Application of lithium orotate in chronic pain management
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Orotate Background and Objectives
Lithium orotate, a compound consisting of lithium and orotic acid, has gained attention in recent years for its potential application in chronic pain management. The background of this compound traces back to the broader use of lithium in psychiatry, which began in the mid-20th century. While lithium carbonate has been the primary form used in medical treatments, lithium orotate has emerged as an alternative due to its unique properties and potential benefits.
The evolution of lithium orotate research has been driven by the need for more effective and safer treatments for chronic pain conditions. Traditional pain management approaches often come with significant side effects and risks of dependency, prompting researchers to explore novel compounds. Lithium orotate's development represents a convergence of psychiatric and pain management research, as scientists investigate the compound's ability to modulate neurotransmitter systems and influence pain perception pathways.
The primary objective of exploring lithium orotate in chronic pain management is to harness its potential neuroprotective and anti-inflammatory properties. Researchers aim to determine whether lithium orotate can effectively reduce pain intensity, improve quality of life, and minimize the need for conventional pain medications in patients suffering from various chronic pain conditions. This includes investigating its efficacy in neuropathic pain, fibromyalgia, and other persistent pain syndromes.
Another key goal is to establish the safety profile of lithium orotate when used for pain management. This involves assessing its bioavailability, optimal dosing regimens, and potential interactions with other medications commonly used in pain treatment. Researchers are particularly interested in comparing the side effect profile of lithium orotate to that of traditional lithium formulations and other pain management drugs.
The technological trend in this field is moving towards personalized medicine approaches. Scientists are exploring how genetic factors and individual patient characteristics may influence the efficacy of lithium orotate in pain management. This includes investigating biomarkers that could predict treatment response and developing targeted delivery systems to enhance the compound's effectiveness while minimizing systemic exposure.
As research progresses, there is a growing focus on understanding the molecular mechanisms by which lithium orotate may modulate pain pathways. This involves studying its effects on neurotransmitter systems, ion channels, and cellular signaling cascades involved in pain processing. By elucidating these mechanisms, researchers hope to identify new targets for pain management and potentially develop more refined lithium-based compounds for chronic pain treatment.
The evolution of lithium orotate research has been driven by the need for more effective and safer treatments for chronic pain conditions. Traditional pain management approaches often come with significant side effects and risks of dependency, prompting researchers to explore novel compounds. Lithium orotate's development represents a convergence of psychiatric and pain management research, as scientists investigate the compound's ability to modulate neurotransmitter systems and influence pain perception pathways.
The primary objective of exploring lithium orotate in chronic pain management is to harness its potential neuroprotective and anti-inflammatory properties. Researchers aim to determine whether lithium orotate can effectively reduce pain intensity, improve quality of life, and minimize the need for conventional pain medications in patients suffering from various chronic pain conditions. This includes investigating its efficacy in neuropathic pain, fibromyalgia, and other persistent pain syndromes.
Another key goal is to establish the safety profile of lithium orotate when used for pain management. This involves assessing its bioavailability, optimal dosing regimens, and potential interactions with other medications commonly used in pain treatment. Researchers are particularly interested in comparing the side effect profile of lithium orotate to that of traditional lithium formulations and other pain management drugs.
The technological trend in this field is moving towards personalized medicine approaches. Scientists are exploring how genetic factors and individual patient characteristics may influence the efficacy of lithium orotate in pain management. This includes investigating biomarkers that could predict treatment response and developing targeted delivery systems to enhance the compound's effectiveness while minimizing systemic exposure.
As research progresses, there is a growing focus on understanding the molecular mechanisms by which lithium orotate may modulate pain pathways. This involves studying its effects on neurotransmitter systems, ion channels, and cellular signaling cascades involved in pain processing. By elucidating these mechanisms, researchers hope to identify new targets for pain management and potentially develop more refined lithium-based compounds for chronic pain treatment.
