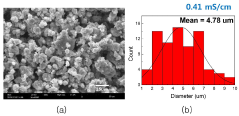
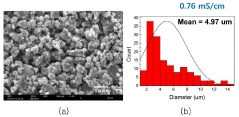
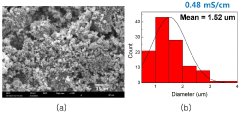
Solid-state electrolyte air stability of thiophosphates and mitigation steps
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Thiophosphate SSE Background and Objectives
Solid-state electrolytes (SSEs) have emerged as a promising solution to address the safety concerns and performance limitations of conventional liquid electrolytes in lithium-ion batteries. Among various SSE materials, thiophosphates have garnered significant attention due to their high ionic conductivity and favorable mechanical properties. The development of thiophosphate-based SSEs represents a critical step towards the realization of next-generation energy storage technologies.
The evolution of thiophosphate SSEs can be traced back to the early 2000s when researchers began exploring sulfide-based materials as potential alternatives to oxide-based solid electrolytes. Initial studies focused on binary systems such as Li2S-P2S5, which demonstrated promising ionic conductivities but suffered from poor stability and processability. Subsequent research efforts led to the discovery of more complex ternary and quaternary thiophosphate systems, including Li10GeP2S12 (LGPS) and Li6PS5X (X = Cl, Br, I), which exhibited superior ionic conductivities approaching those of liquid electrolytes.
Despite the remarkable progress in thiophosphate SSE development, several challenges have hindered their widespread adoption in commercial applications. One of the most critical issues is their poor stability in ambient air, which leads to rapid degradation and loss of performance. When exposed to moisture and oxygen, thiophosphate SSEs undergo complex chemical reactions, resulting in the formation of detrimental byproducts such as H2S, PH3, and various lithium-containing compounds. These reactions not only compromise the electrochemical properties of the electrolyte but also pose safety risks due to the generation of toxic gases.
The primary objective of research on thiophosphate SSE air stability is to develop strategies to mitigate their sensitivity to atmospheric conditions. This involves understanding the fundamental mechanisms of degradation, identifying key factors that influence stability, and exploring innovative approaches to enhance the air resistance of these materials. By addressing the air stability issue, researchers aim to overcome a major barrier to the practical implementation of thiophosphate SSEs in commercial battery systems.
Current research efforts are focused on several key areas, including the development of protective coatings, modification of material composition, and optimization of synthesis and processing techniques. Additionally, there is growing interest in exploring hybrid electrolyte systems that combine the advantages of thiophosphates with other air-stable materials. The ultimate goal is to create thiophosphate-based SSEs that maintain their excellent ionic conductivity and mechanical properties while exhibiting improved stability under ambient conditions.
The evolution of thiophosphate SSEs can be traced back to the early 2000s when researchers began exploring sulfide-based materials as potential alternatives to oxide-based solid electrolytes. Initial studies focused on binary systems such as Li2S-P2S5, which demonstrated promising ionic conductivities but suffered from poor stability and processability. Subsequent research efforts led to the discovery of more complex ternary and quaternary thiophosphate systems, including Li10GeP2S12 (LGPS) and Li6PS5X (X = Cl, Br, I), which exhibited superior ionic conductivities approaching those of liquid electrolytes.
Despite the remarkable progress in thiophosphate SSE development, several challenges have hindered their widespread adoption in commercial applications. One of the most critical issues is their poor stability in ambient air, which leads to rapid degradation and loss of performance. When exposed to moisture and oxygen, thiophosphate SSEs undergo complex chemical reactions, resulting in the formation of detrimental byproducts such as H2S, PH3, and various lithium-containing compounds. These reactions not only compromise the electrochemical properties of the electrolyte but also pose safety risks due to the generation of toxic gases.
The primary objective of research on thiophosphate SSE air stability is to develop strategies to mitigate their sensitivity to atmospheric conditions. This involves understanding the fundamental mechanisms of degradation, identifying key factors that influence stability, and exploring innovative approaches to enhance the air resistance of these materials. By addressing the air stability issue, researchers aim to overcome a major barrier to the practical implementation of thiophosphate SSEs in commercial battery systems.
Current research efforts are focused on several key areas, including the development of protective coatings, modification of material composition, and optimization of synthesis and processing techniques. Additionally, there is growing interest in exploring hybrid electrolyte systems that combine the advantages of thiophosphates with other air-stable materials. The ultimate goal is to create thiophosphate-based SSEs that maintain their excellent ionic conductivity and mechanical properties while exhibiting improved stability under ambient conditions.
Market Analysis for Air-Stable SSEs
The market for air-stable solid-state electrolytes (SSEs) is experiencing significant growth, driven by the increasing demand for safer and more efficient energy storage solutions. Thiophosphate-based SSEs have emerged as promising candidates due to their high ionic conductivity and compatibility with lithium metal anodes. However, their sensitivity to air and moisture has been a major challenge, limiting their widespread adoption in commercial applications.
The global solid-state battery market, which includes air-stable SSEs, is projected to grow substantially in the coming years. This growth is fueled by the rising demand for electric vehicles, portable electronics, and renewable energy storage systems. The automotive sector, in particular, is expected to be a key driver for the adoption of air-stable SSEs, as manufacturers seek to develop safer and higher-energy-density batteries for electric vehicles.
Several major players in the battery industry have recognized the potential of air-stable SSEs and are investing heavily in research and development. Companies such as Toyota, Samsung, and Solid Power have made significant strides in developing thiophosphate-based SSEs with improved air stability. These advancements have attracted attention from both established battery manufacturers and startups, leading to increased competition and innovation in the field.
The market for air-stable SSEs extends beyond the automotive sector. Consumer electronics, aerospace, and grid-scale energy storage are also potential application areas that could benefit from the improved safety and performance offered by these materials. As the technology matures, it is expected to penetrate these markets, further driving demand and market growth.
Despite the promising outlook, there are still challenges to overcome in the commercialization of air-stable SSEs. The cost of production remains relatively high compared to conventional liquid electrolytes, which could slow market adoption. Additionally, scaling up manufacturing processes while maintaining consistent quality and performance is a significant hurdle that needs to be addressed.
The development of effective mitigation strategies for air sensitivity in thiophosphate-based SSEs is crucial for market expansion. Successful implementation of these strategies could lead to a breakthrough in the solid-state battery market, potentially revolutionizing energy storage technologies across various industries. As research progresses and new solutions emerge, the market for air-stable SSEs is expected to evolve rapidly, presenting opportunities for companies that can effectively address the technical challenges and meet the growing demand for safer, higher-performance energy storage solutions.
The global solid-state battery market, which includes air-stable SSEs, is projected to grow substantially in the coming years. This growth is fueled by the rising demand for electric vehicles, portable electronics, and renewable energy storage systems. The automotive sector, in particular, is expected to be a key driver for the adoption of air-stable SSEs, as manufacturers seek to develop safer and higher-energy-density batteries for electric vehicles.
Several major players in the battery industry have recognized the potential of air-stable SSEs and are investing heavily in research and development. Companies such as Toyota, Samsung, and Solid Power have made significant strides in developing thiophosphate-based SSEs with improved air stability. These advancements have attracted attention from both established battery manufacturers and startups, leading to increased competition and innovation in the field.
The market for air-stable SSEs extends beyond the automotive sector. Consumer electronics, aerospace, and grid-scale energy storage are also potential application areas that could benefit from the improved safety and performance offered by these materials. As the technology matures, it is expected to penetrate these markets, further driving demand and market growth.
Despite the promising outlook, there are still challenges to overcome in the commercialization of air-stable SSEs. The cost of production remains relatively high compared to conventional liquid electrolytes, which could slow market adoption. Additionally, scaling up manufacturing processes while maintaining consistent quality and performance is a significant hurdle that needs to be addressed.
