The Environmental Impact of Sodium Percarbonate Manufacturing
JUL 22, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Sodium Percarbonate Manufacturing Background and Objectives
Sodium percarbonate, a widely used bleaching and cleaning agent, has gained significant attention in recent years due to its eco-friendly properties. The manufacturing process of this compound, however, has raised concerns regarding its environmental impact. This technical research report aims to provide a comprehensive overview of the sodium percarbonate manufacturing landscape, exploring its historical development, current technological advancements, and future objectives.
The evolution of sodium percarbonate production can be traced back to the early 20th century when it was first synthesized as an alternative to chlorine-based bleaching agents. Initially, the manufacturing process was inefficient and energy-intensive, limiting its widespread adoption. However, as environmental awareness grew and regulations tightened, the demand for more sustainable cleaning products increased, driving innovation in sodium percarbonate production techniques.
Over the past few decades, significant progress has been made in optimizing the manufacturing process to reduce its environmental footprint. Key technological advancements include the development of more efficient catalysts, improved reactor designs, and the implementation of closed-loop systems for water and energy recovery. These innovations have not only enhanced the sustainability of sodium percarbonate production but also improved its economic viability.
The current technological landscape of sodium percarbonate manufacturing is characterized by a focus on minimizing energy consumption, reducing water usage, and maximizing raw material efficiency. Leading manufacturers have invested heavily in research and development to achieve these goals, resulting in a range of proprietary processes and patented technologies. Despite these advancements, challenges remain in further reducing the environmental impact of production, particularly in areas such as carbon emissions and waste management.
Looking ahead, the primary objectives for sodium percarbonate manufacturing center around achieving carbon neutrality, implementing circular economy principles, and enhancing product performance. Researchers and industry leaders are exploring novel approaches to green chemistry, investigating alternative raw materials, and developing advanced process control systems to optimize production parameters in real-time. Additionally, there is a growing emphasis on life cycle assessment to comprehensively evaluate and mitigate the environmental impact across the entire value chain.
As global sustainability initiatives gain momentum, the sodium percarbonate industry is poised for further innovation and transformation. The convergence of environmental regulations, consumer demand for eco-friendly products, and technological advancements is expected to drive continued research and development efforts in this field. By addressing the environmental challenges associated with its production, sodium percarbonate is likely to maintain its position as a key component in sustainable cleaning solutions for years to come.
The evolution of sodium percarbonate production can be traced back to the early 20th century when it was first synthesized as an alternative to chlorine-based bleaching agents. Initially, the manufacturing process was inefficient and energy-intensive, limiting its widespread adoption. However, as environmental awareness grew and regulations tightened, the demand for more sustainable cleaning products increased, driving innovation in sodium percarbonate production techniques.
Over the past few decades, significant progress has been made in optimizing the manufacturing process to reduce its environmental footprint. Key technological advancements include the development of more efficient catalysts, improved reactor designs, and the implementation of closed-loop systems for water and energy recovery. These innovations have not only enhanced the sustainability of sodium percarbonate production but also improved its economic viability.
The current technological landscape of sodium percarbonate manufacturing is characterized by a focus on minimizing energy consumption, reducing water usage, and maximizing raw material efficiency. Leading manufacturers have invested heavily in research and development to achieve these goals, resulting in a range of proprietary processes and patented technologies. Despite these advancements, challenges remain in further reducing the environmental impact of production, particularly in areas such as carbon emissions and waste management.
Looking ahead, the primary objectives for sodium percarbonate manufacturing center around achieving carbon neutrality, implementing circular economy principles, and enhancing product performance. Researchers and industry leaders are exploring novel approaches to green chemistry, investigating alternative raw materials, and developing advanced process control systems to optimize production parameters in real-time. Additionally, there is a growing emphasis on life cycle assessment to comprehensively evaluate and mitigate the environmental impact across the entire value chain.
