Volumetric Additive Manufacturing For Personalized Medical Devices
SEP 12, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
VAM Technology Background and Objectives
Volumetric Additive Manufacturing (VAM) represents a paradigm shift in 3D printing technology, emerging from the convergence of photopolymerization techniques and advanced optical engineering. Unlike traditional layer-by-layer additive manufacturing methods, VAM enables the simultaneous solidification of an entire volume of photosensitive resin, dramatically reducing production time from hours to minutes while maintaining high resolution and structural integrity.
The evolution of VAM technology can be traced back to early experiments with stereolithography in the 1980s, followed by significant advancements in digital light processing and continuous liquid interface production in the 2000s. The breakthrough in volumetric printing occurred around 2017-2019, when researchers successfully demonstrated the feasibility of computed tomography-inspired approaches for creating complex 3D structures in a single exposure process.
Current VAM technologies primarily utilize computed axial lithography (CAL), holographic techniques, or tomographic reconstruction methods to project patterned light into photopolymer resins. These approaches have demonstrated the ability to produce intricate structures with feature sizes down to tens of micrometers, making them particularly suitable for medical device applications requiring precise geometries and biocompatibility.
The primary technical objective of VAM for personalized medical devices is to enable rapid, patient-specific manufacturing of implants, prosthetics, and anatomical models with complex internal structures that precisely match individual patient anatomy. This includes achieving biocompatibility with various tissue types, incorporating functional gradients of mechanical properties, and potentially integrating active components or drug delivery systems.
Secondary objectives include reducing production costs compared to traditional manufacturing methods, minimizing material waste, and developing streamlined workflows that integrate medical imaging data directly into the manufacturing process. The technology aims to bridge the gap between diagnostic imaging and therapeutic intervention by enabling same-day production of custom medical devices.
Long-term technical goals for VAM in medical applications include multi-material printing capabilities to mimic heterogeneous tissue properties, integration with biodegradable and bioactive materials, and development of in-situ printing techniques for direct application during surgical procedures. Researchers are also exploring methods to overcome current limitations in build volume, resolution trade-offs, and material constraints to expand the range of possible applications.
The convergence of VAM with advances in medical imaging, computational design, and biomaterials science presents unprecedented opportunities for personalized healthcare interventions, potentially transforming treatment approaches for conditions ranging from craniofacial reconstruction to orthopedic implants and tissue engineering scaffolds.
The evolution of VAM technology can be traced back to early experiments with stereolithography in the 1980s, followed by significant advancements in digital light processing and continuous liquid interface production in the 2000s. The breakthrough in volumetric printing occurred around 2017-2019, when researchers successfully demonstrated the feasibility of computed tomography-inspired approaches for creating complex 3D structures in a single exposure process.
Current VAM technologies primarily utilize computed axial lithography (CAL), holographic techniques, or tomographic reconstruction methods to project patterned light into photopolymer resins. These approaches have demonstrated the ability to produce intricate structures with feature sizes down to tens of micrometers, making them particularly suitable for medical device applications requiring precise geometries and biocompatibility.
The primary technical objective of VAM for personalized medical devices is to enable rapid, patient-specific manufacturing of implants, prosthetics, and anatomical models with complex internal structures that precisely match individual patient anatomy. This includes achieving biocompatibility with various tissue types, incorporating functional gradients of mechanical properties, and potentially integrating active components or drug delivery systems.
Secondary objectives include reducing production costs compared to traditional manufacturing methods, minimizing material waste, and developing streamlined workflows that integrate medical imaging data directly into the manufacturing process. The technology aims to bridge the gap between diagnostic imaging and therapeutic intervention by enabling same-day production of custom medical devices.
Long-term technical goals for VAM in medical applications include multi-material printing capabilities to mimic heterogeneous tissue properties, integration with biodegradable and bioactive materials, and development of in-situ printing techniques for direct application during surgical procedures. Researchers are also exploring methods to overcome current limitations in build volume, resolution trade-offs, and material constraints to expand the range of possible applications.
The convergence of VAM with advances in medical imaging, computational design, and biomaterials science presents unprecedented opportunities for personalized healthcare interventions, potentially transforming treatment approaches for conditions ranging from craniofacial reconstruction to orthopedic implants and tissue engineering scaffolds.