Market Analysis for Chronic Pain Management
The chronic pain management market has been experiencing significant growth due to the increasing prevalence of chronic pain conditions worldwide. Chronic pain affects millions of individuals globally, with estimates suggesting that approximately 20% of adults suffer from chronic pain. This widespread issue has created a substantial demand for effective pain management solutions, driving market expansion and innovation in treatment options.
The market for chronic pain management encompasses a wide range of products and services, including pharmaceuticals, medical devices, and alternative therapies. Traditional pain medications, such as opioids and nonsteroidal anti-inflammatory drugs (NSAIDs), have long dominated the market. However, concerns over opioid addiction and the side effects of long-term NSAID use have led to a growing interest in alternative treatment options.
In recent years, there has been a shift towards more holistic and multidisciplinary approaches to chronic pain management. This trend has opened up opportunities for novel therapies and interventions, including nutraceuticals and dietary supplements. Lithium orotate, a mineral supplement, has gained attention as a potential alternative for managing chronic pain conditions.
The market for lithium orotate in chronic pain management is still in its early stages but shows promise for growth. As patients and healthcare providers seek safer and more effective alternatives to traditional pain medications, the demand for innovative solutions like lithium orotate is expected to increase. This presents an opportunity for companies to develop and market lithium orotate-based products specifically tailored for chronic pain management.
Several factors are driving the potential growth of lithium orotate in the chronic pain management market. First, there is a growing body of research suggesting the potential benefits of lithium in managing various types of pain, including neuropathic pain and fibromyalgia. Second, the increasing focus on personalized medicine and targeted therapies is creating a favorable environment for niche products like lithium orotate. Third, the rising awareness of the importance of mental health in pain management aligns well with lithium's known mood-stabilizing properties.
However, challenges exist in the market adoption of lithium orotate for chronic pain management. These include the need for more extensive clinical trials to establish efficacy and safety, regulatory hurdles in different countries, and competition from established pain management therapies. Additionally, educating healthcare providers and patients about the potential benefits and proper use of lithium orotate will be crucial for market penetration.
Despite these challenges, the market potential for lithium orotate in chronic pain management remains promising. As research continues to evolve and more evidence emerges supporting its efficacy, lithium orotate could carve out a significant niche in the broader chronic pain management market. Companies investing in this area may find opportunities to differentiate themselves and address unmet needs in pain management, particularly for patients seeking alternatives to traditional pharmacological interventions.
The market for chronic pain management encompasses a wide range of products and services, including pharmaceuticals, medical devices, and alternative therapies. Traditional pain medications, such as opioids and nonsteroidal anti-inflammatory drugs (NSAIDs), have long dominated the market. However, concerns over opioid addiction and the side effects of long-term NSAID use have led to a growing interest in alternative treatment options.
In recent years, there has been a shift towards more holistic and multidisciplinary approaches to chronic pain management. This trend has opened up opportunities for novel therapies and interventions, including nutraceuticals and dietary supplements. Lithium orotate, a mineral supplement, has gained attention as a potential alternative for managing chronic pain conditions.
The market for lithium orotate in chronic pain management is still in its early stages but shows promise for growth. As patients and healthcare providers seek safer and more effective alternatives to traditional pain medications, the demand for innovative solutions like lithium orotate is expected to increase. This presents an opportunity for companies to develop and market lithium orotate-based products specifically tailored for chronic pain management.
Several factors are driving the potential growth of lithium orotate in the chronic pain management market. First, there is a growing body of research suggesting the potential benefits of lithium in managing various types of pain, including neuropathic pain and fibromyalgia. Second, the increasing focus on personalized medicine and targeted therapies is creating a favorable environment for niche products like lithium orotate. Third, the rising awareness of the importance of mental health in pain management aligns well with lithium's known mood-stabilizing properties.
However, challenges exist in the market adoption of lithium orotate for chronic pain management. These include the need for more extensive clinical trials to establish efficacy and safety, regulatory hurdles in different countries, and competition from established pain management therapies. Additionally, educating healthcare providers and patients about the potential benefits and proper use of lithium orotate will be crucial for market penetration.
