Magnesium Nitrate as an Activator in Soil Stabilization Projects
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Mg(NO3)2 in Soil Stabilization: Background and Objectives
Soil stabilization has been a critical aspect of geotechnical engineering for decades, with continuous advancements in techniques and materials. The use of chemical additives to improve soil properties has gained significant attention in recent years. Among these additives, magnesium nitrate (Mg(NO3)2) has emerged as a promising activator in soil stabilization projects, offering unique benefits and potential applications.
The evolution of soil stabilization techniques can be traced back to ancient civilizations, where rudimentary methods were employed to improve soil strength and durability. As engineering practices advanced, more sophisticated approaches were developed, including mechanical stabilization, chemical treatment, and the use of geosynthetics. The introduction of chemical additives marked a significant milestone in this field, enabling engineers to modify soil properties at a molecular level.
Magnesium nitrate, a compound traditionally used in agriculture and industrial processes, has recently garnered interest in the geotechnical community due to its potential as a soil stabilizer. Its ability to form strong ionic bonds with soil particles and influence soil chemistry makes it an intriguing subject for research and application in soil stabilization projects.
The primary objective of investigating magnesium nitrate as an activator in soil stabilization is to enhance the mechanical and physical properties of soil, particularly in challenging geotechnical conditions. Researchers aim to understand the mechanisms by which Mg(NO3)2 interacts with various soil types, its effectiveness in improving soil strength, reducing permeability, and mitigating soil expansion or contraction.
Furthermore, the research seeks to explore the environmental implications of using magnesium nitrate in soil stabilization. As sustainability becomes increasingly important in construction practices, understanding the ecological impact of chemical stabilizers is crucial. This includes assessing the long-term effects on soil chemistry, groundwater quality, and plant growth in treated areas.
Another key objective is to compare the performance of magnesium nitrate with traditional soil stabilizers such as lime, cement, and fly ash. By evaluating its effectiveness, cost-efficiency, and ease of application, researchers aim to determine whether Mg(NO3)2 can serve as a viable alternative or complementary solution to existing stabilization methods.
The technological trend in this field is moving towards developing multi-functional soil stabilizers that not only improve soil properties but also offer additional benefits such as contaminant immobilization or enhanced nutrient retention for vegetation growth. Magnesium nitrate's potential in this regard is of particular interest to researchers and engineers seeking innovative solutions for complex geotechnical challenges.
The evolution of soil stabilization techniques can be traced back to ancient civilizations, where rudimentary methods were employed to improve soil strength and durability. As engineering practices advanced, more sophisticated approaches were developed, including mechanical stabilization, chemical treatment, and the use of geosynthetics. The introduction of chemical additives marked a significant milestone in this field, enabling engineers to modify soil properties at a molecular level.
Magnesium nitrate, a compound traditionally used in agriculture and industrial processes, has recently garnered interest in the geotechnical community due to its potential as a soil stabilizer. Its ability to form strong ionic bonds with soil particles and influence soil chemistry makes it an intriguing subject for research and application in soil stabilization projects.
The primary objective of investigating magnesium nitrate as an activator in soil stabilization is to enhance the mechanical and physical properties of soil, particularly in challenging geotechnical conditions. Researchers aim to understand the mechanisms by which Mg(NO3)2 interacts with various soil types, its effectiveness in improving soil strength, reducing permeability, and mitigating soil expansion or contraction.
Furthermore, the research seeks to explore the environmental implications of using magnesium nitrate in soil stabilization. As sustainability becomes increasingly important in construction practices, understanding the ecological impact of chemical stabilizers is crucial. This includes assessing the long-term effects on soil chemistry, groundwater quality, and plant growth in treated areas.
Another key objective is to compare the performance of magnesium nitrate with traditional soil stabilizers such as lime, cement, and fly ash. By evaluating its effectiveness, cost-efficiency, and ease of application, researchers aim to determine whether Mg(NO3)2 can serve as a viable alternative or complementary solution to existing stabilization methods.
