Applying Luminol in Advanced Bioassay Development
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Luminol Bioassay Evolution
The evolution of luminol-based bioassays represents a significant advancement in the field of analytical chemistry and biomedical research. Initially developed in the early 20th century, luminol's chemiluminescent properties were primarily utilized in forensic science for blood detection. However, its application in bioassays has undergone remarkable transformations over the years.
In the 1970s, researchers began exploring luminol's potential in biological assays, recognizing its high sensitivity and low background interference. This period marked the inception of luminol-based immunoassays, where the compound was used as a reporter molecule in antibody-antigen reactions. These early applications laid the foundation for more sophisticated bioassay techniques.
The 1980s and 1990s saw a surge in the development of enhanced luminol derivatives and optimized reaction conditions. Scientists focused on improving the quantum yield and stability of the luminol reaction, leading to the introduction of compounds like isoluminol and aminobutylethyl isoluminol (ABEI). These advancements significantly increased the sensitivity and reliability of luminol-based assays.
The turn of the millennium brought about the integration of luminol bioassays with emerging technologies. Microfluidic systems and lab-on-a-chip devices began incorporating luminol-based detection methods, enabling miniaturization and automation of bioassays. This integration not only enhanced the speed and efficiency of analyses but also reduced sample and reagent consumption.
In recent years, the application of luminol in advanced bioassays has expanded into novel areas. Nanotechnology has played a crucial role in this evolution, with the development of luminol-functionalized nanoparticles and nanocomposites. These materials have demonstrated enhanced catalytic activity and improved signal amplification, pushing the limits of detection to unprecedented levels.
The advent of multiplexed assays has further revolutionized luminol-based bioanalysis. By combining luminol with other chemiluminescent compounds or fluorescent markers, researchers have created sophisticated multi-analyte detection systems. This approach has found particular utility in high-throughput screening and complex diagnostic applications.
Most recently, the integration of luminol bioassays with artificial intelligence and machine learning algorithms has opened new frontiers. These advanced computational techniques enable real-time data analysis, pattern recognition, and predictive modeling, significantly enhancing the interpretative power of luminol-based assays.
As we look to the future, the evolution of luminol bioassays continues to be driven by interdisciplinary collaborations and technological convergence. Emerging fields such as single-molecule detection, in vivo imaging, and point-of-care diagnostics are likely to benefit from further refinements in luminol-based detection systems, promising even more sensitive, specific, and versatile bioassay platforms.
In the 1970s, researchers began exploring luminol's potential in biological assays, recognizing its high sensitivity and low background interference. This period marked the inception of luminol-based immunoassays, where the compound was used as a reporter molecule in antibody-antigen reactions. These early applications laid the foundation for more sophisticated bioassay techniques.
The 1980s and 1990s saw a surge in the development of enhanced luminol derivatives and optimized reaction conditions. Scientists focused on improving the quantum yield and stability of the luminol reaction, leading to the introduction of compounds like isoluminol and aminobutylethyl isoluminol (ABEI). These advancements significantly increased the sensitivity and reliability of luminol-based assays.
The turn of the millennium brought about the integration of luminol bioassays with emerging technologies. Microfluidic systems and lab-on-a-chip devices began incorporating luminol-based detection methods, enabling miniaturization and automation of bioassays. This integration not only enhanced the speed and efficiency of analyses but also reduced sample and reagent consumption.
In recent years, the application of luminol in advanced bioassays has expanded into novel areas. Nanotechnology has played a crucial role in this evolution, with the development of luminol-functionalized nanoparticles and nanocomposites. These materials have demonstrated enhanced catalytic activity and improved signal amplification, pushing the limits of detection to unprecedented levels.
The advent of multiplexed assays has further revolutionized luminol-based bioanalysis. By combining luminol with other chemiluminescent compounds or fluorescent markers, researchers have created sophisticated multi-analyte detection systems. This approach has found particular utility in high-throughput screening and complex diagnostic applications.
Most recently, the integration of luminol bioassays with artificial intelligence and machine learning algorithms has opened new frontiers. These advanced computational techniques enable real-time data analysis, pattern recognition, and predictive modeling, significantly enhancing the interpretative power of luminol-based assays.