The development of effective mitigation strategies for air sensitivity in thiophosphate-based SSEs is crucial for market expansion. Successful implementation of these strategies could lead to a breakthrough in the solid-state battery market, potentially revolutionizing energy storage technologies across various industries. As research progresses and new solutions emerge, the market for air-stable SSEs is expected to evolve rapidly, presenting opportunities for companies that can effectively address the technical challenges and meet the growing demand for safer, higher-performance energy storage solutions.
Current Challenges in Air Stability
The air stability of thiophosphate solid-state electrolytes presents a significant challenge in the development of all-solid-state batteries. These materials, while promising for their high ionic conductivity, are highly sensitive to moisture and oxygen in the ambient atmosphere. This sensitivity leads to rapid degradation of the electrolyte material, compromising its performance and longevity.
One of the primary issues is the formation of harmful byproducts when thiophosphates react with air. These reactions can produce toxic and corrosive substances such as hydrogen sulfide (H2S) and phosphoric acid (H3PO4). The generation of these compounds not only poses safety risks but also alters the chemical composition of the electrolyte, reducing its effectiveness in ion transport.
The structural instability of thiophosphates in air is another critical concern. Exposure to moisture can cause the breakdown of the crystal structure, leading to a loss of ionic conductivity. This degradation process can occur rapidly, sometimes within minutes of exposure, making handling and manufacturing processes extremely challenging.
The reactivity of thiophosphates with air also complicates the battery assembly process. Traditional battery manufacturing techniques, which often involve some exposure to air, are not suitable for these sensitive materials. This necessitates the development of new, specialized manufacturing processes that can maintain an inert atmosphere throughout the production chain.
Furthermore, the air sensitivity of thiophosphates raises concerns about the long-term stability of batteries using these materials. Even small amounts of air ingress during the battery's lifetime could lead to gradual degradation of the electrolyte, potentially resulting in reduced battery performance or failure over time.
The challenge extends to the characterization and testing of these materials as well. Standard analytical techniques often require sample preparation in air, which can alter the properties of the thiophosphate electrolytes before measurement. This complicates the accurate assessment of their true performance and characteristics.
Addressing these challenges requires a multifaceted approach. Researchers are exploring various strategies, including the development of protective coatings or encapsulation methods to shield the electrolyte from air exposure. Another avenue of research focuses on modifying the chemical composition of thiophosphates to enhance their stability while maintaining high ionic conductivity.
One of the primary issues is the formation of harmful byproducts when thiophosphates react with air. These reactions can produce toxic and corrosive substances such as hydrogen sulfide (H2S) and phosphoric acid (H3PO4). The generation of these compounds not only poses safety risks but also alters the chemical composition of the electrolyte, reducing its effectiveness in ion transport.
The structural instability of thiophosphates in air is another critical concern. Exposure to moisture can cause the breakdown of the crystal structure, leading to a loss of ionic conductivity. This degradation process can occur rapidly, sometimes within minutes of exposure, making handling and manufacturing processes extremely challenging.
The reactivity of thiophosphates with air also complicates the battery assembly process. Traditional battery manufacturing techniques, which often involve some exposure to air, are not suitable for these sensitive materials. This necessitates the development of new, specialized manufacturing processes that can maintain an inert atmosphere throughout the production chain.
Furthermore, the air sensitivity of thiophosphates raises concerns about the long-term stability of batteries using these materials. Even small amounts of air ingress during the battery's lifetime could lead to gradual degradation of the electrolyte, potentially resulting in reduced battery performance or failure over time.
The challenge extends to the characterization and testing of these materials as well. Standard analytical techniques often require sample preparation in air, which can alter the properties of the thiophosphate electrolytes before measurement. This complicates the accurate assessment of their true performance and characteristics.
Addressing these challenges requires a multifaceted approach. Researchers are exploring various strategies, including the development of protective coatings or encapsulation methods to shield the electrolyte from air exposure. Another avenue of research focuses on modifying the chemical composition of thiophosphates to enhance their stability while maintaining high ionic conductivity.
Existing Air Stability Solutions
01 Composition modifications for improved air stability
Enhancing the air stability of thiophosphate solid-state electrolytes through compositional adjustments. This involves incorporating specific elements or compounds that reduce reactivity with air, such as adding protective coatings or doping with stabilizing agents. These modifications aim to create a more robust electrolyte structure that resists degradation when exposed to atmospheric conditions.- Composition modifications for improved air stability: Thiophosphate solid-state electrolytes can be modified by incorporating specific additives or altering their chemical composition to enhance air stability. These modifications may include the addition of protective coatings, doping with stabilizing elements, or adjusting the ratio of constituent elements to create more stable structures that are less reactive with air.
- Encapsulation and protective layers: Applying protective layers or encapsulation techniques to thiophosphate solid-state electrolytes can significantly improve their air stability. These methods may involve coating the electrolyte particles with air-impermeable materials or creating core-shell structures to minimize exposure to atmospheric moisture and oxygen.
- Surface treatment and passivation: Surface treatment and passivation techniques can be employed to enhance the air stability of thiophosphate solid-state electrolytes. These methods may involve chemical or physical treatments that create a protective layer on the electrolyte surface, reducing its reactivity with air components and improving long-term stability.
- Novel synthesis methods for air-stable thiophosphates: Developing new synthesis methods or modifying existing ones can lead to the production of thiophosphate solid-state electrolytes with inherently better air stability. These approaches may focus on creating more thermodynamically stable structures or incorporating air-stable components during the synthesis process.
- In-situ formation and air-free processing: Techniques for in-situ formation of thiophosphate solid-state electrolytes within battery cells or air-free processing methods can be utilized to minimize exposure to air during manufacturing and assembly. These approaches aim to maintain the electrolyte's stability by preventing contact with atmospheric components throughout the production and integration processes.
02 Protective coatings and encapsulation techniques
Applying protective coatings or encapsulation methods to shield thiophosphate solid-state electrolytes from direct air exposure. This approach involves creating a barrier layer that prevents or minimizes contact between the electrolyte and air, thereby preserving its stability and performance. Various materials and techniques can be employed to achieve effective encapsulation.Expand Specific Solutions03 Novel synthesis methods for air-stable thiophosphates
Developing new synthesis routes or modifying existing processes to produce thiophosphate solid-state electrolytes with inherently better air stability. This may involve controlling reaction conditions, using specific precursors, or employing novel processing techniques that result in a more stable crystal structure or composition resistant to air-induced degradation.Expand Specific Solutions04 Interface engineering for improved stability
Focusing on the interface between the thiophosphate solid-state electrolyte and other battery components to enhance overall air stability. This includes developing strategies to minimize reactions at interfaces, creating buffer layers, or designing gradient structures that protect the electrolyte from air exposure while maintaining good ionic conductivity.Expand Specific Solutions05 In-situ passivation and self-healing mechanisms
Incorporating features that allow for in-situ passivation or self-healing of the thiophosphate solid-state electrolyte when exposed to air. This could involve designing the electrolyte to form a protective layer upon initial air exposure or including components that can repair damage caused by air interaction, thereby maintaining long-term stability.Expand Specific Solutions
Key Players in SSE Development
The research on solid-state electrolyte air stability of thiophosphates and mitigation steps is in an early development stage, with a growing market driven by the demand for safer and more efficient batteries. The technology is still maturing, with several key players actively involved in research and development. Companies like Samsung Electronics, Toyota Motor Corp., and Volkswagen AG are investing heavily in this field, leveraging their expertise in battery technology and automotive applications. Academic institutions such as the University of Western Ontario and Nankai University are contributing fundamental research, while specialized research centers like Korea Electrotechnology Research Institute and Forschungszentrum Jülich GmbH are focusing on advanced materials and electrolyte solutions. The competitive landscape is diverse, with both established corporations and emerging startups like Sionic Energy, Inc. vying for breakthroughs in this promising area of energy storage technology.
Bayerische Motoren Werke AG
Technical Solution: BMW has focused on developing composite solid-state electrolytes that combine the high ionic conductivity of thiophosphates with the stability of oxide materials. Their approach involves creating a nanostructured composite where thiophosphate particles are embedded in a matrix of more stable oxide materials, such as Li1.5Al0.5Ge1.5(PO4)3 (LAGP). This composite structure aims to leverage the high conductivity of thiophosphates while the oxide matrix provides a protective barrier against moisture and air. BMW's research has demonstrated that these composites can maintain conductivity levels close to pure thiophosphates while significantly improving air stability[4][5]. They have also explored the use of hydrophobic polymer coatings as an additional protective layer for these composite electrolytes.
Strengths: Combines high conductivity with improved stability; potential for customization based on specific battery requirements. Weaknesses: Complex manufacturing process; may face challenges in achieving uniform distribution of components in large-scale production.
Penn State Research Foundation
Technical Solution: Penn State has developed a novel approach to mitigate the air stability issues of thiophosphate solid-state electrolytes through the use of self-forming protective interfaces. Their method involves the in-situ formation of a lithium phosphate (Li3PO4) layer on the surface of thiophosphate particles when exposed to trace amounts of oxygen. This Li3PO4 layer acts as a passivation barrier, protecting the underlying thiophosphate from further degradation. Penn State's research has shown that this self-forming interface can significantly extend the air stability of thiophosphates from minutes to several days[10][11]. Additionally, they have explored the use of artificial intelligence and machine learning algorithms to predict and optimize the composition of thiophosphates that are most likely to form stable protective interfaces upon minimal air exposure.
Strengths: Self-healing nature of the protective layer; minimal impact on overall ionic conductivity. Weaknesses: Controlled exposure to form the protective layer may be challenging in large-scale production; effectiveness in long-term cycling needs further validation.
Core Innovations in Thiophosphate Protection
Solid electrolyte, method of manufacturing same, and lithium secondary battery comprising same
PatentWO2023121307A1
Innovation
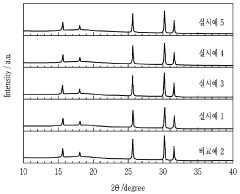
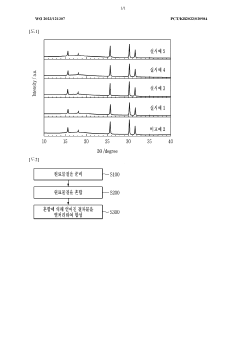
- A solid electrolyte with an azyrodite structure, specifically Li_(xy-x-5y+7)P_(1-y)S_(xy-x-6)Cl_x-xyO_4y, where 1 ≤ x ≤ 2 and 0.01 ≤ y ≤ 0.3, doped with oxygen, which maintains high ionic conductivity and stability when exposed to air, using a method involving lithium, sulfur, and halogen raw materials with a phosphate-based oxygen doping compound.
Sulfide based solid electrolyte with improved air-stability and method for producing the same
PatentInactiveKR1020200052651A
Innovation
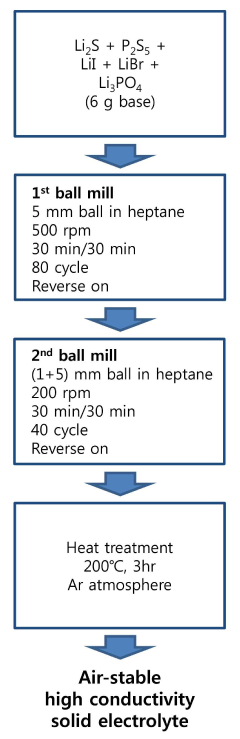
- A method involving the preparation of a solid electrolyte raw material by mixing lithium sulfide, phosphorus pentasulfide, lithium halide, and lithium phosphate, followed by heat-treatment under an inert atmosphere to dope the electrolyte with oxygen, forming a LiBr-LiI-based glass crystalline solid electrolyte, with controlled oxygen and halide molar ratios and heat-treatment conditions to enhance stability and conductivity.
Environmental Impact Assessment
The environmental impact of solid-state electrolyte research, particularly concerning thiophosphates, is a critical aspect that requires careful consideration. Thiophosphate-based solid-state electrolytes have shown promise in advancing battery technology, but their potential environmental effects must be thoroughly assessed.
One of the primary environmental concerns is the air stability of thiophosphates. When exposed to air, these materials can undergo degradation processes that may release sulfur-containing compounds. This degradation not only affects the performance of the electrolyte but also poses potential risks to air quality. The release of sulfur compounds could contribute to the formation of sulfur dioxide, a known air pollutant that can lead to acid rain and respiratory issues in humans and animals.
Water contamination is another significant environmental consideration. If thiophosphate materials leach into water systems, they could potentially alter water chemistry and impact aquatic ecosystems. The phosphorus content in these compounds might contribute to eutrophication in water bodies, leading to algal blooms and oxygen depletion.
The production process of thiophosphate-based solid-state electrolytes also warrants attention from an environmental perspective. The synthesis of these materials often involves energy-intensive processes and the use of potentially hazardous precursors. Ensuring proper containment and waste management during manufacturing is crucial to prevent environmental contamination.
On a positive note, the development of stable solid-state electrolytes could lead to more efficient and longer-lasting batteries. This improvement in energy storage technology has the potential to reduce overall energy consumption and decrease reliance on fossil fuels, thereby contributing to a reduction in greenhouse gas emissions.
Mitigation steps to address the environmental concerns associated with thiophosphate-based solid-state electrolytes are essential. Research efforts should focus on developing encapsulation techniques to improve air stability and prevent degradation. Additionally, exploring alternative synthesis methods that use less toxic precursors and require less energy could reduce the environmental footprint of production processes.
Proper disposal and recycling protocols for batteries containing these materials must be established to prevent environmental contamination at the end of their life cycle. This includes developing efficient recycling technologies to recover valuable materials and minimize waste.
In conclusion, while thiophosphate-based solid-state electrolytes offer promising advancements in battery technology, their potential environmental impacts must be carefully managed. Balancing the benefits of improved energy storage with responsible environmental stewardship is crucial for the sustainable development of this technology.
One of the primary environmental concerns is the air stability of thiophosphates. When exposed to air, these materials can undergo degradation processes that may release sulfur-containing compounds. This degradation not only affects the performance of the electrolyte but also poses potential risks to air quality. The release of sulfur compounds could contribute to the formation of sulfur dioxide, a known air pollutant that can lead to acid rain and respiratory issues in humans and animals.
Water contamination is another significant environmental consideration. If thiophosphate materials leach into water systems, they could potentially alter water chemistry and impact aquatic ecosystems. The phosphorus content in these compounds might contribute to eutrophication in water bodies, leading to algal blooms and oxygen depletion.
The production process of thiophosphate-based solid-state electrolytes also warrants attention from an environmental perspective. The synthesis of these materials often involves energy-intensive processes and the use of potentially hazardous precursors. Ensuring proper containment and waste management during manufacturing is crucial to prevent environmental contamination.
On a positive note, the development of stable solid-state electrolytes could lead to more efficient and longer-lasting batteries. This improvement in energy storage technology has the potential to reduce overall energy consumption and decrease reliance on fossil fuels, thereby contributing to a reduction in greenhouse gas emissions.
Mitigation steps to address the environmental concerns associated with thiophosphate-based solid-state electrolytes are essential. Research efforts should focus on developing encapsulation techniques to improve air stability and prevent degradation. Additionally, exploring alternative synthesis methods that use less toxic precursors and require less energy could reduce the environmental footprint of production processes.
Proper disposal and recycling protocols for batteries containing these materials must be established to prevent environmental contamination at the end of their life cycle. This includes developing efficient recycling technologies to recover valuable materials and minimize waste.
In conclusion, while thiophosphate-based solid-state electrolytes offer promising advancements in battery technology, their potential environmental impacts must be carefully managed. Balancing the benefits of improved energy storage with responsible environmental stewardship is crucial for the sustainable development of this technology.
Safety Regulations for SSE Manufacturing
The manufacturing of solid-state electrolytes (SSEs), particularly thiophosphates, requires stringent safety regulations due to their air sensitivity and potential hazards. These regulations aim to protect workers, ensure product quality, and minimize environmental impact. Key safety measures include controlled atmosphere processing, proper handling and storage protocols, and comprehensive employee training.
Controlled atmosphere processing is crucial for thiophosphate SSE manufacturing. Facilities must be equipped with inert gas glove boxes or dry rooms with extremely low humidity levels (<0.1% relative humidity). These environments prevent moisture and oxygen exposure, which can degrade the electrolyte materials. Continuous monitoring of atmospheric conditions is mandatory, with automated systems to detect and respond to any deviations from safe parameters.
Personal protective equipment (PPE) is essential for workers handling thiophosphate SSEs. This includes chemical-resistant gloves, protective eyewear, and respirators with appropriate filters. Specialized clothing that minimizes static electricity buildup is also required to prevent potential ignition of flammable materials. Regular inspection and replacement of PPE must be documented and enforced.
Storage and transportation of thiophosphate SSEs demand specific safety protocols. Materials must be kept in hermetically sealed containers under an inert atmosphere. Storage areas should be temperature-controlled and equipped with fire suppression systems designed for chemical fires. Transportation of these materials requires specialized packaging that maintains an inert environment and complies with hazardous materials shipping regulations.
Waste management is a critical aspect of SSE manufacturing safety. Proper disposal methods for thiophosphate waste must be implemented, including neutralization procedures and containment strategies. Recycling and recovery processes should be developed to minimize environmental impact and reduce raw material costs.
Employee training programs are fundamental to ensuring compliance with safety regulations. Workers must receive comprehensive instruction on material handling, emergency procedures, and the use of safety equipment. Regular refresher courses and safety drills should be conducted to maintain a high level of preparedness.
Environmental monitoring systems are required to detect any leaks or emissions of thiophosphate materials. These systems should include air quality sensors and regular testing of surrounding soil and water sources. Mitigation plans must be in place to address potential contamination events rapidly and effectively.
Lastly, documentation and record-keeping are essential components of safety regulations. Detailed logs of manufacturing processes, safety incidents, and corrective actions must be maintained. Regular audits and inspections by both internal and external authorities should be conducted to ensure ongoing compliance with all applicable safety standards and regulations.
Controlled atmosphere processing is crucial for thiophosphate SSE manufacturing. Facilities must be equipped with inert gas glove boxes or dry rooms with extremely low humidity levels (<0.1% relative humidity). These environments prevent moisture and oxygen exposure, which can degrade the electrolyte materials. Continuous monitoring of atmospheric conditions is mandatory, with automated systems to detect and respond to any deviations from safe parameters.
Personal protective equipment (PPE) is essential for workers handling thiophosphate SSEs. This includes chemical-resistant gloves, protective eyewear, and respirators with appropriate filters. Specialized clothing that minimizes static electricity buildup is also required to prevent potential ignition of flammable materials. Regular inspection and replacement of PPE must be documented and enforced.
Storage and transportation of thiophosphate SSEs demand specific safety protocols. Materials must be kept in hermetically sealed containers under an inert atmosphere. Storage areas should be temperature-controlled and equipped with fire suppression systems designed for chemical fires. Transportation of these materials requires specialized packaging that maintains an inert environment and complies with hazardous materials shipping regulations.
Waste management is a critical aspect of SSE manufacturing safety. Proper disposal methods for thiophosphate waste must be implemented, including neutralization procedures and containment strategies. Recycling and recovery processes should be developed to minimize environmental impact and reduce raw material costs.
Employee training programs are fundamental to ensuring compliance with safety regulations. Workers must receive comprehensive instruction on material handling, emergency procedures, and the use of safety equipment. Regular refresher courses and safety drills should be conducted to maintain a high level of preparedness.
Environmental monitoring systems are required to detect any leaks or emissions of thiophosphate materials. These systems should include air quality sensors and regular testing of surrounding soil and water sources. Mitigation plans must be in place to address potential contamination events rapidly and effectively.
Lastly, documentation and record-keeping are essential components of safety regulations. Detailed logs of manufacturing processes, safety incidents, and corrective actions must be maintained. Regular audits and inspections by both internal and external authorities should be conducted to ensure ongoing compliance with all applicable safety standards and regulations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!