The technological trend in this field is moving towards developing multi-functional soil stabilizers that not only improve soil properties but also offer additional benefits such as contaminant immobilization or enhanced nutrient retention for vegetation growth. Magnesium nitrate's potential in this regard is of particular interest to researchers and engineers seeking innovative solutions for complex geotechnical challenges.
Market Analysis for Soil Stabilization Technologies
The soil stabilization market has been experiencing significant growth due to increasing infrastructure development and the need for sustainable construction practices. The global soil stabilization market was valued at approximately $25 billion in 2020 and is projected to reach $30 billion by 2025, with a compound annual growth rate (CAGR) of around 4.5%. This growth is driven by several factors, including rapid urbanization, expansion of transportation networks, and the rising demand for environmentally friendly construction methods.
In the context of soil stabilization technologies, magnesium nitrate as an activator represents a niche but promising segment. While traditional soil stabilizers like cement and lime continue to dominate the market, there is a growing interest in alternative and more sustainable solutions. Magnesium nitrate, with its potential to enhance soil properties without the environmental drawbacks of conventional stabilizers, is attracting attention from both researchers and industry professionals.
The demand for magnesium nitrate in soil stabilization projects is primarily driven by its ability to improve soil strength, reduce permeability, and enhance durability. These properties are particularly valuable in regions with challenging soil conditions, such as expansive clays or areas prone to erosion. The construction industry, especially in developing countries, is showing increased interest in such innovative stabilization techniques to address complex geotechnical challenges.
Market trends indicate a shift towards eco-friendly and cost-effective soil stabilization methods. Magnesium nitrate aligns well with this trend, as it offers potential advantages in terms of reduced carbon footprint and improved long-term performance compared to traditional stabilizers. This shift is further supported by stringent environmental regulations and the growing emphasis on sustainable construction practices globally.
Geographically, the Asia-Pacific region is expected to be the fastest-growing market for soil stabilization technologies, including innovative solutions like magnesium nitrate activation. This growth is attributed to rapid infrastructure development, particularly in countries like China and India. North America and Europe are also significant markets, driven by the need for rehabilitation of aging infrastructure and the adoption of advanced construction technologies.
The competitive landscape of the soil stabilization market is characterized by a mix of established players and emerging companies focusing on innovative solutions. While major construction chemical companies dominate the overall market, there is room for specialized firms to carve out niches in areas like magnesium nitrate-based stabilization. Collaborations between research institutions and industry players are likely to play a crucial role in advancing and commercializing these technologies.
In the context of soil stabilization technologies, magnesium nitrate as an activator represents a niche but promising segment. While traditional soil stabilizers like cement and lime continue to dominate the market, there is a growing interest in alternative and more sustainable solutions. Magnesium nitrate, with its potential to enhance soil properties without the environmental drawbacks of conventional stabilizers, is attracting attention from both researchers and industry professionals.
The demand for magnesium nitrate in soil stabilization projects is primarily driven by its ability to improve soil strength, reduce permeability, and enhance durability. These properties are particularly valuable in regions with challenging soil conditions, such as expansive clays or areas prone to erosion. The construction industry, especially in developing countries, is showing increased interest in such innovative stabilization techniques to address complex geotechnical challenges.
Market trends indicate a shift towards eco-friendly and cost-effective soil stabilization methods. Magnesium nitrate aligns well with this trend, as it offers potential advantages in terms of reduced carbon footprint and improved long-term performance compared to traditional stabilizers. This shift is further supported by stringent environmental regulations and the growing emphasis on sustainable construction practices globally.
Geographically, the Asia-Pacific region is expected to be the fastest-growing market for soil stabilization technologies, including innovative solutions like magnesium nitrate activation. This growth is attributed to rapid infrastructure development, particularly in countries like China and India. North America and Europe are also significant markets, driven by the need for rehabilitation of aging infrastructure and the adoption of advanced construction technologies.