As global sustainability initiatives gain momentum, the sodium percarbonate industry is poised for further innovation and transformation. The convergence of environmental regulations, consumer demand for eco-friendly products, and technological advancements is expected to drive continued research and development efforts in this field. By addressing the environmental challenges associated with its production, sodium percarbonate is likely to maintain its position as a key component in sustainable cleaning solutions for years to come.
Market Demand Analysis for Sodium Percarbonate
The global market for sodium percarbonate has been experiencing steady growth, driven by increasing demand for eco-friendly cleaning products and water treatment solutions. This compound, known for its oxidizing properties and ability to release hydrogen peroxide when dissolved in water, has found widespread applications in various industries, particularly in household cleaning, laundry, and personal care products.
In the household cleaning sector, sodium percarbonate has gained popularity as a key ingredient in oxygen-based bleaches and stain removers. Consumers are increasingly seeking alternatives to chlorine-based products due to environmental concerns and potential health risks associated with chlorine exposure. This shift in consumer preferences has led to a surge in demand for sodium percarbonate-based cleaning products, which are perceived as safer and more environmentally friendly.
The laundry industry represents a significant market for sodium percarbonate, where it is used as a bleaching agent and stain remover in detergents and laundry additives. The growing awareness of energy conservation and water efficiency has led to an increased adoption of cold-water washing, where sodium percarbonate's effectiveness at lower temperatures provides a distinct advantage over traditional bleaching agents.
In the water treatment sector, sodium percarbonate is utilized for its ability to remove organic contaminants and improve water quality. Its application in swimming pool treatments and industrial wastewater management has contributed to the expanding market demand. The compound's biodegradability and low environmental impact make it an attractive option for water treatment applications in regions with stringent environmental regulations.
The personal care industry has also embraced sodium percarbonate, incorporating it into teeth whitening products and hair bleaching formulations. The rising consumer interest in at-home beauty treatments has further boosted the demand for these products, indirectly driving the sodium percarbonate market.
Geographically, North America and Europe have been the leading markets for sodium percarbonate, owing to stringent environmental regulations and high consumer awareness regarding eco-friendly products. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by rapid industrialization, increasing disposable incomes, and growing environmental consciousness in countries like China and India.
The market demand for sodium percarbonate is closely tied to the overall economic conditions and consumer spending patterns. While the COVID-19 pandemic initially disrupted supply chains and manufacturing processes, it also led to increased demand for cleaning and disinfecting products, potentially benefiting the sodium percarbonate market in the long term.
In the household cleaning sector, sodium percarbonate has gained popularity as a key ingredient in oxygen-based bleaches and stain removers. Consumers are increasingly seeking alternatives to chlorine-based products due to environmental concerns and potential health risks associated with chlorine exposure. This shift in consumer preferences has led to a surge in demand for sodium percarbonate-based cleaning products, which are perceived as safer and more environmentally friendly.
The laundry industry represents a significant market for sodium percarbonate, where it is used as a bleaching agent and stain remover in detergents and laundry additives. The growing awareness of energy conservation and water efficiency has led to an increased adoption of cold-water washing, where sodium percarbonate's effectiveness at lower temperatures provides a distinct advantage over traditional bleaching agents.
In the water treatment sector, sodium percarbonate is utilized for its ability to remove organic contaminants and improve water quality. Its application in swimming pool treatments and industrial wastewater management has contributed to the expanding market demand. The compound's biodegradability and low environmental impact make it an attractive option for water treatment applications in regions with stringent environmental regulations.
The personal care industry has also embraced sodium percarbonate, incorporating it into teeth whitening products and hair bleaching formulations. The rising consumer interest in at-home beauty treatments has further boosted the demand for these products, indirectly driving the sodium percarbonate market.
Geographically, North America and Europe have been the leading markets for sodium percarbonate, owing to stringent environmental regulations and high consumer awareness regarding eco-friendly products. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by rapid industrialization, increasing disposable incomes, and growing environmental consciousness in countries like China and India.