Despite these challenges, the market potential for lithium orotate in chronic pain management remains promising. As research continues to evolve and more evidence emerges supporting its efficacy, lithium orotate could carve out a significant niche in the broader chronic pain management market. Companies investing in this area may find opportunities to differentiate themselves and address unmet needs in pain management, particularly for patients seeking alternatives to traditional pharmacological interventions.
Current Status and Challenges in Lithium Orotate Research
The current status of lithium orotate research in chronic pain management is characterized by a mix of promising findings and significant challenges. While lithium has long been used in psychiatry, its application in pain management, particularly in the form of lithium orotate, is a relatively new area of exploration.
Recent studies have shown potential benefits of lithium orotate in managing chronic pain conditions. Researchers have observed its ability to modulate neurotransmitter systems and reduce inflammation, which are key factors in chronic pain. However, the exact mechanisms of action in pain management are not fully understood, presenting a major challenge for researchers.
One of the primary obstacles in lithium orotate research is the limited number of large-scale, randomized controlled trials. Most existing studies are small in scale or observational, which limits the strength of evidence. This scarcity of robust clinical data hinders the widespread acceptance of lithium orotate as a pain management tool in mainstream medical practice.
Another significant challenge is the regulatory status of lithium orotate. Unlike lithium carbonate, which is FDA-approved for bipolar disorder, lithium orotate is often classified as a dietary supplement. This classification complicates research efforts and clinical applications, as it is subject to less stringent regulations and oversight.
The optimal dosing of lithium orotate for pain management remains unclear. While lower doses are typically used compared to psychiatric applications, determining the most effective and safe dosage for various chronic pain conditions is an ongoing challenge. This is further complicated by individual variations in response and potential interactions with other medications.
Safety concerns also pose a challenge in lithium orotate research. Although it is generally considered to have a better safety profile than other lithium formulations, long-term effects and potential risks, especially in vulnerable populations, are not fully established. This necessitates careful monitoring and further research to ensure its safe use in chronic pain management.
The multifaceted nature of chronic pain adds another layer of complexity to lithium orotate research. Different types of chronic pain may respond differently to lithium orotate, and identifying which specific pain conditions are most responsive to this treatment is an ongoing challenge.
Despite these challenges, the field is advancing. Researchers are exploring innovative approaches, including combination therapies and personalized treatment protocols. There is also growing interest in understanding the potential neuroprotective effects of lithium orotate, which could have implications beyond pain management.
Recent studies have shown potential benefits of lithium orotate in managing chronic pain conditions. Researchers have observed its ability to modulate neurotransmitter systems and reduce inflammation, which are key factors in chronic pain. However, the exact mechanisms of action in pain management are not fully understood, presenting a major challenge for researchers.
One of the primary obstacles in lithium orotate research is the limited number of large-scale, randomized controlled trials. Most existing studies are small in scale or observational, which limits the strength of evidence. This scarcity of robust clinical data hinders the widespread acceptance of lithium orotate as a pain management tool in mainstream medical practice.
Another significant challenge is the regulatory status of lithium orotate. Unlike lithium carbonate, which is FDA-approved for bipolar disorder, lithium orotate is often classified as a dietary supplement. This classification complicates research efforts and clinical applications, as it is subject to less stringent regulations and oversight.
The optimal dosing of lithium orotate for pain management remains unclear. While lower doses are typically used compared to psychiatric applications, determining the most effective and safe dosage for various chronic pain conditions is an ongoing challenge. This is further complicated by individual variations in response and potential interactions with other medications.
Safety concerns also pose a challenge in lithium orotate research. Although it is generally considered to have a better safety profile than other lithium formulations, long-term effects and potential risks, especially in vulnerable populations, are not fully established. This necessitates careful monitoring and further research to ensure its safe use in chronic pain management.
The multifaceted nature of chronic pain adds another layer of complexity to lithium orotate research. Different types of chronic pain may respond differently to lithium orotate, and identifying which specific pain conditions are most responsive to this treatment is an ongoing challenge.
Despite these challenges, the field is advancing. Researchers are exploring innovative approaches, including combination therapies and personalized treatment protocols. There is also growing interest in understanding the potential neuroprotective effects of lithium orotate, which could have implications beyond pain management.
Existing Lithium Orotate Treatment Protocols
01 Use of lithium orotate for pain management
Lithium orotate is being explored as a potential treatment for various types of pain. Its unique properties may help in reducing inflammation and modulating neurotransmitter activity, which could contribute to pain relief. This compound is being investigated for its efficacy in managing chronic pain conditions and improving overall pain management strategies.- Use of lithium orotate for pain management: Lithium orotate is being explored as a potential treatment for various types of pain. Its unique properties may help in reducing inflammation and modulating pain signals in the nervous system, offering a novel approach to pain management.
- Combination therapy with lithium orotate: Lithium orotate is being studied in combination with other pain management techniques or medications to enhance overall efficacy. This approach may provide synergistic effects, potentially leading to improved pain relief and reduced side effects compared to traditional treatments alone.
- Targeted delivery systems for lithium orotate: Development of novel delivery systems for lithium orotate to improve its efficacy in pain management. These may include transdermal patches, controlled-release formulations, or targeted drug delivery methods to enhance the compound's bioavailability and reduce potential side effects.
- Lithium orotate in neurological pain management: Investigation of lithium orotate's potential in managing neurological pain conditions, such as neuropathic pain or migraine headaches. Its neuroprotective properties and ability to modulate neurotransmitter systems may offer benefits in these specific pain disorders.
- Monitoring and personalization of lithium orotate therapy: Development of methods for monitoring lithium orotate levels and personalizing treatment regimens for individual patients. This may involve the use of biomarkers, genetic testing, or advanced imaging techniques to optimize dosing and improve treatment outcomes in pain management.
02 Combination therapy with lithium orotate for enhanced pain relief
Research is being conducted on combining lithium orotate with other pain management techniques or medications to achieve better pain relief outcomes. This approach may involve integrating lithium orotate into multimodal pain management strategies, potentially leading to more effective and comprehensive pain treatment protocols.Expand Specific Solutions03 Lithium orotate in medical devices for pain management
Innovative medical devices incorporating lithium orotate are being developed for pain management applications. These devices may include implantable systems, transdermal delivery mechanisms, or other novel approaches to administer lithium orotate for pain relief, potentially offering new options for patients with chronic pain conditions.Expand Specific Solutions04 Personalized pain management using lithium orotate
Advancements in personalized medicine are being applied to pain management strategies involving lithium orotate. This approach aims to tailor the use of lithium orotate based on individual patient characteristics, genetic profiles, and specific pain conditions, potentially improving treatment efficacy and reducing side effects.Expand Specific Solutions05 Monitoring and optimizing lithium orotate therapy for pain
Systems and methods are being developed to monitor and optimize the use of lithium orotate in pain management. These may include advanced diagnostic tools, real-time monitoring devices, and data analysis techniques to assess treatment efficacy, adjust dosages, and manage potential side effects, ensuring safe and effective use of lithium orotate for pain relief.Expand Specific Solutions
Key Players in Lithium Orotate Development
The application of lithium orotate in chronic pain management is in an early developmental stage, with a relatively small market size but growing interest. The technology's maturity is still evolving, with several key players exploring its potential. Companies like Grünenthal GmbH, Purdue Pharma LP, and Vertex Pharmaceuticals, Inc. are likely at the forefront of research in this area, given their expertise in pain management and drug development. While not yet mainstream, the use of lithium orotate for chronic pain shows promise, with ongoing studies and clinical trials expected to further elucidate its efficacy and safety profile in the coming years.
Grünenthal GmbH
Technical Solution: Grünenthal has developed a novel approach to chronic pain management using lithium orotate. Their research focuses on the neuroprotective and anti-inflammatory properties of lithium orotate, which may help reduce chronic pain by modulating neurotransmitter systems and reducing neuroinflammation[1]. The company has conducted preclinical studies demonstrating that low-dose lithium orotate can effectively cross the blood-brain barrier and accumulate in neural tissues, potentially offering a more targeted approach to pain management compared to traditional lithium carbonate[3]. Grünenthal is currently exploring the use of lithium orotate in combination with other analgesics to create synergistic effects and reduce the required dosages of opioids in chronic pain treatment[5].