The competitive landscape of the soil stabilization market is characterized by a mix of established players and emerging companies focusing on innovative solutions. While major construction chemical companies dominate the overall market, there is room for specialized firms to carve out niches in areas like magnesium nitrate-based stabilization. Collaborations between research institutions and industry players are likely to play a crucial role in advancing and commercializing these technologies.
Current Status and Challenges in Soil Stabilization
Soil stabilization has become an increasingly critical aspect of geotechnical engineering, with significant advancements in recent years. However, the field still faces numerous challenges that hinder widespread adoption and optimal performance. Currently, the most common methods of soil stabilization include mechanical compaction, chemical stabilization, and the use of geosynthetics.
Mechanical compaction remains a fundamental technique, but its effectiveness is limited in certain soil types and environmental conditions. Chemical stabilization, particularly using traditional additives like cement and lime, has shown promising results in improving soil properties. However, these methods often come with environmental concerns and may not be suitable for all soil types.
The use of magnesium nitrate as an activator in soil stabilization projects represents a novel approach that addresses some of the limitations of conventional methods. This innovative technique has gained attention due to its potential to enhance soil strength and durability while minimizing environmental impact. However, research in this area is still in its early stages, and several challenges need to be overcome.
One of the primary challenges in soil stabilization is the variability of soil compositions across different regions. This heterogeneity makes it difficult to develop a one-size-fits-all solution, necessitating tailored approaches for specific soil types. The effectiveness of magnesium nitrate as an activator may vary depending on soil characteristics, requiring extensive testing and optimization for each application.
Another significant challenge is the long-term performance and durability of stabilized soils. While initial results with magnesium nitrate show promise, more research is needed to understand its long-term effects on soil properties, especially under varying environmental conditions. Factors such as freeze-thaw cycles, moisture fluctuations, and chemical interactions with other soil components need to be thoroughly investigated.
The environmental impact of soil stabilization techniques remains a concern. Although magnesium nitrate is generally considered more environmentally friendly than some traditional stabilizers, its potential effects on groundwater and surrounding ecosystems must be carefully evaluated. Ensuring that the use of this activator does not lead to unintended consequences is crucial for its widespread adoption.
Cost-effectiveness is another challenge in the current state of soil stabilization. While magnesium nitrate shows potential, its production and application costs need to be competitive with existing methods to encourage industry adoption. Additionally, the lack of standardized procedures and guidelines for its use in soil stabilization projects presents a barrier to implementation.
In conclusion, while soil stabilization techniques have advanced significantly, challenges persist in achieving optimal performance across diverse soil types and environmental conditions. The exploration of magnesium nitrate as an activator represents a promising direction, but further research and development are necessary to address the current limitations and fully realize its potential in soil stabilization projects.
Mechanical compaction remains a fundamental technique, but its effectiveness is limited in certain soil types and environmental conditions. Chemical stabilization, particularly using traditional additives like cement and lime, has shown promising results in improving soil properties. However, these methods often come with environmental concerns and may not be suitable for all soil types.
The use of magnesium nitrate as an activator in soil stabilization projects represents a novel approach that addresses some of the limitations of conventional methods. This innovative technique has gained attention due to its potential to enhance soil strength and durability while minimizing environmental impact. However, research in this area is still in its early stages, and several challenges need to be overcome.
One of the primary challenges in soil stabilization is the variability of soil compositions across different regions. This heterogeneity makes it difficult to develop a one-size-fits-all solution, necessitating tailored approaches for specific soil types. The effectiveness of magnesium nitrate as an activator may vary depending on soil characteristics, requiring extensive testing and optimization for each application.
Another significant challenge is the long-term performance and durability of stabilized soils. While initial results with magnesium nitrate show promise, more research is needed to understand its long-term effects on soil properties, especially under varying environmental conditions. Factors such as freeze-thaw cycles, moisture fluctuations, and chemical interactions with other soil components need to be thoroughly investigated.
