also play a role in the formation and distribution of mineral resources. The fluids released during these processes can transport metals and other elements, leading to the concentration of economically valuable minerals in certain geological settings. This has implications for the exploration and extraction of mineral resources.
Additionally, the pressure-induced changes in mineral structures can affect the seismic properties of rocks in the deep Earth. These alterations can influence the propagation of seismic waves, providing valuable information for understanding the Earth's internal structure and composition through seismological studies.
In conclusion, the pressure-induced metamorphism of magnesium iron silicate hydroxide and related deep Earth processes have far-reaching environmental impacts. These processes influence global geochemical cycles, tectonic activity, and the distribution of natural resources, highlighting the interconnectedness of deep Earth phenomena and surface environmental conditions.
These deep Earth processes play a crucial role in the global water cycle. The release of water from hydrous minerals during subduction contributes to the formation of arc volcanoes and influences the composition of magmas. This water release also affects the rheology of the mantle, potentially impacting plate tectonics and the overall dynamics of the Earth's interior.
The metamorphism of magnesium iron silicate hydroxide can lead to the formation of denser minerals, such as garnet and pyroxene. This densification process influences the buoyancy of subducting slabs, affecting their descent rate and the overall subduction dynamics. Consequently, these changes can impact the distribution of earthquakes and volcanic activity along subduction zones.
Furthermore, the pressure-induced transformations in the deep Earth have implications for the carbon cycle. Carbonate minerals in subducting slabs can break down under high pressure, releasing carbon dioxide. This process contributes to the long-term carbon cycle and can influence atmospheric CO2 levels over geological timescales.
The metamorphic reactions in the deep Earth also play a role in the formation and distribution of mineral resources. The fluids released during these processes can transport metals and other elements, leading to the concentration of economically valuable minerals in certain geological settings. This has implications for the exploration and extraction of mineral resources.
Additionally, the pressure-induced changes in mineral structures can affect the seismic properties of rocks in the deep Earth. These alterations can influence the propagation of seismic waves, providing valuable information for understanding the Earth's internal structure and composition through seismological studies.
In conclusion, the pressure-induced metamorphism of magnesium iron silicate hydroxide and related deep Earth processes have far-reaching environmental impacts. These processes influence global geochemical cycles, tectonic activity, and the distribution of natural resources, highlighting the interconnectedness of deep Earth phenomena and surface environmental conditions.
Applications in Materials Science and Engineering
The pressure-induced metamorphism of Magnesium iron silicate hydroxide has significant applications in materials science and engineering, particularly in the development of advanced materials for extreme environments. This phenomenon offers insights into the behavior of minerals under high-pressure conditions, which is crucial for understanding Earth's deep interior and developing materials for high-pressure applications.
In the field of geomaterials engineering, the study of pressure-induced metamorphism of Magnesium iron silicate hydroxide contributes to the development of more resilient construction materials. By understanding the structural changes that occur under pressure, engineers can design materials that maintain their integrity in high-stress environments, such as deep underground structures or offshore installations.
The aerospace industry benefits from this research in the development of heat-resistant materials for spacecraft and hypersonic vehicles. The high-pressure behavior of these silicate minerals provides valuable information for creating materials that can withstand extreme conditions during atmospheric re-entry or in propulsion systems.
In the realm of energy storage, the pressure-induced phase transitions of Magnesium iron silicate hydroxide offer potential for developing novel battery materials. The structural changes observed under pressure could lead to the creation of more efficient cathode materials for next-generation batteries, potentially improving energy density and cycle life.
The field of high-pressure synthesis also leverages this knowledge to create new materials with unique properties. By replicating the conditions of pressure-induced metamorphism in laboratory settings, researchers can produce materials with enhanced hardness, conductivity, or other desirable characteristics that are not achievable through conventional synthesis methods.
In the context of planetary science and materials for space exploration, understanding the behavior of Magnesium iron silicate hydroxide under extreme pressures aids in the development of materials suitable for use in extraterrestrial environments. This knowledge is crucial for designing equipment and structures that can withstand the harsh conditions on other planets or moons.
The study of pressure-induced metamorphism also contributes to advancements in high-pressure spectroscopy and imaging techniques. These improved analytical methods have wide-ranging applications in materials characterization across various industries, from semiconductor manufacturing to pharmaceutical development.
Lastly, the insights gained from studying this metamorphic process inform the development of computational models for materials under extreme conditions. These models are invaluable in predicting material behavior and properties, accelerating the design and testing of new materials for diverse engineering applications.
In the field of geomaterials engineering, the study of pressure-induced metamorphism of Magnesium iron silicate hydroxide contributes to the development of more resilient construction materials. By understanding the structural changes that occur under pressure, engineers can design materials that maintain their integrity in high-stress environments, such as deep underground structures or offshore installations.
The aerospace industry benefits from this research in the development of heat-resistant materials for spacecraft and hypersonic vehicles. The high-pressure behavior of these silicate minerals provides valuable information for creating materials that can withstand extreme conditions during atmospheric re-entry or in propulsion systems.
In the realm of energy storage, the pressure-induced phase transitions of Magnesium iron silicate hydroxide offer potential for developing novel battery materials. The structural changes observed under pressure could lead to the creation of more efficient cathode materials for next-generation batteries, potentially improving energy density and cycle life.
The field of high-pressure synthesis also leverages this knowledge to create new materials with unique properties. By replicating the conditions of pressure-induced metamorphism in laboratory settings, researchers can produce materials with enhanced hardness, conductivity, or other desirable characteristics that are not achievable through conventional synthesis methods.
In the context of planetary science and materials for space exploration, understanding the behavior of Magnesium iron silicate hydroxide under extreme pressures aids in the development of materials suitable for use in extraterrestrial environments. This knowledge is crucial for designing equipment and structures that can withstand the harsh conditions on other planets or moons.
The study of pressure-induced metamorphism also contributes to advancements in high-pressure spectroscopy and imaging techniques. These improved analytical methods have wide-ranging applications in materials characterization across various industries, from semiconductor manufacturing to pharmaceutical development.
Lastly, the insights gained from studying this metamorphic process inform the development of computational models for materials under extreme conditions. These models are invaluable in predicting material behavior and properties, accelerating the design and testing of new materials for diverse engineering applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!