Strengths: Innovative approach targeting neuroinflammation, potential for reduced side effects compared to traditional lithium treatments. Weaknesses: Limited clinical data on long-term efficacy and safety in chronic pain management.
Nicox SA
Technical Solution: Nicox SA has developed a unique approach to chronic pain management by combining lithium orotate with nitric oxide-donating compounds. Their proprietary technology, known as NONO-lithium, aims to enhance the analgesic and anti-inflammatory effects of lithium orotate while leveraging the vasodilatory properties of nitric oxide[2]. Preclinical studies have shown that this combination may provide superior pain relief in models of neuropathic pain compared to lithium orotate alone[4]. The company is currently conducting phase II clinical trials to evaluate the efficacy and safety of their NONO-lithium formulation in patients with chronic lower back pain and osteoarthritis[6].
Strengths: Novel combination therapy potentially offering enhanced pain relief and improved tissue perfusion. Weaknesses: Complex mechanism of action may lead to unexpected interactions or side effects.
Core Innovations in Lithium Orotate Formulations
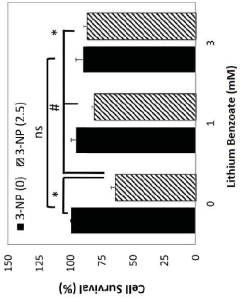
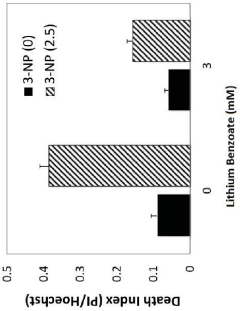
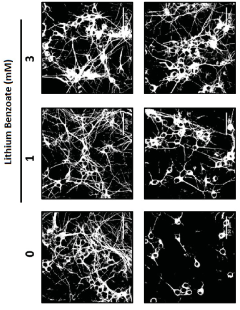
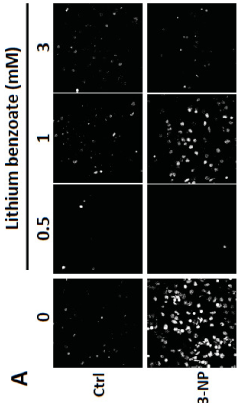
Use of lithium benzoate for treating central nervous system disorders
PatentInactiveIN202018043784A
Innovation
- Lithium benzoate is administered as a compound, either alone or formulated with a pharmaceutically acceptable carrier, to treat CNS diseases and alleviate pain, demonstrating protective effects in various in vitro and in vivo models by rescuing neuron toxicity, enhancing mitochondrial function, and reducing oxidative stress and reactive oxygen species production.
Lithium fluoride nanoparticles for the protection of chondrocytes in osteoarthritis
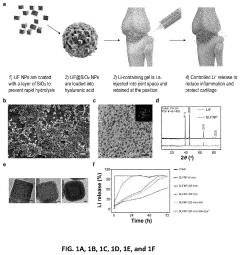
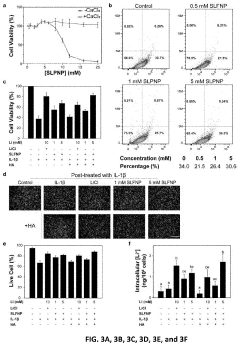
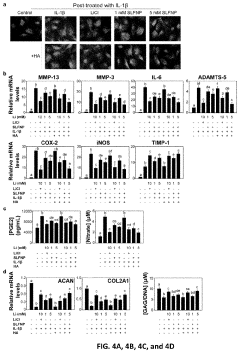
PatentPendingUS20230277472A1
Innovation
- Development of silica-coated lithium fluoride nanoparticles (SLFNP) with a therapeutically effective lithium core and a silica shell for controlled release, encapsulated in a hyaluronic acid matrix to provide sustained lithium delivery and minimize systemic toxicity.
Safety and Efficacy Considerations
The application of lithium orotate in chronic pain management requires careful consideration of both safety and efficacy aspects. Lithium orotate, a compound formed from lithium and orotic acid, has gained attention for its potential therapeutic benefits in managing chronic pain conditions. However, its use necessitates a thorough evaluation of its safety profile and effectiveness.
From a safety perspective, lithium orotate appears to have a more favorable profile compared to traditional lithium carbonate used in psychiatric treatments. The orotate form allows for lower dosages while maintaining therapeutic effects, potentially reducing the risk of side effects associated with higher lithium levels. Nevertheless, long-term studies on the safety of lithium orotate specifically for chronic pain management are limited, warranting cautious application and ongoing monitoring.
Patients using lithium orotate for chronic pain should undergo regular blood tests to monitor lithium levels and kidney function, as lithium can affect renal function over time. Additionally, potential interactions with other medications, particularly those commonly prescribed for pain management, must be carefully evaluated to prevent adverse effects or reduced efficacy of either treatment.
Regarding efficacy, preliminary research suggests that lithium orotate may offer benefits in managing various chronic pain conditions, including fibromyalgia, neuropathic pain, and inflammatory pain disorders. Its proposed mechanisms of action include modulation of neurotransmitter systems, anti-inflammatory effects, and neuroprotective properties. These multifaceted actions may contribute to its potential effectiveness in addressing the complex nature of chronic pain.
Clinical studies have reported improvements in pain intensity, quality of life, and associated symptoms such as depression and anxiety in some patients using lithium orotate. However, the body of evidence remains limited, with most studies being small-scale or observational. Larger, well-designed randomized controlled trials are necessary to establish the true efficacy of lithium orotate in chronic pain management across different patient populations and pain conditions.
It is crucial to note that individual responses to lithium orotate may vary, and its effectiveness may depend on factors such as the underlying cause of chronic pain, dosage, duration of treatment, and concurrent therapies. Healthcare providers should carefully weigh the potential benefits against the risks when considering lithium orotate as a treatment option for chronic pain, taking into account the patient's medical history, current medications, and overall health status.
In conclusion, while lithium orotate shows promise in the field of chronic pain management, its application requires a balanced approach that prioritizes both safety and efficacy. Ongoing research and clinical experience will be essential in refining its use and determining its place in the broader spectrum of chronic pain treatments.
From a safety perspective, lithium orotate appears to have a more favorable profile compared to traditional lithium carbonate used in psychiatric treatments. The orotate form allows for lower dosages while maintaining therapeutic effects, potentially reducing the risk of side effects associated with higher lithium levels. Nevertheless, long-term studies on the safety of lithium orotate specifically for chronic pain management are limited, warranting cautious application and ongoing monitoring.
Patients using lithium orotate for chronic pain should undergo regular blood tests to monitor lithium levels and kidney function, as lithium can affect renal function over time. Additionally, potential interactions with other medications, particularly those commonly prescribed for pain management, must be carefully evaluated to prevent adverse effects or reduced efficacy of either treatment.
Regarding efficacy, preliminary research suggests that lithium orotate may offer benefits in managing various chronic pain conditions, including fibromyalgia, neuropathic pain, and inflammatory pain disorders. Its proposed mechanisms of action include modulation of neurotransmitter systems, anti-inflammatory effects, and neuroprotective properties. These multifaceted actions may contribute to its potential effectiveness in addressing the complex nature of chronic pain.
Clinical studies have reported improvements in pain intensity, quality of life, and associated symptoms such as depression and anxiety in some patients using lithium orotate. However, the body of evidence remains limited, with most studies being small-scale or observational. Larger, well-designed randomized controlled trials are necessary to establish the true efficacy of lithium orotate in chronic pain management across different patient populations and pain conditions.
It is crucial to note that individual responses to lithium orotate may vary, and its effectiveness may depend on factors such as the underlying cause of chronic pain, dosage, duration of treatment, and concurrent therapies. Healthcare providers should carefully weigh the potential benefits against the risks when considering lithium orotate as a treatment option for chronic pain, taking into account the patient's medical history, current medications, and overall health status.
In conclusion, while lithium orotate shows promise in the field of chronic pain management, its application requires a balanced approach that prioritizes both safety and efficacy. Ongoing research and clinical experience will be essential in refining its use and determining its place in the broader spectrum of chronic pain treatments.
Regulatory Framework for Lithium Orotate Use
The regulatory framework for lithium orotate use in chronic pain management is complex and varies significantly across different jurisdictions. In the United States, lithium orotate is not approved by the Food and Drug Administration (FDA) for medical use, including pain management. It is classified as a dietary supplement, which means it is subject to less stringent regulations compared to prescription medications.
Under the Dietary Supplement Health and Education Act (DSHEA) of 1994, manufacturers of lithium orotate are responsible for ensuring the safety of their products before marketing them. However, they are not required to provide evidence of efficacy or obtain FDA approval before selling the supplement. This regulatory approach has led to a proliferation of lithium orotate products in the market, often marketed for various health benefits, including pain relief.
In contrast, the European Union (EU) has stricter regulations regarding lithium orotate. The European Food Safety Authority (EFSA) has not approved any health claims for lithium orotate, and its use as a food supplement is subject to national regulations within each EU member state. Some countries have banned or restricted its sale altogether.
In Canada, lithium orotate is not approved as a Natural Health Product by Health Canada, and its sale for medicinal purposes is not permitted. However, it may be available as a non-medicinal ingredient in certain products, subject to specific regulatory requirements.
The lack of consistent regulations across countries poses challenges for researchers and healthcare providers interested in exploring the potential of lithium orotate for chronic pain management. Clinical trials and research studies must navigate these regulatory differences, often limiting the scope and applicability of their findings.
Furthermore, the absence of standardized dosing guidelines and quality control measures for lithium orotate supplements raises concerns about safety and efficacy. Without regulatory oversight, there is a risk of variability in product quality and potency, which could impact its effectiveness in pain management and potentially lead to adverse effects.
As interest in alternative pain management strategies grows, there is increasing pressure on regulatory bodies to reassess the status of lithium orotate. Some advocates argue for more comprehensive studies and potential reclassification as a regulated medication, which would require rigorous clinical trials and safety assessments. However, such changes would necessitate significant shifts in regulatory frameworks and likely face resistance from both supplement manufacturers and proponents of alternative medicine.
Under the Dietary Supplement Health and Education Act (DSHEA) of 1994, manufacturers of lithium orotate are responsible for ensuring the safety of their products before marketing them. However, they are not required to provide evidence of efficacy or obtain FDA approval before selling the supplement. This regulatory approach has led to a proliferation of lithium orotate products in the market, often marketed for various health benefits, including pain relief.
In contrast, the European Union (EU) has stricter regulations regarding lithium orotate. The European Food Safety Authority (EFSA) has not approved any health claims for lithium orotate, and its use as a food supplement is subject to national regulations within each EU member state. Some countries have banned or restricted its sale altogether.
In Canada, lithium orotate is not approved as a Natural Health Product by Health Canada, and its sale for medicinal purposes is not permitted. However, it may be available as a non-medicinal ingredient in certain products, subject to specific regulatory requirements.
The lack of consistent regulations across countries poses challenges for researchers and healthcare providers interested in exploring the potential of lithium orotate for chronic pain management. Clinical trials and research studies must navigate these regulatory differences, often limiting the scope and applicability of their findings.
Furthermore, the absence of standardized dosing guidelines and quality control measures for lithium orotate supplements raises concerns about safety and efficacy. Without regulatory oversight, there is a risk of variability in product quality and potency, which could impact its effectiveness in pain management and potentially lead to adverse effects.
As interest in alternative pain management strategies grows, there is increasing pressure on regulatory bodies to reassess the status of lithium orotate. Some advocates argue for more comprehensive studies and potential reclassification as a regulated medication, which would require rigorous clinical trials and safety assessments. However, such changes would necessitate significant shifts in regulatory frameworks and likely face resistance from both supplement manufacturers and proponents of alternative medicine.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!