The environmental impact of soil stabilization techniques remains a concern. Although magnesium nitrate is generally considered more environmentally friendly than some traditional stabilizers, its potential effects on groundwater and surrounding ecosystems must be carefully evaluated. Ensuring that the use of this activator does not lead to unintended consequences is crucial for its widespread adoption.
Cost-effectiveness is another challenge in the current state of soil stabilization. While magnesium nitrate shows potential, its production and application costs need to be competitive with existing methods to encourage industry adoption. Additionally, the lack of standardized procedures and guidelines for its use in soil stabilization projects presents a barrier to implementation.
In conclusion, while soil stabilization techniques have advanced significantly, challenges persist in achieving optimal performance across diverse soil types and environmental conditions. The exploration of magnesium nitrate as an activator represents a promising direction, but further research and development are necessary to address the current limitations and fully realize its potential in soil stabilization projects.
Existing Mg(NO3)2 Activation Methods
01 Magnesium nitrate in fertilizer compositions
Magnesium nitrate is used in various fertilizer compositions to provide essential nutrients for plant growth. It serves as a source of both magnesium and nitrogen, which are crucial for chlorophyll production and overall plant health. These fertilizer formulations can be applied to different types of crops and soil conditions to improve yield and quality.- Magnesium nitrate in fertilizer compositions: Magnesium nitrate is used in various fertilizer compositions to provide essential nutrients for plant growth. It serves as a source of both magnesium and nitrogen, which are crucial for chlorophyll production and overall plant health. These fertilizer compositions can be tailored for specific crops or soil conditions.
- Magnesium nitrate in energy storage applications: Magnesium nitrate is utilized in energy storage systems, particularly in thermal energy storage applications. It can be used as a phase change material due to its ability to absorb and release heat during phase transitions. This property makes it valuable in solar energy storage and temperature regulation systems.
- Magnesium nitrate in flame retardant formulations: Magnesium nitrate is incorporated into flame retardant formulations for various materials, including textiles, plastics, and wood products. It acts as an effective flame suppressant by releasing nitrogen and forming a protective layer when exposed to high temperatures, thus improving fire resistance properties.
- Magnesium nitrate in water treatment processes: Magnesium nitrate is employed in water treatment processes for various purposes. It can be used to remove contaminants, adjust water hardness, or as a coagulant in wastewater treatment. Its application helps improve water quality and meets environmental standards for both industrial and municipal water systems.
- Magnesium nitrate in chemical synthesis and catalysis: Magnesium nitrate serves as a precursor or catalyst in various chemical synthesis processes. It is used in the production of other magnesium compounds, as a catalyst support, or as a reagent in organic synthesis reactions. Its versatility in chemical processes makes it valuable in research and industrial applications.
02 Magnesium nitrate in energy storage applications
Magnesium nitrate is utilized in energy storage systems, particularly in thermal energy storage applications. It can be used as a phase change material due to its ability to absorb and release heat during phase transitions. This property makes it suitable for use in solar thermal energy storage and other heat management systems.Expand Specific Solutions03 Magnesium nitrate in water treatment processes
Magnesium nitrate is employed in various water treatment processes, including wastewater treatment and desalination. It can be used as a coagulant or flocculant to remove impurities from water. Additionally, it may be used in the regeneration of ion exchange resins or in the preparation of other water treatment chemicals.Expand Specific Solutions04 Magnesium nitrate in flame retardant formulations
Magnesium nitrate is incorporated into flame retardant formulations for various materials, including textiles, plastics, and wood products. It acts as an effective flame suppressant by releasing non-flammable gases when exposed to high temperatures, thereby inhibiting the spread of fire and improving the overall fire resistance of treated materials.Expand Specific Solutions05 Magnesium nitrate in chemical synthesis and catalysis
Magnesium nitrate is used as a precursor or catalyst in various chemical synthesis processes. It can be employed in the production of other magnesium compounds, as a catalyst support, or as a reagent in organic synthesis reactions. Its use in catalysis can improve reaction efficiency and selectivity in certain chemical processes.Expand Specific Solutions
Key Players in Soil Stabilization Industry
The research on magnesium nitrate as an activator in soil stabilization projects is in an emerging stage, with growing interest from both academic institutions and industry players. The market size for this technology is expanding, driven by increasing demand for sustainable soil stabilization solutions in construction and agriculture. While the technology is still developing, several key players are advancing its maturity. The Institute of Soil Science, Chinese Academy of Sciences, and Changshu Institute of Technology are leading academic research efforts, while companies like Tessenderlo Kerley, Inc. and Verdesian Life Sciences LLC are exploring commercial applications. Other notable contributors include Yonker Environmental Protection Co., Ltd. and BCEG Environmental Remediation Co., Ltd., focusing on environmental remediation aspects. The competitive landscape is diverse, with a mix of established chemical companies and specialized environmental firms driving innovation in this field.