As we look to the future, the evolution of luminol bioassays continues to be driven by interdisciplinary collaborations and technological convergence. Emerging fields such as single-molecule detection, in vivo imaging, and point-of-care diagnostics are likely to benefit from further refinements in luminol-based detection systems, promising even more sensitive, specific, and versatile bioassay platforms.
Market Demand Analysis
The market demand for advanced bioassay development utilizing luminol has been steadily increasing in recent years. This growth is primarily driven by the expanding fields of medical diagnostics, forensic science, and environmental monitoring. Luminol, known for its chemiluminescent properties, has become a crucial component in developing highly sensitive and specific bioassays.
In the medical diagnostics sector, there is a growing need for rapid and accurate testing methods. Luminol-based bioassays offer significant advantages in terms of sensitivity and speed, making them particularly valuable for early disease detection and monitoring. The global in vitro diagnostics market, which includes bioassays, is projected to experience substantial growth, with luminol-based technologies playing a key role in this expansion.
Forensic science represents another significant market for luminol applications in bioassays. Law enforcement agencies and forensic laboratories worldwide are increasingly adopting luminol-based techniques for crime scene investigations, particularly in detecting trace amounts of blood. This demand is expected to continue rising as forensic methodologies become more sophisticated and the need for reliable evidence collection grows.
Environmental monitoring is an emerging field where luminol-based bioassays are gaining traction. With increasing concerns about water and soil contamination, there is a rising demand for sensitive detection methods for various pollutants and toxins. Luminol-based assays offer a promising solution for rapid on-site testing, attracting interest from environmental agencies and industrial sectors concerned with regulatory compliance.
The pharmaceutical and biotechnology industries are also driving market demand for advanced bioassays incorporating luminol. These sectors require highly sensitive analytical tools for drug discovery, development, and quality control processes. Luminol-based assays provide the necessary sensitivity and versatility for a wide range of applications in these fields.
Academic and research institutions contribute significantly to the market demand as well. The ongoing research into new applications of luminol in bioassays continues to expand the potential market. This research-driven demand not only fuels current market growth but also paves the way for future commercial applications.
Despite the positive market outlook, challenges such as the need for standardization and the competition from alternative technologies exist. However, the unique properties of luminol and its potential for further optimization in bioassay development continue to drive market interest and investment in this technology.
In the medical diagnostics sector, there is a growing need for rapid and accurate testing methods. Luminol-based bioassays offer significant advantages in terms of sensitivity and speed, making them particularly valuable for early disease detection and monitoring. The global in vitro diagnostics market, which includes bioassays, is projected to experience substantial growth, with luminol-based technologies playing a key role in this expansion.
Forensic science represents another significant market for luminol applications in bioassays. Law enforcement agencies and forensic laboratories worldwide are increasingly adopting luminol-based techniques for crime scene investigations, particularly in detecting trace amounts of blood. This demand is expected to continue rising as forensic methodologies become more sophisticated and the need for reliable evidence collection grows.
Environmental monitoring is an emerging field where luminol-based bioassays are gaining traction. With increasing concerns about water and soil contamination, there is a rising demand for sensitive detection methods for various pollutants and toxins. Luminol-based assays offer a promising solution for rapid on-site testing, attracting interest from environmental agencies and industrial sectors concerned with regulatory compliance.
The pharmaceutical and biotechnology industries are also driving market demand for advanced bioassays incorporating luminol. These sectors require highly sensitive analytical tools for drug discovery, development, and quality control processes. Luminol-based assays provide the necessary sensitivity and versatility for a wide range of applications in these fields.
Academic and research institutions contribute significantly to the market demand as well. The ongoing research into new applications of luminol in bioassays continues to expand the potential market. This research-driven demand not only fuels current market growth but also paves the way for future commercial applications.
Despite the positive market outlook, challenges such as the need for standardization and the competition from alternative technologies exist. However, the unique properties of luminol and its potential for further optimization in bioassay development continue to drive market interest and investment in this technology.
Current Challenges
The application of luminol in advanced bioassay development faces several significant challenges that researchers and developers must address to fully harness its potential. One of the primary obstacles is the optimization of luminol's chemiluminescent reaction for specific bioassay applications. While luminol's light-emitting properties make it an attractive option for sensitive detection methods, fine-tuning the reaction conditions to achieve optimal signal-to-noise ratios in complex biological matrices remains a considerable hurdle.
Another challenge lies in the stability and shelf-life of luminol-based reagents. The sensitivity of luminol to environmental factors such as light, temperature, and pH can lead to degradation over time, potentially compromising the reliability and reproducibility of bioassays. Developing robust formulations and storage protocols that maintain the integrity of luminol-based reagents is crucial for the widespread adoption of these advanced bioassay techniques.
The integration of luminol-based detection systems with existing bioassay platforms presents additional technical difficulties. Compatibility issues may arise when incorporating luminol chemistry into microfluidic devices, high-throughput screening systems, or point-of-care diagnostic tools. Overcoming these integration challenges requires interdisciplinary collaboration between chemists, biologists, and engineers to design seamless and efficient assay workflows.
Furthermore, the potential for interference from endogenous substances in biological samples poses a significant challenge in luminol-based bioassays. Compounds naturally present in blood, urine, or tissue samples may quench or enhance the chemiluminescent signal, leading to false positives or negatives. Developing strategies to mitigate these matrix effects, such as sample pre-treatment methods or the use of selective catalysts, is essential for improving the accuracy and reliability of luminol-based detection systems.
The quantification and standardization of luminol-based assays also present ongoing challenges. Establishing consistent protocols for signal measurement, calibration, and data interpretation across different laboratories and instruments is crucial for the widespread adoption of these advanced bioassays. This includes addressing variations in luminol purity, reagent concentrations, and detection equipment sensitivity that can impact assay performance and reproducibility.
Lastly, regulatory considerations and validation requirements pose significant hurdles in the development and commercialization of luminol-based bioassays, particularly for clinical diagnostic applications. Meeting stringent regulatory standards for sensitivity, specificity, and reproducibility requires extensive validation studies and quality control measures, which can be time-consuming and resource-intensive for developers.
Another challenge lies in the stability and shelf-life of luminol-based reagents. The sensitivity of luminol to environmental factors such as light, temperature, and pH can lead to degradation over time, potentially compromising the reliability and reproducibility of bioassays. Developing robust formulations and storage protocols that maintain the integrity of luminol-based reagents is crucial for the widespread adoption of these advanced bioassay techniques.
The integration of luminol-based detection systems with existing bioassay platforms presents additional technical difficulties. Compatibility issues may arise when incorporating luminol chemistry into microfluidic devices, high-throughput screening systems, or point-of-care diagnostic tools. Overcoming these integration challenges requires interdisciplinary collaboration between chemists, biologists, and engineers to design seamless and efficient assay workflows.
Furthermore, the potential for interference from endogenous substances in biological samples poses a significant challenge in luminol-based bioassays. Compounds naturally present in blood, urine, or tissue samples may quench or enhance the chemiluminescent signal, leading to false positives or negatives. Developing strategies to mitigate these matrix effects, such as sample pre-treatment methods or the use of selective catalysts, is essential for improving the accuracy and reliability of luminol-based detection systems.
The quantification and standardization of luminol-based assays also present ongoing challenges. Establishing consistent protocols for signal measurement, calibration, and data interpretation across different laboratories and instruments is crucial for the widespread adoption of these advanced bioassays. This includes addressing variations in luminol purity, reagent concentrations, and detection equipment sensitivity that can impact assay performance and reproducibility.
Lastly, regulatory considerations and validation requirements pose significant hurdles in the development and commercialization of luminol-based bioassays, particularly for clinical diagnostic applications. Meeting stringent regulatory standards for sensitivity, specificity, and reproducibility requires extensive validation studies and quality control measures, which can be time-consuming and resource-intensive for developers.
Existing Luminol Methods
01 Luminol in forensic applications
Luminol is widely used in forensic science for detecting trace amounts of blood at crime scenes. When mixed with an oxidizing agent, it produces a bright blue chemiluminescence in the presence of hemoglobin. This reaction is highly sensitive and can detect blood even after cleaning attempts, making it valuable for crime scene investigations.- Luminol in chemiluminescence detection: Luminol is widely used in chemiluminescence detection methods for various applications. It produces a bright blue light when oxidized, making it useful for detecting blood traces in forensic investigations, as well as in analytical chemistry for detecting certain substances or reactions.
- Luminol-based biosensors and immunoassays: Luminol is incorporated into biosensors and immunoassay systems for detecting specific biological molecules or pathogens. These systems often combine luminol with enzymes or other reagents to create highly sensitive and selective detection methods for medical diagnostics and environmental monitoring.
- Enhanced luminol formulations: Researchers have developed enhanced luminol formulations by combining it with other compounds or modifying its structure. These improvements aim to increase sensitivity, stability, or specificity of luminol-based detection methods, leading to more efficient and reliable analytical techniques.
- Luminol in environmental monitoring: Luminol-based systems are employed in environmental monitoring applications, such as detecting pollutants in water or air. These methods often involve integrating luminol into portable devices or automated systems for real-time or on-site analysis of environmental samples.
- Industrial applications of luminol: Luminol finds applications in various industrial processes, including quality control in manufacturing, leak detection in pipelines, and monitoring chemical reactions. Its chemiluminescent properties are utilized to develop sensitive and rapid detection methods for specific industrial needs.
02 Luminol-based detection systems
Various detection systems incorporate luminol for its chemiluminescent properties. These systems are used in environmental monitoring, food safety testing, and medical diagnostics. The high sensitivity of luminol allows for the detection of minute quantities of target substances, making it useful in a wide range of analytical applications.Expand Specific Solutions03 Luminol derivatives and modifications
Research has focused on developing luminol derivatives and modifications to enhance its properties or tailor it for specific applications. These modifications can improve sensitivity, stability, or specificity of the luminol reaction, expanding its utility in various fields such as biosensors and imaging techniques.Expand Specific Solutions04 Luminol in biomedical research
Luminol has found applications in biomedical research, particularly in studying cellular processes involving reactive oxygen species. It can be used to detect and quantify the production of hydrogen peroxide and other oxidants in biological systems, providing insights into cellular metabolism and oxidative stress.Expand Specific Solutions05 Industrial and environmental applications of luminol
Luminol is utilized in various industrial and environmental applications, including water quality monitoring, detection of metal contaminants, and assessment of antioxidant capacity in food products. Its chemiluminescent properties make it suitable for developing rapid and sensitive detection methods for a range of analytes in complex matrices.Expand Specific Solutions
Key Industry Players
The field of applying Luminol in advanced bioassay development is in a growth phase, with increasing market potential as bioanalytical techniques evolve. The global market for bioassays is expanding, driven by pharmaceutical research and diagnostic applications. Technologically, the use of Luminol in bioassays is advancing, with companies like MetrioPharm AG and EpimAb Biotherapeutics leading innovation in this area. Academic institutions such as Washington University in St. Louis and Beijing Institute of Technology are contributing to research advancements. While the technology is not yet fully mature, it shows promise for enhancing sensitivity and specificity in various bioanalytical applications, attracting interest from both established players like Beckman Coulter and emerging biotech firms.
Cyanagen Srl
Technical Solution: Cyanagen Srl has developed advanced luminol-based chemiluminescent substrates for bioassay applications. Their SuperSignal™ West Femto Maximum Sensitivity Substrate utilizes an enhanced luminol formulation to achieve femtogram-level protein detection in Western blotting[1]. The company has also created SuperSignal™ ELISA Femto Maximum Sensitivity Substrate, which employs a modified luminol molecule to enable ultra-sensitive detection in ELISA assays, with a lower detection limit up to 0.5 pg/mL for some antigens[2]. Cyanagen's luminol derivatives feature improved quantum yield and extended light emission, allowing for longer integration times and enhanced sensitivity in bioassay development[3].
Strengths: Ultra-high sensitivity enabling detection of low abundance proteins; Extended signal duration for flexible imaging times. Weaknesses: May require specialized equipment for optimal performance; Higher cost compared to traditional colorimetric substrates.
Applied Biosystems LLC
Technical Solution: Applied Biosystems has integrated luminol-based chemiluminescence into their advanced bioassay platforms. Their CLIA-certified immunoassay systems utilize enhanced luminol formulations to achieve high sensitivity and broad dynamic range[4]. The company's Luminex® xMAP® Technology incorporates luminol-based detection in a multiplex format, allowing simultaneous quantification of up to 100 analytes in a single well[5]. Applied Biosystems has also developed the Attune™ CytPix™ Flow Cytometer, which combines traditional flow cytometry with luminol-based imaging to provide both quantitative and visual data on cellular populations[6].
Strengths: Integration of luminol-based detection into high-throughput, multiplex platforms; Combination of quantitative and visual data in flow cytometry. Weaknesses: Complex systems may require specialized training; Higher initial investment compared to traditional bioassay methods.
Core Luminol Innovations
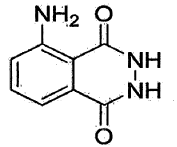
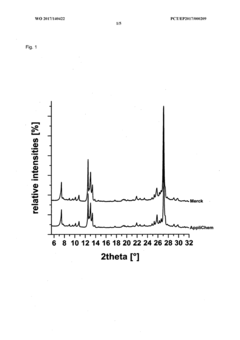
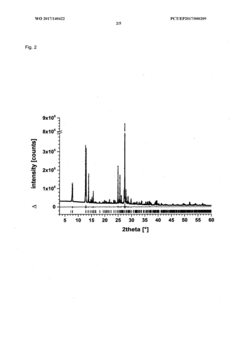
Method for producing a crystalline form of 5-amino-2,3-dihydrophthalazine-1,4-dione
PatentWO2017140422A1
Innovation
- A method involving dissolving 5-amino-2,3-dihydrophthalazine-1,4-dione in a refluxing ethanol-water solution, cooling, separating the precipitated crystals, and drying to produce a phase-pure crystalline form of luminol, which can be resuspended and washed for enhanced purity.
Method for solubilizing 5-amino-2,3-dihydro-1,4-phthalazinedione
PatentPendingUS20250051288A1
Innovation
- A solubilization method using a combination of phosphatidylcholine, medium-chained triglycerides, lysophosphatidylcholine, C2 to C4 alcohols, and glyceryl stearate or saturated/unsaturated fatty acids to solubilize 5-amino-2,3-dihydro-1,4-phthalazinedione or its salts, without the need for polysorbate solubilizers.
Regulatory Compliance
The application of luminol in advanced bioassay development necessitates careful consideration of regulatory compliance to ensure the safety, efficacy, and reliability of the resulting assays. Regulatory bodies such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe have established stringent guidelines for the development and validation of bioassays used in clinical and research settings.
For luminol-based bioassays, compliance with Good Laboratory Practice (GLP) regulations is crucial. These regulations ensure the quality and integrity of non-clinical laboratory studies and data. Researchers must maintain detailed records of all experimental procedures, including reagent preparation, instrument calibration, and data analysis. Additionally, standard operating procedures (SOPs) should be established and followed rigorously to ensure reproducibility and consistency in luminol-based assay performance.
The validation of luminol-based bioassays is a critical aspect of regulatory compliance. This process involves demonstrating the accuracy, precision, sensitivity, and specificity of the assay. Validation studies should include assessments of linearity, range, limit of detection, and limit of quantification. Furthermore, stability testing of luminol reagents and any associated substrates or enhancers is essential to ensure the reliability of the assay over time.
Regulatory bodies also require thorough documentation of the assay development process, including the rationale for selecting luminol as the chemiluminescent agent. This documentation should include a comprehensive risk assessment that addresses potential safety concerns associated with luminol use, such as its potential for skin irritation or environmental impact.
For bioassays intended for clinical diagnostics, compliance with the In Vitro Diagnostic Regulation (IVDR) in Europe or the FDA's regulations for in vitro diagnostic devices is mandatory. These regulations impose additional requirements on the manufacturing process, quality management systems, and post-market surveillance of luminol-based diagnostic assays.
Researchers must also consider the ethical implications of using luminol-based bioassays, particularly when applied to human or animal subjects. Compliance with ethical guidelines, such as obtaining informed consent and ensuring the welfare of research participants, is essential for regulatory approval.
As luminol-based bioassays may involve the handling of biological materials, adherence to biosafety regulations is critical. This includes proper handling, storage, and disposal of potentially hazardous materials, as well as ensuring appropriate laboratory safety measures are in place.
Lastly, researchers should stay informed about evolving regulatory requirements and guidelines specific to chemiluminescent assays and bioanalytical method validation. Regular communication with regulatory authorities and participation in relevant scientific conferences can help ensure ongoing compliance and facilitate the successful development and implementation of luminol-based advanced bioassays.
For luminol-based bioassays, compliance with Good Laboratory Practice (GLP) regulations is crucial. These regulations ensure the quality and integrity of non-clinical laboratory studies and data. Researchers must maintain detailed records of all experimental procedures, including reagent preparation, instrument calibration, and data analysis. Additionally, standard operating procedures (SOPs) should be established and followed rigorously to ensure reproducibility and consistency in luminol-based assay performance.
The validation of luminol-based bioassays is a critical aspect of regulatory compliance. This process involves demonstrating the accuracy, precision, sensitivity, and specificity of the assay. Validation studies should include assessments of linearity, range, limit of detection, and limit of quantification. Furthermore, stability testing of luminol reagents and any associated substrates or enhancers is essential to ensure the reliability of the assay over time.
Regulatory bodies also require thorough documentation of the assay development process, including the rationale for selecting luminol as the chemiluminescent agent. This documentation should include a comprehensive risk assessment that addresses potential safety concerns associated with luminol use, such as its potential for skin irritation or environmental impact.
For bioassays intended for clinical diagnostics, compliance with the In Vitro Diagnostic Regulation (IVDR) in Europe or the FDA's regulations for in vitro diagnostic devices is mandatory. These regulations impose additional requirements on the manufacturing process, quality management systems, and post-market surveillance of luminol-based diagnostic assays.
Researchers must also consider the ethical implications of using luminol-based bioassays, particularly when applied to human or animal subjects. Compliance with ethical guidelines, such as obtaining informed consent and ensuring the welfare of research participants, is essential for regulatory approval.
As luminol-based bioassays may involve the handling of biological materials, adherence to biosafety regulations is critical. This includes proper handling, storage, and disposal of potentially hazardous materials, as well as ensuring appropriate laboratory safety measures are in place.
Lastly, researchers should stay informed about evolving regulatory requirements and guidelines specific to chemiluminescent assays and bioanalytical method validation. Regular communication with regulatory authorities and participation in relevant scientific conferences can help ensure ongoing compliance and facilitate the successful development and implementation of luminol-based advanced bioassays.
Biosafety Considerations
When applying luminol in advanced bioassay development, biosafety considerations are paramount to ensure the protection of researchers, laboratory personnel, and the environment. Luminol, while generally considered safe, can pose potential risks if not handled properly. The primary concern is its oxidizing properties, which can cause skin and eye irritation upon direct contact. Therefore, appropriate personal protective equipment (PPE) such as gloves, lab coats, and safety goggles should be mandatory when working with luminol solutions.
The preparation and handling of luminol solutions should be conducted in well-ventilated areas or under fume hoods to minimize inhalation risks. Although luminol is not highly volatile, prolonged exposure to its vapors may cause respiratory irritation. Proper storage of luminol and its precursors is essential to prevent accidental spills or exposure. It should be kept in tightly sealed containers, away from heat sources and incompatible materials.
Disposal of luminol-containing waste requires careful consideration. While luminol itself is not considered highly toxic to aquatic life, the chemicals used in conjunction with it in bioassays may pose environmental risks. Proper waste management protocols should be established, including the segregation of chemical waste and its disposal through approved channels.
In the context of bioassay development, the interaction between luminol and biological samples must be carefully evaluated. Although luminol is not known to interfere significantly with most biological processes, its potential effects on living cells or organisms used in bioassays should be thoroughly investigated. This is particularly important when developing in vivo or ex vivo assays where luminol may come into direct contact with living tissues.
Biosafety considerations also extend to the handling of biological samples used in luminol-based bioassays. Proper containment measures should be implemented to prevent contamination and ensure the integrity of both the samples and the assay results. This may include the use of biosafety cabinets for handling potentially infectious materials and strict adherence to aseptic techniques.
Researchers should be trained in the safe handling of luminol and associated chemicals, as well as in emergency procedures in case of accidental exposure or spills. Regular safety audits and updates to laboratory protocols are essential to maintain a safe working environment. Additionally, any new applications of luminol in bioassay development should undergo a thorough risk assessment to identify and mitigate potential biosafety concerns before implementation.
The preparation and handling of luminol solutions should be conducted in well-ventilated areas or under fume hoods to minimize inhalation risks. Although luminol is not highly volatile, prolonged exposure to its vapors may cause respiratory irritation. Proper storage of luminol and its precursors is essential to prevent accidental spills or exposure. It should be kept in tightly sealed containers, away from heat sources and incompatible materials.
Disposal of luminol-containing waste requires careful consideration. While luminol itself is not considered highly toxic to aquatic life, the chemicals used in conjunction with it in bioassays may pose environmental risks. