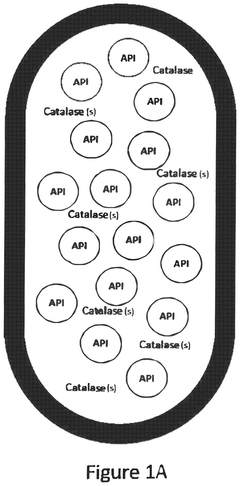
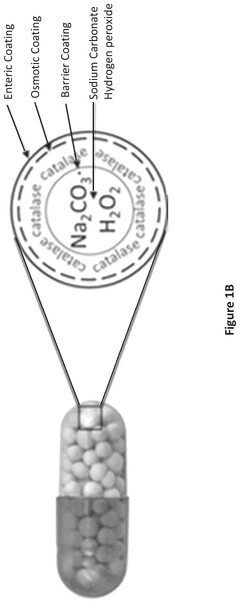
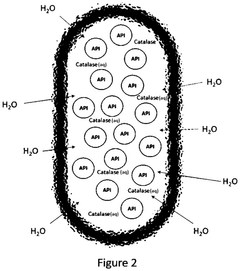
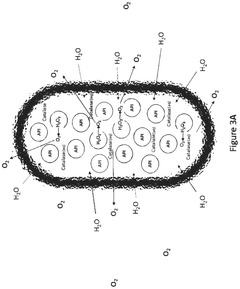
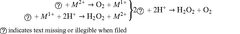Role of Sodium Percarbonate in Veterinary Antiseptic Solutions
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Percarbonate in Veterinary Antiseptics: Background and Objectives
Sodium percarbonate has emerged as a significant component in veterinary antiseptic solutions, marking a notable advancement in animal healthcare. This compound, a stable adduct of hydrogen peroxide and sodium carbonate, has gained attention for its potent oxidizing properties and broad-spectrum antimicrobial activity. The evolution of veterinary antiseptics has been driven by the need for more effective, safe, and environmentally friendly solutions to combat pathogens in animal care settings.
The primary objective of incorporating sodium percarbonate into veterinary antiseptics is to enhance the efficacy of disinfection while minimizing potential harm to animals and the environment. This aligns with the growing emphasis on sustainable and eco-friendly practices in veterinary medicine. Sodium percarbonate's ability to release active oxygen upon dissolution makes it an attractive alternative to traditional chlorine-based disinfectants, which have raised concerns due to their potential toxicity and environmental impact.
The development of sodium percarbonate-based antiseptics represents a convergence of several technological trends in the veterinary field. These include the pursuit of multi-functional formulations that can simultaneously cleanse, disinfect, and deodorize, as well as the demand for products that are effective against a wide range of pathogens, including bacteria, viruses, and fungi. The increasing prevalence of antibiotic-resistant microorganisms has further underscored the importance of developing alternative antimicrobial agents.
Historically, the use of hydrogen peroxide in veterinary antiseptics has been limited by its instability and short shelf life. Sodium percarbonate addresses these challenges by providing a stable, solid form of hydrogen peroxide that can be easily incorporated into various formulations. This innovation has opened up new possibilities for the development of more effective and user-friendly veterinary care products.
The integration of sodium percarbonate into veterinary antiseptics also reflects a broader trend towards evidence-based veterinary medicine. Researchers and manufacturers are increasingly focused on conducting rigorous studies to demonstrate the efficacy and safety of new antiseptic formulations. This scientific approach aims to provide veterinarians and animal care professionals with reliable tools for preventing and controlling infections in diverse clinical settings.
As the veterinary industry continues to evolve, the role of sodium percarbonate in antiseptic solutions is expected to expand. Future research and development efforts are likely to focus on optimizing formulations, exploring synergistic combinations with other active ingredients, and investigating novel applications in animal health. The ongoing pursuit of more effective and sustainable antiseptic solutions underscores the dynamic nature of veterinary technology and its potential to improve animal welfare on a global scale.
The primary objective of incorporating sodium percarbonate into veterinary antiseptics is to enhance the efficacy of disinfection while minimizing potential harm to animals and the environment. This aligns with the growing emphasis on sustainable and eco-friendly practices in veterinary medicine. Sodium percarbonate's ability to release active oxygen upon dissolution makes it an attractive alternative to traditional chlorine-based disinfectants, which have raised concerns due to their potential toxicity and environmental impact.
The development of sodium percarbonate-based antiseptics represents a convergence of several technological trends in the veterinary field. These include the pursuit of multi-functional formulations that can simultaneously cleanse, disinfect, and deodorize, as well as the demand for products that are effective against a wide range of pathogens, including bacteria, viruses, and fungi. The increasing prevalence of antibiotic-resistant microorganisms has further underscored the importance of developing alternative antimicrobial agents.
Historically, the use of hydrogen peroxide in veterinary antiseptics has been limited by its instability and short shelf life. Sodium percarbonate addresses these challenges by providing a stable, solid form of hydrogen peroxide that can be easily incorporated into various formulations. This innovation has opened up new possibilities for the development of more effective and user-friendly veterinary care products.
The integration of sodium percarbonate into veterinary antiseptics also reflects a broader trend towards evidence-based veterinary medicine. Researchers and manufacturers are increasingly focused on conducting rigorous studies to demonstrate the efficacy and safety of new antiseptic formulations. This scientific approach aims to provide veterinarians and animal care professionals with reliable tools for preventing and controlling infections in diverse clinical settings.
As the veterinary industry continues to evolve, the role of sodium percarbonate in antiseptic solutions is expected to expand. Future research and development efforts are likely to focus on optimizing formulations, exploring synergistic combinations with other active ingredients, and investigating novel applications in animal health. The ongoing pursuit of more effective and sustainable antiseptic solutions underscores the dynamic nature of veterinary technology and its potential to improve animal welfare on a global scale.
Market Analysis of Veterinary Antiseptic Solutions
The veterinary antiseptic solutions market has been experiencing steady growth due to increasing pet ownership, rising awareness about animal health, and the growing demand for livestock healthcare. This market segment is a crucial part of the broader veterinary care industry, which is projected to reach significant value in the coming years.
Sodium percarbonate plays a vital role in this market as an active ingredient in many veterinary antiseptic solutions. Its effectiveness as a disinfectant and its ability to release hydrogen peroxide upon dissolution make it a popular choice for various applications in animal healthcare. The compound's versatility allows it to be used in both small animal and livestock settings, contributing to its widespread adoption.
The market for veterinary antiseptic solutions containing sodium percarbonate is driven by several factors. Firstly, the increasing focus on preventive healthcare for animals has led to a higher demand for effective antiseptic products. Veterinarians and pet owners alike are recognizing the importance of proper wound care and infection prevention, boosting the use of these solutions.
Additionally, the rise in intensive farming practices has created a need for robust antiseptic solutions in livestock management. Sodium percarbonate-based products offer an efficient and cost-effective means of maintaining hygiene in large-scale animal husbandry operations, further driving market growth.
The market is also influenced by regulatory factors. Stringent regulations regarding animal welfare and food safety have led to increased use of approved antiseptic solutions in both companion animal care and food-producing animal sectors. Sodium percarbonate's status as a generally recognized as safe (GRAS) substance by regulatory bodies has contributed to its widespread acceptance in veterinary antiseptic formulations.
Geographically, North America and Europe currently dominate the veterinary antiseptic solutions market, owing to high pet ownership rates and advanced veterinary care infrastructure. However, emerging markets in Asia-Pacific and Latin America are showing rapid growth potential due to increasing disposable incomes and growing awareness about animal health.
The competitive landscape of the market is characterized by a mix of large pharmaceutical companies and specialized veterinary care product manufacturers. These players are continuously investing in research and development to improve the efficacy of their antiseptic solutions and expand their product portfolios.
Looking ahead, the market for veterinary antiseptic solutions, including those containing sodium percarbonate, is expected to continue its growth trajectory. Factors such as the humanization of pets, advancements in veterinary medicine, and the increasing focus on food safety in animal-derived products are likely to sustain this trend. As the market evolves, we may see a rise in demand for more specialized and targeted antiseptic solutions, potentially leading to new formulations and applications of sodium percarbonate in veterinary care.
Sodium percarbonate plays a vital role in this market as an active ingredient in many veterinary antiseptic solutions. Its effectiveness as a disinfectant and its ability to release hydrogen peroxide upon dissolution make it a popular choice for various applications in animal healthcare. The compound's versatility allows it to be used in both small animal and livestock settings, contributing to its widespread adoption.
The market for veterinary antiseptic solutions containing sodium percarbonate is driven by several factors. Firstly, the increasing focus on preventive healthcare for animals has led to a higher demand for effective antiseptic products. Veterinarians and pet owners alike are recognizing the importance of proper wound care and infection prevention, boosting the use of these solutions.
Additionally, the rise in intensive farming practices has created a need for robust antiseptic solutions in livestock management. Sodium percarbonate-based products offer an efficient and cost-effective means of maintaining hygiene in large-scale animal husbandry operations, further driving market growth.
The market is also influenced by regulatory factors. Stringent regulations regarding animal welfare and food safety have led to increased use of approved antiseptic solutions in both companion animal care and food-producing animal sectors. Sodium percarbonate's status as a generally recognized as safe (GRAS) substance by regulatory bodies has contributed to its widespread acceptance in veterinary antiseptic formulations.
Geographically, North America and Europe currently dominate the veterinary antiseptic solutions market, owing to high pet ownership rates and advanced veterinary care infrastructure. However, emerging markets in Asia-Pacific and Latin America are showing rapid growth potential due to increasing disposable incomes and growing awareness about animal health.
The competitive landscape of the market is characterized by a mix of large pharmaceutical companies and specialized veterinary care product manufacturers. These players are continuously investing in research and development to improve the efficacy of their antiseptic solutions and expand their product portfolios.
Looking ahead, the market for veterinary antiseptic solutions, including those containing sodium percarbonate, is expected to continue its growth trajectory. Factors such as the humanization of pets, advancements in veterinary medicine, and the increasing focus on food safety in animal-derived products are likely to sustain this trend. As the market evolves, we may see a rise in demand for more specialized and targeted antiseptic solutions, potentially leading to new formulations and applications of sodium percarbonate in veterinary care.
Current Challenges in Veterinary Antiseptic Formulations
The veterinary antiseptic industry faces several significant challenges in formulating effective and safe solutions. One of the primary concerns is the development of antimicrobial resistance, which has become increasingly prevalent in both human and animal medicine. This resistance poses a substantial threat to the efficacy of current antiseptic formulations, necessitating the exploration of novel active ingredients and combination therapies.
Another critical challenge is the need for broad-spectrum efficacy against a diverse range of pathogens, including bacteria, fungi, and viruses. Veterinary antiseptics must be capable of addressing a wide variety of infections across different animal species, each with unique physiological characteristics and susceptibilities. This requirement often leads to complex formulations that may compromise stability or increase production costs.
The safety profile of antiseptic solutions remains a paramount concern, particularly regarding potential toxicity to animals and environmental impact. Many traditional antiseptic compounds have been associated with adverse effects, such as tissue irritation or systemic toxicity when absorbed through the skin or ingested. Formulating products that maintain high efficacy while minimizing these risks is an ongoing challenge for manufacturers.
Stability and shelf-life of antiseptic formulations present additional hurdles. Many active ingredients are susceptible to degradation over time or when exposed to light, heat, or moisture. This instability can lead to reduced efficacy and potentially harmful by-products. Developing stable formulations that maintain their potency under various storage conditions and throughout their intended shelf-life is crucial for ensuring product reliability and safety.
The regulatory landscape for veterinary antiseptics is becoming increasingly stringent, with growing emphasis on demonstrating both efficacy and safety through rigorous scientific studies. Meeting these regulatory requirements while maintaining cost-effectiveness and market competitiveness adds another layer of complexity to the formulation process.
Furthermore, there is a growing demand for eco-friendly and sustainable antiseptic solutions in the veterinary field. This trend challenges manufacturers to develop formulations that are biodegradable, sourced from renewable materials, and have minimal environmental impact. Balancing these sustainability goals with the need for robust antimicrobial activity and product stability presents a significant technical challenge.
Lastly, the diversity of application methods and environments in veterinary practice necessitates the development of versatile formulations. Antiseptics must be effective when applied as sprays, wipes, dips, or in wound dressings, and they must maintain their efficacy in the presence of organic matter, varying pH levels, and different temperatures. Creating formulations that perform consistently across these diverse conditions remains an ongoing challenge in the industry.
Another critical challenge is the need for broad-spectrum efficacy against a diverse range of pathogens, including bacteria, fungi, and viruses. Veterinary antiseptics must be capable of addressing a wide variety of infections across different animal species, each with unique physiological characteristics and susceptibilities. This requirement often leads to complex formulations that may compromise stability or increase production costs.
The safety profile of antiseptic solutions remains a paramount concern, particularly regarding potential toxicity to animals and environmental impact. Many traditional antiseptic compounds have been associated with adverse effects, such as tissue irritation or systemic toxicity when absorbed through the skin or ingested. Formulating products that maintain high efficacy while minimizing these risks is an ongoing challenge for manufacturers.
Stability and shelf-life of antiseptic formulations present additional hurdles. Many active ingredients are susceptible to degradation over time or when exposed to light, heat, or moisture. This instability can lead to reduced efficacy and potentially harmful by-products. Developing stable formulations that maintain their potency under various storage conditions and throughout their intended shelf-life is crucial for ensuring product reliability and safety.
The regulatory landscape for veterinary antiseptics is becoming increasingly stringent, with growing emphasis on demonstrating both efficacy and safety through rigorous scientific studies. Meeting these regulatory requirements while maintaining cost-effectiveness and market competitiveness adds another layer of complexity to the formulation process.
Furthermore, there is a growing demand for eco-friendly and sustainable antiseptic solutions in the veterinary field. This trend challenges manufacturers to develop formulations that are biodegradable, sourced from renewable materials, and have minimal environmental impact. Balancing these sustainability goals with the need for robust antimicrobial activity and product stability presents a significant technical challenge.
Lastly, the diversity of application methods and environments in veterinary practice necessitates the development of versatile formulations. Antiseptics must be effective when applied as sprays, wipes, dips, or in wound dressings, and they must maintain their efficacy in the presence of organic matter, varying pH levels, and different temperatures. Creating formulations that perform consistently across these diverse conditions remains an ongoing challenge in the industry.
Existing Sodium Percarbonate-based Antiseptic Solutions
01 Synthesis and production of sodium percarbonate
Various methods for synthesizing and producing sodium percarbonate are described. These methods involve the reaction of sodium carbonate with hydrogen peroxide under specific conditions to form sodium percarbonate crystals. The processes may include steps such as crystallization, drying, and stabilization to improve the quality and stability of the final product.- Synthesis and production of sodium percarbonate: Various methods for synthesizing and producing sodium percarbonate are described. These processes typically involve the reaction of sodium carbonate with hydrogen peroxide under controlled conditions. The production methods aim to improve yield, purity, and stability of the final product.
- Stabilization of sodium percarbonate: Techniques for enhancing the stability of sodium percarbonate are discussed. These may include coating the particles, adding stabilizing agents, or modifying the crystal structure. Improved stability helps maintain the product's effectiveness during storage and use.
- Applications in cleaning and bleaching: Sodium percarbonate is widely used in cleaning and bleaching applications. It serves as an oxygen-based bleach in laundry detergents and household cleaners. The compound's ability to release hydrogen peroxide in aqueous solutions makes it effective for stain removal and disinfection.
- Formulation in personal care products: Sodium percarbonate is incorporated into various personal care products, such as toothpaste and mouthwash. Its oxidizing properties contribute to teeth whitening and oral hygiene. Formulations may include additional ingredients to enhance efficacy and improve user experience.
- Environmental and safety considerations: Research focuses on the environmental impact and safety aspects of sodium percarbonate use. Studies examine its biodegradability, ecotoxicity, and potential risks associated with handling and storage. Efforts are made to develop more environmentally friendly production processes and safer formulations.
02 Stabilization of sodium percarbonate
Techniques for stabilizing sodium percarbonate to improve its shelf life and performance are discussed. These may include coating the particles with stabilizing agents, incorporating additives, or modifying the crystal structure. Stabilization helps prevent decomposition and maintains the active oxygen content of the compound during storage and use.Expand Specific Solutions03 Applications in cleaning and bleaching
Sodium percarbonate is widely used in cleaning and bleaching applications. It serves as an effective oxygen-based bleaching agent in laundry detergents, dishwashing products, and other household cleaners. The compound releases hydrogen peroxide when dissolved in water, providing stain removal and disinfecting properties.Expand Specific Solutions04 Formulation in personal care products
Sodium percarbonate is incorporated into various personal care products, such as tooth whitening formulations and hair bleaching agents. Its oxygen-releasing properties make it effective for brightening and whitening applications while being relatively gentle compared to other bleaching agents.Expand Specific Solutions05 Environmental and safety considerations
Research and development efforts focus on improving the environmental profile and safety of sodium percarbonate. This includes developing more eco-friendly production methods, reducing impurities, and enhancing the biodegradability of formulations containing sodium percarbonate. Safety measures for handling and storage are also addressed.Expand Specific Solutions
Key Players in Veterinary Antiseptic Industry
The role of sodium percarbonate in veterinary antiseptic solutions is an emerging field with growing market potential. The industry is in its early growth stage, characterized by increasing research and development activities. The global market for veterinary antiseptics is expanding, driven by rising pet ownership and focus on animal health. Technologically, sodium percarbonate-based solutions are gaining traction due to their effectiveness and safety profile. Companies like Ecolab USA, Zoetis Services, and Boehringer Ingelheim Vetmedica are leading players, investing in R&D to develop innovative formulations. However, the technology is still evolving, with ongoing efforts to optimize efficacy and application methods across various veterinary settings.
Solvay SA
Technical Solution: Solvay, a leading chemical company, has developed an innovative sodium percarbonate-based veterinary antiseptic solution that focuses on stability and efficacy. Their technology involves a proprietary stabilization process that significantly extends the shelf life of the product while maintaining its potent antimicrobial properties[10]. Solvay's formulation incorporates nano-sized sodium percarbonate particles, which increase the surface area for reaction and enhance the overall antiseptic efficacy[12]. The company has also developed a unique delivery system that allows for precise dosing and easy application in various veterinary settings. Their solution includes a color indicator that changes as the active oxygen species are released, providing visual confirmation of the antiseptic action[14].
Strengths: Enhanced stability, improved efficacy due to nano-sized particles, and visual indicator for active status. Weaknesses: Potential higher cost due to advanced formulation, and may require specialized equipment for nano-particle production.
Tianjin Ringpu Bio-technology Co., Ltd.
Technical Solution: Tianjin Ringpu Bio-technology has developed a unique veterinary antiseptic solution that combines sodium percarbonate with traditional Chinese herbal extracts. Their approach leverages the oxidizing power of sodium percarbonate while incorporating synergistic antimicrobial and wound-healing properties of selected herbal components[15]. The company's formulation includes a proprietary extraction process that enhances the stability and compatibility of the herbal extracts with sodium percarbonate[17]. This combination results in a broad-spectrum antiseptic with additional benefits such as anti-inflammatory and analgesic effects. Tianjin Ringpu's technology also features a pH-responsive release mechanism, ensuring optimal activity of both the sodium percarbonate and herbal extracts in different physiological environments[19].
Strengths: Unique combination of modern and traditional medicine, additional therapeutic benefits beyond antiseptic action. Weaknesses: Complexity in formulation may lead to higher production costs, potential variability in herbal extract components.
Innovative Applications of Sodium Percarbonate in Veterinary Care
Enteric aerobization therapy
PatentActiveUS12128079B2
Innovation
- Enteric aerobization therapy (EAT) using oxygen carrier molecules, oxygen-containing mixtures, oxygen prodrugs, or oxygen-generating compounds delivered orally to convert the anaerobic intestinal environment to an aerobic one through formulations with catalase, yeast cells, or other catalysts, ensuring sustained oxygen release and stability.
Veterinary Composition Comprising Superoxide Dismutase and at Least One Hydrolysate of Proteins Rich in Bioassimilable Peptides
PatentPendingUS20250213660A1
Innovation
- A veterinary composition comprising superoxide dismutase (SOD) and a protein hydrolysate rich in bioassimilable peptides, administered orally, to prevent, regulate, and treat fear, anxiety, and associated behavior disorders, with a peptide fraction less than 1% having a molecular weight greater than 10,000 Da, and a ratio of SOD to protein hydrolysate between 0.01:100 and 100:1.
Safety and Efficacy Regulations for Veterinary Antiseptics
The regulatory landscape for veterinary antiseptics is complex and multifaceted, with various agencies and organizations involved in ensuring the safety and efficacy of these products. In the United States, the Food and Drug Administration (FDA) plays a primary role in regulating veterinary antiseptics, particularly through its Center for Veterinary Medicine (CVM). The FDA requires manufacturers to demonstrate both safety and efficacy before approving any veterinary antiseptic product for market release.
Safety regulations for veterinary antiseptics focus on several key aspects. These include toxicity assessments to ensure the product does not harm the animal when used as directed, as well as evaluations of potential environmental impacts. Manufacturers must provide comprehensive data on the product's ingredients, including any potential for adverse reactions or long-term effects. Additionally, safety regulations often extend to the handling and storage of these products, with requirements for clear labeling and proper packaging to prevent accidental exposure or misuse.
Efficacy regulations are equally stringent, requiring manufacturers to provide substantial evidence that the antiseptic product is effective for its intended use. This typically involves conducting controlled clinical trials that demonstrate the product's ability to reduce or eliminate harmful microorganisms on animal skin or wounds. The FDA often requires these studies to be conducted under Good Laboratory Practice (GLP) conditions to ensure the reliability and reproducibility of results.
In the European Union, the European Medicines Agency (EMA) oversees the regulation of veterinary antiseptics. The EMA's approach aligns closely with that of the FDA, emphasizing both safety and efficacy. However, there may be some variations in specific requirements or testing protocols between regions, necessitating careful consideration for manufacturers seeking global market access.
Regulatory bodies also consider the potential for antimicrobial resistance when evaluating veterinary antiseptics. This has led to increased scrutiny of products containing antibiotics and a preference for alternative antiseptic compounds that are less likely to contribute to resistance development. As a result, manufacturers are often required to provide data on the long-term effectiveness of their products and any potential for microorganisms to develop resistance over time.
The regulatory framework for veterinary antiseptics also includes post-market surveillance requirements. Manufacturers must continue to monitor and report on the safety and efficacy of their products after they have been approved and released to the market. This ongoing process helps to identify any unforeseen issues or long-term effects that may not have been apparent during initial testing phases.
Safety regulations for veterinary antiseptics focus on several key aspects. These include toxicity assessments to ensure the product does not harm the animal when used as directed, as well as evaluations of potential environmental impacts. Manufacturers must provide comprehensive data on the product's ingredients, including any potential for adverse reactions or long-term effects. Additionally, safety regulations often extend to the handling and storage of these products, with requirements for clear labeling and proper packaging to prevent accidental exposure or misuse.
Efficacy regulations are equally stringent, requiring manufacturers to provide substantial evidence that the antiseptic product is effective for its intended use. This typically involves conducting controlled clinical trials that demonstrate the product's ability to reduce or eliminate harmful microorganisms on animal skin or wounds. The FDA often requires these studies to be conducted under Good Laboratory Practice (GLP) conditions to ensure the reliability and reproducibility of results.
In the European Union, the European Medicines Agency (EMA) oversees the regulation of veterinary antiseptics. The EMA's approach aligns closely with that of the FDA, emphasizing both safety and efficacy. However, there may be some variations in specific requirements or testing protocols between regions, necessitating careful consideration for manufacturers seeking global market access.
Regulatory bodies also consider the potential for antimicrobial resistance when evaluating veterinary antiseptics. This has led to increased scrutiny of products containing antibiotics and a preference for alternative antiseptic compounds that are less likely to contribute to resistance development. As a result, manufacturers are often required to provide data on the long-term effectiveness of their products and any potential for microorganisms to develop resistance over time.
The regulatory framework for veterinary antiseptics also includes post-market surveillance requirements. Manufacturers must continue to monitor and report on the safety and efficacy of their products after they have been approved and released to the market. This ongoing process helps to identify any unforeseen issues or long-term effects that may not have been apparent during initial testing phases.
Environmental Impact of Sodium Percarbonate-based Solutions
The environmental impact of sodium percarbonate-based solutions in veterinary antiseptic applications is a crucial consideration for sustainable veterinary practices. Sodium percarbonate, when dissolved in water, releases hydrogen peroxide and sodium carbonate, both of which have implications for the environment.
One of the primary advantages of sodium percarbonate-based solutions is their relatively low environmental toxicity. Hydrogen peroxide, the active component, readily decomposes into water and oxygen, leaving no harmful residues. This characteristic makes it an environmentally friendly alternative to more persistent antiseptic compounds.
However, the environmental impact is not entirely benign. The release of sodium carbonate can lead to localized increases in pH levels, potentially affecting aquatic ecosystems if large quantities of the solution are discharged into water bodies. This alkalinity shift may disrupt the balance of aquatic flora and fauna, particularly in sensitive environments.
The production process of sodium percarbonate also bears environmental considerations. Manufacturing typically involves the reaction of sodium carbonate with hydrogen peroxide, which requires energy input and may contribute to carbon emissions depending on the energy source used. Additionally, the transportation and packaging of sodium percarbonate-based products contribute to their overall environmental footprint.
On the positive side, the stability of sodium percarbonate in dry form reduces the need for frequent transportation of diluted solutions, potentially lowering the carbon footprint associated with distribution. Furthermore, its effectiveness as an antiseptic at lower concentrations compared to some alternatives may result in reduced chemical usage overall.
The biodegradability of sodium percarbonate-based solutions is another environmental advantage. Unlike some persistent antiseptics that can accumulate in the environment, these solutions break down relatively quickly, minimizing long-term ecological impacts. This property is particularly important in agricultural settings where repeated use of veterinary antiseptics is common.
However, it is essential to consider the potential for oxygen depletion in aquatic environments due to the rapid decomposition of hydrogen peroxide. While this effect is typically localized and short-lived, it could be significant in stagnant or poorly oxygenated water bodies.
In conclusion, while sodium percarbonate-based solutions offer several environmental benefits in veterinary antiseptic applications, their use still requires careful management and consideration of local ecological conditions. Proper disposal practices and judicious application are crucial to minimizing potential negative impacts while maximizing the environmental advantages of this technology.
One of the primary advantages of sodium percarbonate-based solutions is their relatively low environmental toxicity. Hydrogen peroxide, the active component, readily decomposes into water and oxygen, leaving no harmful residues. This characteristic makes it an environmentally friendly alternative to more persistent antiseptic compounds.
However, the environmental impact is not entirely benign. The release of sodium carbonate can lead to localized increases in pH levels, potentially affecting aquatic ecosystems if large quantities of the solution are discharged into water bodies. This alkalinity shift may disrupt the balance of aquatic flora and fauna, particularly in sensitive environments.
The production process of sodium percarbonate also bears environmental considerations. Manufacturing typically involves the reaction of sodium carbonate with hydrogen peroxide, which requires energy input and may contribute to carbon emissions depending on the energy source used. Additionally, the transportation and packaging of sodium percarbonate-based products contribute to their overall environmental footprint.
On the positive side, the stability of sodium percarbonate in dry form reduces the need for frequent transportation of diluted solutions, potentially lowering the carbon footprint associated with distribution. Furthermore, its effectiveness as an antiseptic at lower concentrations compared to some alternatives may result in reduced chemical usage overall.
The biodegradability of sodium percarbonate-based solutions is another environmental advantage. Unlike some persistent antiseptics that can accumulate in the environment, these solutions break down relatively quickly, minimizing long-term ecological impacts. This property is particularly important in agricultural settings where repeated use of veterinary antiseptics is common.
However, it is essential to consider the potential for oxygen depletion in aquatic environments due to the rapid decomposition of hydrogen peroxide. While this effect is typically localized and short-lived, it could be significant in stagnant or poorly oxygenated water bodies.
In conclusion, while sodium percarbonate-based solutions offer several environmental benefits in veterinary antiseptic applications, their use still requires careful management and consideration of local ecological conditions. Proper disposal practices and judicious application are crucial to minimizing potential negative impacts while maximizing the environmental advantages of this technology.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!