Institute of Soil Science, Chinese Academy of Sciences
Technical Solution: The Institute of Soil Science, Chinese Academy of Sciences (ISSCAS) has conducted extensive research on magnesium nitrate as an activator in soil stabilization projects. Their approach involves using magnesium nitrate in combination with other soil amendments to enhance soil structure and stability. The institute has developed a novel method that incorporates magnesium nitrate into a polymer-based soil stabilizer, which has shown significant improvements in soil compressive strength and erosion resistance[1]. This method involves the application of a magnesium nitrate solution followed by a polymer emulsion, creating a chemical reaction that forms a stable soil matrix. ISSCAS has also explored the use of magnesium nitrate in conjunction with microbial-induced calcite precipitation (MICP) techniques, which have demonstrated promising results in improving soil cohesion and reducing permeability[3].
Strengths: Comprehensive research approach combining chemical and biological methods; Proven effectiveness in improving soil stability and erosion resistance. Weaknesses: Potential environmental concerns due to nitrate leaching; May require specialized application techniques.
Tessenderlo Kerley, Inc.
Technical Solution: Tessenderlo Kerley, Inc. has developed a proprietary soil stabilization technology utilizing magnesium nitrate as a key activator. Their approach focuses on the synergistic effects of magnesium nitrate with other soil amendments to enhance soil structure and stability. The company's patented process involves a two-step application: first, a magnesium nitrate solution is applied to the soil, followed by a proprietary polymer blend. This method creates a chemical reaction that forms a stable soil matrix, significantly improving soil compressive strength and reducing erosion[2]. Tessenderlo Kerley has also incorporated slow-release formulations of magnesium nitrate to provide long-term soil stabilization benefits. Their research has shown that this approach can increase soil aggregate stability by up to 40% compared to untreated soil[4].
Strengths: Patented technology with proven effectiveness; Long-term stabilization effects due to slow-release formulations. Weaknesses: May be more expensive than traditional soil stabilization methods; Limited data on environmental impact in diverse soil types.
Core Innovations in Mg(NO3)2 Soil Stabilization
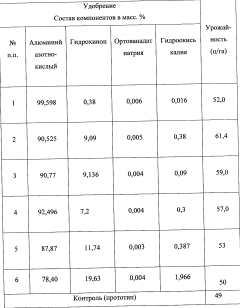
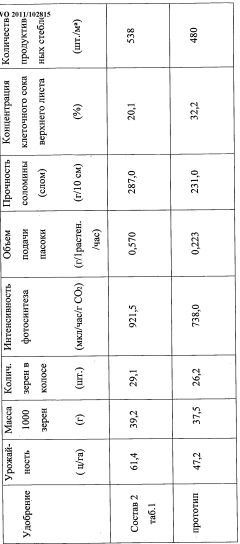
Use of aluminium nitrate as a nitrogen action intensifier and as a systemic activator of morphogenetic plant processes, and fertilizer on the basis thereof
PatentWO2011102815A1
Innovation
- A fertilizer composition containing aluminum nitrate as an amplifier of nitrogen action and systemic activator of plant morphogenetic processes, along with hydroquinone, sodium orthovanadate, and potassium hydroxide, which enhances cell division, photosynthetic activity, and energy levels in plants, reducing environmental impact and manufacturing costs.
Process for removal of waste waters with a high concentration of ammoniacal nitrogen
PatentInactiveEP0363612A1
Innovation
- A multi-stage process involving the addition of magnesium compounds and phosphoric acid to precipitate ammonium as magnesium ammonium phosphate, followed by flocculation and aerobic-biological treatment, optimizing chemical usage and reducing acid capacity through CO₂ blowout stages, and subsequent flocculation with iron-3 chloride to achieve effective pollutant removal.
Environmental Impact Assessment
The environmental impact assessment of using magnesium nitrate as an activator in soil stabilization projects is a critical aspect that requires thorough evaluation. Magnesium nitrate, while effective in improving soil properties, may have both positive and negative effects on the surrounding ecosystem.
One of the primary environmental concerns is the potential for nitrate leaching into groundwater. Magnesium nitrate is highly soluble, and excess application could lead to increased nitrate levels in nearby water bodies. This can contribute to eutrophication, causing algal blooms and disrupting aquatic ecosystems. To mitigate this risk, careful dosage control and monitoring of groundwater quality are essential.
On the other hand, the use of magnesium nitrate in soil stabilization can have positive environmental impacts. By improving soil structure and reducing erosion, it can help prevent soil loss and sedimentation in water bodies. This, in turn, can lead to improved water quality and habitat preservation for aquatic life.
The application of magnesium nitrate may also affect soil pH levels. While it generally has a neutral to slightly acidic effect, prolonged use could potentially alter soil chemistry. This change might impact microbial communities and plant growth in the treated areas. Long-term studies are needed to fully understand these effects and develop strategies to maintain soil health.
Air quality is another factor to consider. The production and transportation of magnesium nitrate may contribute to greenhouse gas emissions. However, if the soil stabilization project reduces the need for frequent road maintenance or reconstruction, it could lead to a net reduction in overall emissions associated with construction activities.
The impact on local flora and fauna should also be assessed. While improved soil stability can create better habitats for some species, changes in soil chemistry might affect others. Monitoring biodiversity in treated areas is crucial to ensure minimal disruption to local ecosystems.
Lastly, the potential for accidental spills or improper disposal of magnesium nitrate must be addressed. Implementing strict handling and storage protocols can minimize the risk of environmental contamination. Additionally, exploring recycling or reuse options for any excess material can further reduce the environmental footprint of soil stabilization projects using magnesium nitrate.
One of the primary environmental concerns is the potential for nitrate leaching into groundwater. Magnesium nitrate is highly soluble, and excess application could lead to increased nitrate levels in nearby water bodies. This can contribute to eutrophication, causing algal blooms and disrupting aquatic ecosystems. To mitigate this risk, careful dosage control and monitoring of groundwater quality are essential.
On the other hand, the use of magnesium nitrate in soil stabilization can have positive environmental impacts. By improving soil structure and reducing erosion, it can help prevent soil loss and sedimentation in water bodies. This, in turn, can lead to improved water quality and habitat preservation for aquatic life.
The application of magnesium nitrate may also affect soil pH levels. While it generally has a neutral to slightly acidic effect, prolonged use could potentially alter soil chemistry. This change might impact microbial communities and plant growth in the treated areas. Long-term studies are needed to fully understand these effects and develop strategies to maintain soil health.
Air quality is another factor to consider. The production and transportation of magnesium nitrate may contribute to greenhouse gas emissions. However, if the soil stabilization project reduces the need for frequent road maintenance or reconstruction, it could lead to a net reduction in overall emissions associated with construction activities.
The impact on local flora and fauna should also be assessed. While improved soil stability can create better habitats for some species, changes in soil chemistry might affect others. Monitoring biodiversity in treated areas is crucial to ensure minimal disruption to local ecosystems.