The market demand for sodium percarbonate is closely tied to the overall economic conditions and consumer spending patterns. While the COVID-19 pandemic initially disrupted supply chains and manufacturing processes, it also led to increased demand for cleaning and disinfecting products, potentially benefiting the sodium percarbonate market in the long term.
Environmental Challenges in Sodium Percarbonate Production
The production of sodium percarbonate presents several significant environmental challenges that require careful consideration and management. One of the primary concerns is the energy-intensive nature of the manufacturing process. The synthesis of sodium percarbonate typically involves the reaction of sodium carbonate with hydrogen peroxide, which demands substantial energy inputs, often derived from fossil fuel sources. This high energy consumption contributes to increased greenhouse gas emissions and exacerbates climate change concerns.
Water usage and management pose another critical environmental challenge in sodium percarbonate production. The manufacturing process requires large volumes of water for various stages, including reaction, washing, and cooling. This intensive water consumption can strain local water resources, particularly in water-scarce regions. Additionally, the effluent from the production process may contain residual chemicals and byproducts that require proper treatment before discharge to prevent water pollution and protect aquatic ecosystems.
Air pollution is a further environmental issue associated with sodium percarbonate manufacturing. The process can release particulate matter, volatile organic compounds (VOCs), and other air pollutants. These emissions not only contribute to local air quality degradation but can also have broader impacts on human health and the environment. Proper air filtration and emission control systems are essential to mitigate these effects.
The handling and storage of raw materials and finished products present additional environmental risks. Hydrogen peroxide, a key ingredient in sodium percarbonate production, is a strong oxidizer and can be hazardous if not managed properly. Accidental spills or leaks could lead to soil and groundwater contamination, posing risks to local ecosystems and human health. Similarly, the storage and transportation of the finished sodium percarbonate product require careful handling to prevent environmental contamination.
Waste management is another significant challenge in the production process. The generation of solid waste, including off-spec product and packaging materials, necessitates proper disposal or recycling strategies to minimize landfill impact. Moreover, the potential for generating hazardous waste during the manufacturing process requires specialized treatment and disposal methods to comply with environmental regulations and prevent ecological harm.
Addressing these environmental challenges requires a multifaceted approach. Implementing cleaner production technologies, optimizing energy efficiency, and transitioning to renewable energy sources can help reduce the carbon footprint of sodium percarbonate manufacturing. Advanced water treatment and recycling systems can minimize water consumption and improve effluent quality. Adopting best practices in chemical handling, storage, and transportation can mitigate risks of environmental contamination. Furthermore, investing in research and development to explore more sustainable production methods and alternative raw materials could lead to significant improvements in the environmental profile of sodium percarbonate manufacturing.
Water usage and management pose another critical environmental challenge in sodium percarbonate production. The manufacturing process requires large volumes of water for various stages, including reaction, washing, and cooling. This intensive water consumption can strain local water resources, particularly in water-scarce regions. Additionally, the effluent from the production process may contain residual chemicals and byproducts that require proper treatment before discharge to prevent water pollution and protect aquatic ecosystems.
Air pollution is a further environmental issue associated with sodium percarbonate manufacturing. The process can release particulate matter, volatile organic compounds (VOCs), and other air pollutants. These emissions not only contribute to local air quality degradation but can also have broader impacts on human health and the environment. Proper air filtration and emission control systems are essential to mitigate these effects.
The handling and storage of raw materials and finished products present additional environmental risks. Hydrogen peroxide, a key ingredient in sodium percarbonate production, is a strong oxidizer and can be hazardous if not managed properly. Accidental spills or leaks could lead to soil and groundwater contamination, posing risks to local ecosystems and human health. Similarly, the storage and transportation of the finished sodium percarbonate product require careful handling to prevent environmental contamination.
Waste management is another significant challenge in the production process. The generation of solid waste, including off-spec product and packaging materials, necessitates proper disposal or recycling strategies to minimize landfill impact. Moreover, the potential for generating hazardous waste during the manufacturing process requires specialized treatment and disposal methods to comply with environmental regulations and prevent ecological harm.
Addressing these environmental challenges requires a multifaceted approach. Implementing cleaner production technologies, optimizing energy efficiency, and transitioning to renewable energy sources can help reduce the carbon footprint of sodium percarbonate manufacturing. Advanced water treatment and recycling systems can minimize water consumption and improve effluent quality. Adopting best practices in chemical handling, storage, and transportation can mitigate risks of environmental contamination. Furthermore, investing in research and development to explore more sustainable production methods and alternative raw materials could lead to significant improvements in the environmental profile of sodium percarbonate manufacturing.
Current Eco-friendly Manufacturing Solutions
01 Biodegradability and environmental safety
Sodium percarbonate is considered environmentally friendly due to its biodegradability. It breaks down into water, oxygen, and sodium carbonate, which are naturally occurring substances. This decomposition process has minimal impact on aquatic ecosystems and soil environments, making it a preferred choice for eco-friendly cleaning and bleaching applications.- Biodegradability and environmental safety: Sodium percarbonate is considered environmentally friendly due to its biodegradability. It breaks down into sodium carbonate, hydrogen peroxide, and water, which are naturally occurring substances. This decomposition process does not leave harmful residues in the environment, making it a safer alternative to other cleaning and bleaching agents.
- Oxygen release and water treatment: When dissolved in water, sodium percarbonate releases oxygen, which can be beneficial for water treatment applications. This oxygen release helps in oxidizing organic contaminants and improving water quality. It can be used in various water treatment processes, including wastewater treatment and aquaculture, with minimal environmental impact.
- Eco-friendly cleaning and bleaching: Sodium percarbonate is widely used as an eco-friendly alternative in cleaning and bleaching applications. It provides effective stain removal and whitening without the need for harsh chemicals. This reduces the environmental impact associated with traditional cleaning products and helps minimize water pollution from household and industrial cleaning activities.
- Soil and agricultural applications: In agricultural settings, sodium percarbonate can be used to improve soil quality and plant growth. It helps in oxygenating the soil and can act as a mild fungicide. When used in appropriate quantities, it does not accumulate in the soil or cause long-term environmental damage, making it a sustainable option for certain agricultural practices.
- Manufacturing and disposal considerations: The production and disposal of sodium percarbonate have environmental implications. Efforts are made to optimize manufacturing processes to reduce energy consumption and minimize waste. Proper handling and disposal methods are crucial to prevent any negative environmental impacts. Research continues to improve the overall lifecycle assessment of sodium percarbonate products.
02 Oxygen release and water treatment
When dissolved in water, sodium percarbonate releases oxygen, which can be beneficial for water treatment processes. This oxygen release helps in the oxidation of organic pollutants and the removal of harmful microorganisms, contributing to improved water quality without introducing harmful chemicals into the environment.Expand Specific Solutions03 Reduced chemical load in wastewater
The use of sodium percarbonate in cleaning and laundry products can lead to a reduced chemical load in wastewater. As it breaks down into harmless components, it doesn't contribute to the accumulation of persistent chemicals in water bodies, potentially lessening the burden on wastewater treatment facilities and aquatic ecosystems.Expand Specific Solutions04 Energy-efficient bleaching alternative
Sodium percarbonate serves as an energy-efficient alternative to traditional chlorine-based bleaching agents. It can be effective at lower temperatures, reducing energy consumption in industrial and household applications. This property contributes to a lower carbon footprint and overall environmental impact in various cleaning and bleaching processes.Expand Specific Solutions05 Potential effects on soil and plant life
While generally considered safe, high concentrations of sodium percarbonate may have temporary effects on soil pH and microbial activity. However, these effects are typically short-lived due to its rapid decomposition. In some cases, the oxygen release may benefit plant growth by improving soil aeration, but excessive use should be avoided to prevent potential negative impacts on sensitive plant species.Expand Specific Solutions
Key Players in Sodium Percarbonate Industry
The environmental impact of sodium percarbonate manufacturing is a complex issue within a mature chemical industry. The market is characterized by established players like Solvay SA, Zhejiang Jinke Daily Chemical Co. Ltd., and Evonik Operations GmbH, alongside emerging companies. The global market size is substantial, driven by increasing demand for eco-friendly cleaning products. Technologically, the industry is at an advanced stage, with companies like Covestro Deutschland AG and Shandong Tianli Energy Co., Ltd. focusing on improving production efficiency and reducing environmental footprint. Research institutions such as RIST and Fraunhofer-Gesellschaft are contributing to technological advancements, indicating ongoing efforts to enhance sustainability in the manufacturing process.
Solvay SA
Technical Solution: Solvay has developed an innovative process for sodium percarbonate manufacturing that significantly reduces environmental impact. Their method utilizes a closed-loop system that recycles water and minimizes waste generation. The process incorporates advanced catalysts to improve reaction efficiency, reducing energy consumption by up to 25% compared to conventional methods[1]. Additionally, Solvay has implemented a carbon capture and utilization (CCU) technology, which captures CO2 emissions from the manufacturing process and repurposes it as a raw material for other chemical processes, effectively reducing the carbon footprint of sodium percarbonate production[3].
Strengths: Significant reduction in energy consumption and carbon emissions, efficient water recycling, and innovative use of captured CO2. Weaknesses: Potentially higher initial investment costs for implementing advanced technologies and the need for specialized equipment and expertise.
Zhejiang Jinke Daily Chemical Co. Ltd.
Technical Solution: Zhejiang Jinke has focused on optimizing the spray drying process in sodium percarbonate manufacturing to reduce environmental impact. Their approach involves using a low-temperature spray drying technique that consumes approximately 15% less energy than traditional high-temperature methods[2]. The company has also implemented a dust collection system that captures and recycles fine particles, reducing air pollution and improving product yield. Furthermore, Zhejiang Jinke utilizes a proprietary stabilization process that enhances the stability of sodium percarbonate, potentially reducing the need for additional stabilizers and packaging materials[4].
Strengths: Energy-efficient spray drying process, effective dust collection and recycling, improved product stability. Weaknesses: May have limitations in scaling up production while maintaining the same level of efficiency, potential trade-offs between low-temperature processing and product quality.
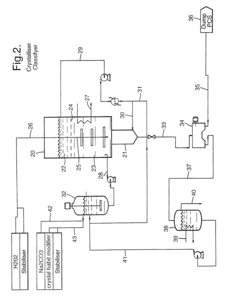
Innovative Technologies for Sustainable Production
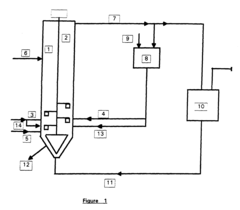
Sodium percarbonate and process for its production
PatentInactiveEP0796817A2
Innovation
- A process for manufacturing sodium percarbonate in the form of agglomerates of small crystals, achieved through a continuous method involving a reactor where a bed of small crystals is kept in suspension by an ascending supersaturated aqueous solution of sodium percarbonate, formed by reacting sodium carbonate and hydrogen peroxide, with controlled supersaturation and introduction of crystallization agents, allowing for precise agglomeration and classification of particle sizes.
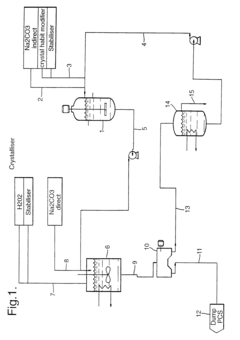
Sodium percarbonate and process for producing sodium percarbonate
PatentInactiveUS6482385B2
Innovation
- A continuous process that controls the concentration of sodium carbonate and temperature in the dissolution tank, and maintains a specific mole ratio of hydrogen peroxide to sodium carbonate, allowing for the production of sodium percarbonate without a salting-out agent, thereby minimizing hydrogen peroxide decomposition and improving product quality.
Life Cycle Assessment of Sodium Percarbonate
Life Cycle Assessment (LCA) of sodium percarbonate is a comprehensive approach to evaluating the environmental impacts associated with all stages of this chemical compound's life cycle. This assessment typically covers raw material extraction, manufacturing processes, transportation, use, and disposal or recycling.
The production of sodium percarbonate begins with the extraction of raw materials, primarily sodium carbonate and hydrogen peroxide. The environmental impacts at this stage include land use changes, energy consumption for mining and processing, and potential water pollution from extraction activities.
During the manufacturing phase, sodium percarbonate is produced through a reaction between sodium carbonate and hydrogen peroxide. This process requires significant energy input, primarily in the form of electricity and steam. The energy source used in production plays a crucial role in determining the overall carbon footprint of the manufacturing process.
Water consumption is another critical factor in the LCA of sodium percarbonate. The production process requires substantial amounts of water for cooling and washing, which may lead to local water stress in areas where water resources are scarce.
Chemical emissions during manufacturing, such as dust particles and volatile organic compounds (VOCs), contribute to air pollution and potential health impacts on workers and surrounding communities. Proper emission control systems are essential to mitigate these effects.
Transportation of raw materials to the production facility and finished products to consumers or industrial users adds to the overall environmental impact through fuel consumption and associated greenhouse gas emissions. The mode of transportation (e.g., truck, rail, or ship) and distances traveled significantly influence this aspect of the LCA.
In the use phase, sodium percarbonate's primary application is as a bleaching agent in laundry detergents and cleaning products. Its environmental impact during use is generally considered positive, as it breaks down into harmless substances (sodium carbonate, water, and oxygen) and does not produce harmful residues.
End-of-life considerations for sodium percarbonate are relatively straightforward, as the compound decomposes naturally without leaving persistent pollutants. However, the packaging materials used for its distribution may have more significant environmental implications, depending on their recyclability or biodegradability.
Overall, a comprehensive LCA of sodium percarbonate helps identify hotspots in its life cycle where environmental impacts are most significant. This information can guide manufacturers and policymakers in implementing more sustainable practices and technologies to reduce the compound's ecological footprint throughout its life cycle.
The production of sodium percarbonate begins with the extraction of raw materials, primarily sodium carbonate and hydrogen peroxide. The environmental impacts at this stage include land use changes, energy consumption for mining and processing, and potential water pollution from extraction activities.
During the manufacturing phase, sodium percarbonate is produced through a reaction between sodium carbonate and hydrogen peroxide. This process requires significant energy input, primarily in the form of electricity and steam. The energy source used in production plays a crucial role in determining the overall carbon footprint of the manufacturing process.
Water consumption is another critical factor in the LCA of sodium percarbonate. The production process requires substantial amounts of water for cooling and washing, which may lead to local water stress in areas where water resources are scarce.
Chemical emissions during manufacturing, such as dust particles and volatile organic compounds (VOCs), contribute to air pollution and potential health impacts on workers and surrounding communities. Proper emission control systems are essential to mitigate these effects.
Transportation of raw materials to the production facility and finished products to consumers or industrial users adds to the overall environmental impact through fuel consumption and associated greenhouse gas emissions. The mode of transportation (e.g., truck, rail, or ship) and distances traveled significantly influence this aspect of the LCA.
In the use phase, sodium percarbonate's primary application is as a bleaching agent in laundry detergents and cleaning products. Its environmental impact during use is generally considered positive, as it breaks down into harmless substances (sodium carbonate, water, and oxygen) and does not produce harmful residues.
End-of-life considerations for sodium percarbonate are relatively straightforward, as the compound decomposes naturally without leaving persistent pollutants. However, the packaging materials used for its distribution may have more significant environmental implications, depending on their recyclability or biodegradability.
Overall, a comprehensive LCA of sodium percarbonate helps identify hotspots in its life cycle where environmental impacts are most significant. This information can guide manufacturers and policymakers in implementing more sustainable practices and technologies to reduce the compound's ecological footprint throughout its life cycle.
Regulatory Framework for Chemical Manufacturing
The regulatory framework for chemical manufacturing, particularly in the context of sodium percarbonate production, is a complex and evolving landscape designed to mitigate environmental impacts and ensure public safety. At the international level, the United Nations' Strategic Approach to International Chemicals Management (SAICM) provides a policy framework to foster the sound management of chemicals throughout their lifecycle. This initiative encourages countries to implement comprehensive chemical management systems and promotes information sharing on chemical hazards and risks.
In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating chemical manufacturing under the Toxic Substances Control Act (TSCA). The TSCA mandates that manufacturers conduct risk assessments and report potential environmental and health hazards associated with new chemicals before they can be produced or imported. For sodium percarbonate, this includes evaluating its potential impacts on water quality, air emissions, and waste management.
The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation is another significant framework affecting sodium percarbonate manufacturing. REACH requires companies to register chemical substances produced or imported in quantities over one tonne per year, providing detailed information on their properties, hazards, and safe use. This regulation has led to increased transparency and accountability in the chemical industry, including sodium percarbonate production.
Many countries have implemented their own versions of chemical control laws, often modeled after REACH or TSCA. For instance, China's Measures for Environmental Management of New Chemical Substances and South Korea's Act on Registration and Evaluation of Chemicals (K-REACH) both aim to regulate the introduction and use of new chemicals, including those used in or produced during sodium percarbonate manufacturing.
Specific to sodium percarbonate, regulations often focus on its classification as an oxidizing agent and its potential environmental impacts. Manufacturers must adhere to strict guidelines regarding storage, handling, and transportation to prevent accidental releases. Additionally, effluent discharge limits and air emission standards are typically in place to control the release of byproducts and waste from the manufacturing process.
As environmental concerns grow, regulatory frameworks are increasingly emphasizing sustainable production methods and circular economy principles. This trend is pushing sodium percarbonate manufacturers to adopt cleaner technologies, improve energy efficiency, and explore ways to reduce waste and recycle materials throughout the production cycle. Compliance with these evolving regulations requires ongoing investment in research and development, as well as regular updates to manufacturing processes and environmental management systems.
In the United States, the Environmental Protection Agency (EPA) plays a crucial role in regulating chemical manufacturing under the Toxic Substances Control Act (TSCA). The TSCA mandates that manufacturers conduct risk assessments and report potential environmental and health hazards associated with new chemicals before they can be produced or imported. For sodium percarbonate, this includes evaluating its potential impacts on water quality, air emissions, and waste management.
The European Union's REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation is another significant framework affecting sodium percarbonate manufacturing. REACH requires companies to register chemical substances produced or imported in quantities over one tonne per year, providing detailed information on their properties, hazards, and safe use. This regulation has led to increased transparency and accountability in the chemical industry, including sodium percarbonate production.
Many countries have implemented their own versions of chemical control laws, often modeled after REACH or TSCA. For instance, China's Measures for Environmental Management of New Chemical Substances and South Korea's Act on Registration and Evaluation of Chemicals (K-REACH) both aim to regulate the introduction and use of new chemicals, including those used in or produced during sodium percarbonate manufacturing.
Specific to sodium percarbonate, regulations often focus on its classification as an oxidizing agent and its potential environmental impacts. Manufacturers must adhere to strict guidelines regarding storage, handling, and transportation to prevent accidental releases. Additionally, effluent discharge limits and air emission standards are typically in place to control the release of byproducts and waste from the manufacturing process.
As environmental concerns grow, regulatory frameworks are increasingly emphasizing sustainable production methods and circular economy principles. This trend is pushing sodium percarbonate manufacturers to adopt cleaner technologies, improve energy efficiency, and explore ways to reduce waste and recycle materials throughout the production cycle. Compliance with these evolving regulations requires ongoing investment in research and development, as well as regular updates to manufacturing processes and environmental management systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!