Market Analysis for Personalized Medical Devices
The global market for personalized medical devices is experiencing unprecedented growth, driven by advancements in Volumetric Additive Manufacturing (VAM) technologies. This market reached approximately $16.8 billion in 2022 and is projected to grow at a CAGR of 14.2% through 2030, potentially reaching $45.6 billion by the end of the forecast period.
The demand for personalized medical devices is primarily fueled by the increasing prevalence of chronic diseases and the aging global population. According to WHO data, chronic diseases account for 71% of all deaths globally, creating substantial demand for customized therapeutic solutions. Additionally, the global population aged 65 and above is expected to double by 2050, further accelerating market growth.
Orthopedic implants currently dominate the personalized medical device market, accounting for roughly 38% of the total market share. This segment includes patient-specific knee replacements, hip implants, and spinal devices. Dental applications follow closely at 27%, with hearing aids and prosthetics representing 18% and 12% respectively.
Regional analysis reveals North America as the current market leader with approximately 42% market share, followed by Europe (31%) and Asia-Pacific (21%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 16.8% annually, driven by improving healthcare infrastructure, increasing disposable income, and growing awareness about advanced medical technologies.
The reimbursement landscape for personalized medical devices remains complex but is gradually evolving. Currently, about 65% of personalized medical devices receive some form of insurance coverage in developed markets, though coverage policies vary significantly across regions and device categories. The trend toward value-based healthcare is expected to improve reimbursement prospects as evidence accumulates demonstrating superior clinical outcomes and cost-effectiveness of personalized solutions.
Consumer willingness to pay premium prices for personalized medical devices is increasing, with surveys indicating that 78% of patients would prefer customized solutions over standard options if given the choice. This preference is particularly strong among younger demographics and higher-income groups.
Key market restraints include high manufacturing costs, regulatory hurdles, and limited accessibility in developing regions. The average cost premium for personalized devices remains 30-40% higher than standard alternatives, creating affordability challenges. However, as VAM technologies mature and economies of scale improve, this cost differential is expected to narrow significantly over the next decade.
The demand for personalized medical devices is primarily fueled by the increasing prevalence of chronic diseases and the aging global population. According to WHO data, chronic diseases account for 71% of all deaths globally, creating substantial demand for customized therapeutic solutions. Additionally, the global population aged 65 and above is expected to double by 2050, further accelerating market growth.
Orthopedic implants currently dominate the personalized medical device market, accounting for roughly 38% of the total market share. This segment includes patient-specific knee replacements, hip implants, and spinal devices. Dental applications follow closely at 27%, with hearing aids and prosthetics representing 18% and 12% respectively.
Regional analysis reveals North America as the current market leader with approximately 42% market share, followed by Europe (31%) and Asia-Pacific (21%). However, the Asia-Pacific region is expected to witness the fastest growth rate of 16.8% annually, driven by improving healthcare infrastructure, increasing disposable income, and growing awareness about advanced medical technologies.
The reimbursement landscape for personalized medical devices remains complex but is gradually evolving. Currently, about 65% of personalized medical devices receive some form of insurance coverage in developed markets, though coverage policies vary significantly across regions and device categories. The trend toward value-based healthcare is expected to improve reimbursement prospects as evidence accumulates demonstrating superior clinical outcomes and cost-effectiveness of personalized solutions.
Consumer willingness to pay premium prices for personalized medical devices is increasing, with surveys indicating that 78% of patients would prefer customized solutions over standard options if given the choice. This preference is particularly strong among younger demographics and higher-income groups.
Key market restraints include high manufacturing costs, regulatory hurdles, and limited accessibility in developing regions. The average cost premium for personalized devices remains 30-40% higher than standard alternatives, creating affordability challenges. However, as VAM technologies mature and economies of scale improve, this cost differential is expected to narrow significantly over the next decade.
Current VAM Challenges in Medical Applications
Despite the promising potential of Volumetric Additive Manufacturing (VAM) for personalized medical devices, several significant challenges currently impede its widespread clinical adoption. Material biocompatibility remains a primary concern, as VAM resins must simultaneously satisfy optical properties for light-based curing while meeting stringent biocompatibility requirements for medical applications. The limited range of FDA-approved photopolymers specifically formulated for VAM significantly restricts the development of implantable or long-term contact medical devices.
Resolution constraints present another major hurdle. While VAM offers impressive fabrication speeds, achieving the microscale features often required for complex medical devices (such as microfluidic channels or cellular scaffolds with precise porosity) remains difficult. Current systems typically achieve resolutions of 50-100 μm, which falls short of the sub-10 μm precision needed for certain specialized medical applications.
Post-processing requirements further complicate the manufacturing workflow. Medical devices produced via VAM frequently require extensive cleaning to remove uncured resin, which can be particularly challenging for complex internal geometries. Additionally, sterilization processes may compromise the mechanical or chemical properties of VAM-produced components, necessitating careful material selection and validation protocols.
Regulatory pathways represent a substantial barrier to commercialization. The novelty of VAM technology means that standardized testing protocols and regulatory frameworks specific to this manufacturing method are still evolving. Medical device manufacturers must navigate complex approval processes with limited precedent, often requiring extensive validation studies to demonstrate equivalence to traditionally manufactured devices.
Scalability and reproducibility challenges also persist. While VAM excels at producing complex geometries, ensuring consistent mechanical properties throughout larger structures remains problematic. Variations in light penetration, resin viscosity, and curing kinetics can lead to heterogeneous material properties, potentially compromising device performance and reliability in clinical settings.
Integration with medical imaging workflows presents additional technical hurdles. Converting patient-specific medical imaging data (from CT or MRI) into VAM-compatible models requires sophisticated software tools and expertise. Current solutions often involve multiple manual steps, increasing the time and cost associated with personalized device production.
Cost considerations further limit adoption, as specialized VAM equipment, biocompatible resins, and the expertise required to operate these systems remain expensive compared to conventional manufacturing methods. Until economies of scale reduce these costs, VAM may remain limited to high-value, specialized medical applications rather than routine clinical use.
Resolution constraints present another major hurdle. While VAM offers impressive fabrication speeds, achieving the microscale features often required for complex medical devices (such as microfluidic channels or cellular scaffolds with precise porosity) remains difficult. Current systems typically achieve resolutions of 50-100 μm, which falls short of the sub-10 μm precision needed for certain specialized medical applications.
Post-processing requirements further complicate the manufacturing workflow. Medical devices produced via VAM frequently require extensive cleaning to remove uncured resin, which can be particularly challenging for complex internal geometries. Additionally, sterilization processes may compromise the mechanical or chemical properties of VAM-produced components, necessitating careful material selection and validation protocols.
Regulatory pathways represent a substantial barrier to commercialization. The novelty of VAM technology means that standardized testing protocols and regulatory frameworks specific to this manufacturing method are still evolving. Medical device manufacturers must navigate complex approval processes with limited precedent, often requiring extensive validation studies to demonstrate equivalence to traditionally manufactured devices.
Scalability and reproducibility challenges also persist. While VAM excels at producing complex geometries, ensuring consistent mechanical properties throughout larger structures remains problematic. Variations in light penetration, resin viscosity, and curing kinetics can lead to heterogeneous material properties, potentially compromising device performance and reliability in clinical settings.
Integration with medical imaging workflows presents additional technical hurdles. Converting patient-specific medical imaging data (from CT or MRI) into VAM-compatible models requires sophisticated software tools and expertise. Current solutions often involve multiple manual steps, increasing the time and cost associated with personalized device production.
Cost considerations further limit adoption, as specialized VAM equipment, biocompatible resins, and the expertise required to operate these systems remain expensive compared to conventional manufacturing methods. Until economies of scale reduce these costs, VAM may remain limited to high-value, specialized medical applications rather than routine clinical use.
Current VAM Solutions for Medical Device Fabrication
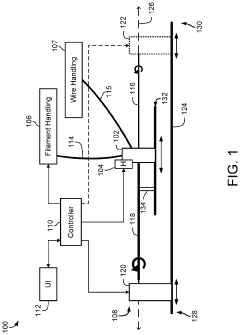
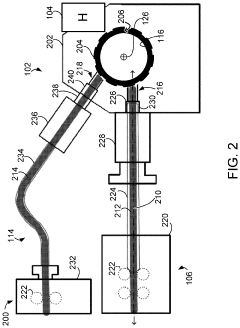
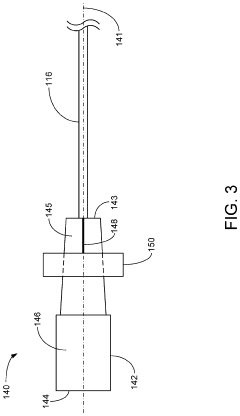
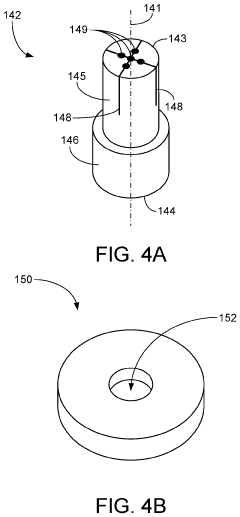
01 Volumetric additive manufacturing techniques and processes
Volumetric additive manufacturing involves creating three-dimensional objects by solidifying material throughout a volume simultaneously, rather than layer by layer. This approach enables faster production times and can create complex geometries without the need for support structures. Various techniques include computed axial lithography, holographic stereolithography, and tomographic volumetric printing, which use controlled light patterns to selectively cure photosensitive resins in a volumetric workspace.- Volumetric additive manufacturing techniques: Volumetric additive manufacturing (VAM) involves creating 3D objects by solidifying material throughout a volume simultaneously, rather than layer by layer. These techniques include computed axial lithography (CAL), tomographic volumetric additive manufacturing, and holographic approaches that use projected light patterns to cure photosensitive resins. This approach enables faster production times compared to traditional layer-by-layer methods and can create complex geometries without support structures.
- Materials for volumetric printing: Specialized materials are essential for successful volumetric additive manufacturing. These include photosensitive resins with specific curing properties, dual-initiator systems that enable precise spatial control of polymerization, and materials with tuned optical properties. Advanced formulations may incorporate nanoparticles, functional additives, or biomaterials to enhance mechanical properties or add functionality to the printed objects while maintaining compatibility with volumetric printing processes.
- Light projection and control systems: Sophisticated light projection and control systems are critical components of volumetric additive manufacturing. These systems utilize digital light processing (DLP), spatial light modulators, or holographic techniques to project specific patterns of light into photosensitive materials. Advanced algorithms control the intensity, wavelength, and spatial distribution of light to achieve precise curing throughout the volume, often employing computed tomography principles to determine the optimal light patterns for creating complex 3D structures.
- Process optimization and control: Process optimization in volumetric additive manufacturing involves sophisticated control algorithms, real-time monitoring systems, and feedback mechanisms to ensure printing accuracy and quality. This includes methods for compensating for optical distortions, managing heat distribution, controlling reaction kinetics, and optimizing exposure parameters. Advanced systems may incorporate machine learning approaches to predict and adjust for material behavior during the printing process, resulting in improved dimensional accuracy and surface finish.
- Applications and specialized implementations: Volumetric additive manufacturing has diverse applications across multiple industries. In the medical field, it enables the rapid production of patient-specific implants, tissue engineering scaffolds, and anatomical models. In electronics, it facilitates the creation of embedded circuits and sensors. Industrial applications include the production of complex components with internal features that would be difficult to manufacture using traditional methods. Specialized implementations may combine volumetric techniques with other manufacturing processes for hybrid approaches that leverage the advantages of multiple technologies.
02 Materials for volumetric additive manufacturing
Specialized materials are essential for successful volumetric additive manufacturing. These include photosensitive resins with specific curing properties, nanocomposites, and hybrid materials that can be precisely solidified using light or other energy sources. The development of these materials focuses on achieving rapid curing times, high resolution, and desired mechanical properties in the final printed objects. Advanced formulations may incorporate functional additives to enhance specific properties such as strength, flexibility, or biocompatibility.Expand Specific Solutions03 Light control systems for volumetric printing
Sophisticated light control systems are crucial for volumetric additive manufacturing. These systems include spatial light modulators, digital light processing units, and holographic projectors that can precisely direct light patterns into the printing volume. Advanced algorithms control the intensity, wavelength, and spatial distribution of light to achieve accurate solidification of the desired geometry. Multi-beam approaches and synchronized projection systems enable higher resolution and faster printing speeds in volumetric manufacturing processes.Expand Specific Solutions04 Integration with computational methods and AI
Volumetric additive manufacturing relies heavily on computational methods and artificial intelligence for process optimization. Advanced algorithms calculate optimal light dose distributions, predict material behavior during curing, and compensate for optical distortions. Machine learning approaches improve print quality by analyzing and adapting to material response patterns. Computational modeling helps in designing complex internal structures and optimizing manufacturing parameters for specific applications, enhancing both efficiency and final product quality.Expand Specific Solutions05 Applications and industry-specific implementations
Volumetric additive manufacturing has diverse applications across multiple industries. In medical fields, it enables rapid production of customized implants, prosthetics, and tissue engineering scaffolds. For industrial applications, it allows for the creation of complex components with internal features that would be difficult to produce using traditional methods. The technology is also being adapted for specific use cases in aerospace, automotive, and consumer products, where complex geometries and rapid production are valuable. Recent developments focus on scaling the technology for larger objects and higher throughput manufacturing.Expand Specific Solutions
Key Industry Players in Medical VAM Technology
Volumetric Additive Manufacturing (VAM) for personalized medical devices is emerging as a transformative technology in the early growth stage of its industry lifecycle. The market is expanding rapidly, projected to reach significant scale as healthcare providers increasingly adopt customized solutions. Technologically, VAM is advancing from experimental to clinical application phases, with varying maturity levels across key players. Academic institutions (MIT, Cornell, EPFL) are driving fundamental research, while specialized companies like Readily3D and Axtra3D are commercializing innovative printing technologies. Established medical device manufacturers (Align Technology, Boston Scientific) are integrating VAM into their production workflows, and healthcare providers (Tan Tock Seng Hospital, Les Hôpitaux Universitaires de Genève) are implementing these solutions in clinical settings. The competitive landscape reflects a dynamic ecosystem of research institutions, startups, and established corporations collaborating to advance personalized medical manufacturing.
Align Technology, Inc.
Technical Solution: Align Technology has developed an advanced volumetric additive manufacturing system specifically for the production of personalized clear dental aligners and orthodontic devices. Their proprietary technology combines digital scanning, 3D modeling, and precision manufacturing to create custom-fit devices for each patient. The process begins with intraoral scanning to create a digital model of the patient's dentition. Align's software then generates a treatment plan with a series of intermediate steps to gradually move teeth to their desired positions. Their volumetric manufacturing system uses a combination of photopolymerization techniques to rapidly produce these aligners with high precision. The company has developed specialized thermoformable materials that provide the right balance of rigidity and flexibility for effective tooth movement while maintaining patient comfort. Align's manufacturing facilities utilize automated production lines with integrated quality control systems that verify dimensional accuracy and material properties of each device. Their technology enables the production of thousands of unique patient-specific devices daily with consistent quality and performance characteristics.
Strengths: Highly automated end-to-end digital workflow from patient scan to finished product; proprietary materials specifically engineered for dental applications; exceptional dimensional accuracy (within 50 microns) critical for effective treatment outcomes. Weaknesses: Technology currently optimized primarily for dental applications rather than broader medical device categories; relatively high cost per device compared to traditional orthodontic approaches; limited material options compared to some other additive manufacturing platforms.
Lawrence Livermore National Security LLC
Technical Solution: Lawrence Livermore National Security (LLNS) has developed a groundbreaking volumetric additive manufacturing technology called Computed Axial Lithography (CAL) for creating personalized medical devices. This approach projects synchronized patterns of light into a rotating volume of photosensitive resin, allowing the entire object to be formed simultaneously rather than layer-by-layer. LLNS's technology can produce complex structures with features as small as 100 microns in a matter of minutes, regardless of object complexity. Their system incorporates advanced algorithms that calculate optimal light patterns to achieve precise control over material properties throughout the printed object. LLNS has developed specialized formulations of biocompatible resins that can be tailored for specific medical applications, including patient-specific implants, drug delivery devices, and anatomical models for surgical planning. Their technology enables the creation of structures with controlled porosity and mechanical gradients that can better mimic natural tissues. LLNS has also pioneered methods for embedding functional components, such as sensors or drug reservoirs, within printed medical devices during the manufacturing process, enabling advanced functionality in personalized healthcare products.
Strengths: Exceptional speed (10-100x faster than conventional methods); ability to create objects with complex internal geometries impossible with traditional manufacturing; excellent surface finish without visible layer lines; minimal post-processing requirements. Weaknesses: Currently limited to photopolymer materials; challenges in scaling to very large build volumes; higher computational requirements for generating projection patterns compared to conventional slicing approaches.
Critical Patents and Research in Medical VAM
Additive manufacturing for medical devices
PatentActiveUS20240009925A1
Innovation
- The use of additive manufacturing systems that allow for the customization of medical devices by printing a jacket over subassemblies on a substrate or mandrel, enabling the inclusion of internal components like electrodes and coils, while maintaining flexibility through the use of a liner between the printed jacket and the lead or wire.
Three-dimensional printing of patient-specific implants
PatentActiveIN2768CHE2015A
Innovation
- A maltodextrin-based liquid binder formulation for 3D inkjet printing, compatible with metal and ceramic powders, is used to create anatomically designed scaffolds with macropores and micropores, enabling vascularization and improved mechanical properties, along with a method involving post-printing treatments like illumination and sintering in an inert atmosphere.
Regulatory Framework for 3D-Printed Medical Devices
The regulatory landscape for 3D-printed medical devices represents a complex and evolving framework that manufacturers must navigate when developing volumetric additive manufacturing solutions for personalized healthcare applications. The FDA has established a classification system for medical devices based on risk levels, with Class I devices requiring minimal regulatory control, while Class II and III devices face progressively stringent requirements including premarket notification (510(k)) or premarket approval (PMA).
For volumetrically manufactured personalized medical devices, the FDA's "Technical Considerations for Additive Manufactured Medical Devices" guidance document provides essential direction regarding design, manufacturing, and testing considerations. This framework emphasizes the importance of process validation, quality control systems, and material characterization specific to additive manufacturing technologies.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have introduced more rigorous requirements for medical device manufacturers, including those utilizing volumetric additive manufacturing. These regulations mandate comprehensive technical documentation, clinical evaluation, and post-market surveillance systems that are particularly relevant for personalized medical devices.
Quality management systems compliant with ISO 13485 standards are fundamental regulatory requirements across major markets. For volumetric additive manufacturing, these systems must address unique considerations such as digital design control, build environment parameters, and post-processing validation protocols that ensure consistent quality in personalized applications.
Regulatory pathways for custom-made devices offer potential routes for personalized medical solutions. However, the scale at which volumetric manufacturing can produce "personalized" devices challenges traditional definitions of "custom-made" in regulatory frameworks, creating a gray area that manufacturers must carefully address.
Data privacy regulations, including HIPAA in the United States and GDPR in Europe, introduce additional compliance requirements when patient-specific data is used to design personalized medical devices. Manufacturers must implement robust data protection measures throughout the digital workflow from patient imaging to final device production.
International harmonization efforts, such as the Medical Device Single Audit Program (MDSAP) and the International Medical Device Regulators Forum (IMDRF), are working to standardize regulatory approaches to emerging technologies like volumetric additive manufacturing, though significant regional differences persist that manufacturers must address in their regulatory strategies.
For volumetrically manufactured personalized medical devices, the FDA's "Technical Considerations for Additive Manufactured Medical Devices" guidance document provides essential direction regarding design, manufacturing, and testing considerations. This framework emphasizes the importance of process validation, quality control systems, and material characterization specific to additive manufacturing technologies.
The European Union's Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) have introduced more rigorous requirements for medical device manufacturers, including those utilizing volumetric additive manufacturing. These regulations mandate comprehensive technical documentation, clinical evaluation, and post-market surveillance systems that are particularly relevant for personalized medical devices.
Quality management systems compliant with ISO 13485 standards are fundamental regulatory requirements across major markets. For volumetric additive manufacturing, these systems must address unique considerations such as digital design control, build environment parameters, and post-processing validation protocols that ensure consistent quality in personalized applications.
Regulatory pathways for custom-made devices offer potential routes for personalized medical solutions. However, the scale at which volumetric manufacturing can produce "personalized" devices challenges traditional definitions of "custom-made" in regulatory frameworks, creating a gray area that manufacturers must carefully address.
Data privacy regulations, including HIPAA in the United States and GDPR in Europe, introduce additional compliance requirements when patient-specific data is used to design personalized medical devices. Manufacturers must implement robust data protection measures throughout the digital workflow from patient imaging to final device production.
International harmonization efforts, such as the Medical Device Single Audit Program (MDSAP) and the International Medical Device Regulators Forum (IMDRF), are working to standardize regulatory approaches to emerging technologies like volumetric additive manufacturing, though significant regional differences persist that manufacturers must address in their regulatory strategies.
Biocompatibility and Material Science Considerations
Biocompatibility represents a critical consideration in volumetric additive manufacturing (VAM) for personalized medical devices. The materials used must not elicit adverse biological responses when in contact with human tissues and fluids. ISO 10993 standards provide comprehensive guidelines for evaluating biocompatibility, including cytotoxicity, sensitization, irritation, and systemic toxicity assessments. For implantable devices manufactured through VAM, long-term biocompatibility testing becomes essential to ensure safety throughout the device's intended lifespan.
Material selection for VAM in medical applications requires balancing mechanical properties, printability, and biological performance. Photopolymerizable resins commonly used in VAM must be carefully formulated to minimize potential toxicity from residual monomers, photoinitiators, and other additives. Recent advances have led to the development of medical-grade resins specifically designed for biocompatible applications, incorporating materials such as modified polyethylene glycol diacrylate (PEGDA) and gelatin methacrylate (GelMA).
Surface characteristics of VAM-produced medical devices significantly influence biocompatibility outcomes. Surface roughness, wettability, and chemical composition can affect protein adsorption, cell adhesion, and subsequent biological responses. Post-processing techniques such as surface treatments and sterilization methods must be optimized to enhance biocompatibility without compromising the structural integrity or dimensional accuracy of the personalized devices.
Biodegradable materials present unique opportunities and challenges in VAM for temporary medical implants. Polycaprolactone (PCL), poly(lactic-co-glycolic acid) (PLGA), and various hydrogels have shown promise for applications requiring controlled degradation profiles. The degradation kinetics must be precisely engineered to match the healing timeline of the specific medical application, while ensuring that degradation byproducts remain non-toxic and can be metabolized or excreted safely.
Composite materials combining polymeric matrices with bioactive fillers represent an emerging frontier in VAM for medical devices. Incorporating hydroxyapatite, bioactive glass, or growth factors can enhance tissue integration and healing responses. However, these additions complicate the photopolymerization process central to VAM, requiring careful optimization of light penetration depth, curing kinetics, and resulting mechanical properties.
Regulatory considerations for materials used in VAM-produced medical devices remain stringent and complex. Manufacturers must demonstrate both biocompatibility and manufacturing consistency through extensive documentation and testing. The FDA's regulatory pathway for 3D-printed medical devices continues to evolve, with particular emphasis on material characterization, process validation, and quality control measures specific to additive manufacturing technologies.
Material selection for VAM in medical applications requires balancing mechanical properties, printability, and biological performance. Photopolymerizable resins commonly used in VAM must be carefully formulated to minimize potential toxicity from residual monomers, photoinitiators, and other additives. Recent advances have led to the development of medical-grade resins specifically designed for biocompatible applications, incorporating materials such as modified polyethylene glycol diacrylate (PEGDA) and gelatin methacrylate (GelMA).
Surface characteristics of VAM-produced medical devices significantly influence biocompatibility outcomes. Surface roughness, wettability, and chemical composition can affect protein adsorption, cell adhesion, and subsequent biological responses. Post-processing techniques such as surface treatments and sterilization methods must be optimized to enhance biocompatibility without compromising the structural integrity or dimensional accuracy of the personalized devices.
Biodegradable materials present unique opportunities and challenges in VAM for temporary medical implants. Polycaprolactone (PCL), poly(lactic-co-glycolic acid) (PLGA), and various hydrogels have shown promise for applications requiring controlled degradation profiles. The degradation kinetics must be precisely engineered to match the healing timeline of the specific medical application, while ensuring that degradation byproducts remain non-toxic and can be metabolized or excreted safely.
Composite materials combining polymeric matrices with bioactive fillers represent an emerging frontier in VAM for medical devices. Incorporating hydroxyapatite, bioactive glass, or growth factors can enhance tissue integration and healing responses. However, these additions complicate the photopolymerization process central to VAM, requiring careful optimization of light penetration depth, curing kinetics, and resulting mechanical properties.
Regulatory considerations for materials used in VAM-produced medical devices remain stringent and complex. Manufacturers must demonstrate both biocompatibility and manufacturing consistency through extensive documentation and testing. The FDA's regulatory pathway for 3D-printed medical devices continues to evolve, with particular emphasis on material characterization, process validation, and quality control measures specific to additive manufacturing technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!