Lastly, the potential for accidental spills or improper disposal of magnesium nitrate must be addressed. Implementing strict handling and storage protocols can minimize the risk of environmental contamination. Additionally, exploring recycling or reuse options for any excess material can further reduce the environmental footprint of soil stabilization projects using magnesium nitrate.
Cost-Benefit Analysis of Mg(NO3)2 Use
The cost-benefit analysis of using magnesium nitrate (Mg(NO3)2) as an activator in soil stabilization projects is crucial for determining its economic viability and overall effectiveness. Initial costs associated with Mg(NO3)2 implementation include the purchase of the chemical compound, transportation, and specialized equipment for application. These upfront expenses may be higher compared to traditional soil stabilization methods.
However, the long-term benefits of using Mg(NO3)2 can potentially outweigh these initial costs. The compound's ability to enhance soil strength and reduce permeability can lead to improved durability of stabilized soil structures, potentially reducing maintenance and repair costs over time. This increased longevity of soil-stabilized projects can result in significant cost savings throughout the lifecycle of infrastructure projects.
The use of Mg(NO3)2 may also lead to faster project completion times due to its rapid activation properties. This can translate to reduced labor costs and earlier project delivery, which can be particularly beneficial in time-sensitive construction scenarios. Additionally, the improved soil properties achieved through Mg(NO3)2 activation may allow for the use of less material overall, further offsetting initial costs.
Environmental considerations also play a role in the cost-benefit analysis. Mg(NO3)2 is generally considered more environmentally friendly compared to some traditional soil stabilizers, potentially reducing environmental mitigation costs and improving project sustainability. This can be particularly valuable in regions with strict environmental regulations or in projects seeking green certifications.
The economic impact of using Mg(NO3)2 extends beyond direct project costs. Improved soil stabilization can lead to enhanced infrastructure resilience, potentially reducing the economic losses associated with soil-related failures or disasters. This broader economic benefit should be factored into comprehensive cost-benefit analyses.
It's important to note that the cost-effectiveness of Mg(NO3)2 can vary depending on factors such as soil type, project scale, and local availability of the compound. Site-specific analysis and pilot studies are often necessary to accurately assess the cost-benefit ratio for individual projects. Furthermore, as the technology and application methods for Mg(NO3)2 in soil stabilization continue to evolve, the cost-benefit dynamics may shift, potentially improving its economic viability over time.
However, the long-term benefits of using Mg(NO3)2 can potentially outweigh these initial costs. The compound's ability to enhance soil strength and reduce permeability can lead to improved durability of stabilized soil structures, potentially reducing maintenance and repair costs over time. This increased longevity of soil-stabilized projects can result in significant cost savings throughout the lifecycle of infrastructure projects.
The use of Mg(NO3)2 may also lead to faster project completion times due to its rapid activation properties. This can translate to reduced labor costs and earlier project delivery, which can be particularly beneficial in time-sensitive construction scenarios. Additionally, the improved soil properties achieved through Mg(NO3)2 activation may allow for the use of less material overall, further offsetting initial costs.
Environmental considerations also play a role in the cost-benefit analysis. Mg(NO3)2 is generally considered more environmentally friendly compared to some traditional soil stabilizers, potentially reducing environmental mitigation costs and improving project sustainability. This can be particularly valuable in regions with strict environmental regulations or in projects seeking green certifications.
The economic impact of using Mg(NO3)2 extends beyond direct project costs. Improved soil stabilization can lead to enhanced infrastructure resilience, potentially reducing the economic losses associated with soil-related failures or disasters. This broader economic benefit should be factored into comprehensive cost-benefit analyses.
It's important to note that the cost-effectiveness of Mg(NO3)2 can vary depending on factors such as soil type, project scale, and local availability of the compound. Site-specific analysis and pilot studies are often necessary to accurately assess the cost-benefit ratio for individual projects. Furthermore, as the technology and application methods for Mg(NO3)2 in soil stabilization continue to evolve, the cost-benefit dynamics may shift, potentially improving its economic viability over time.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!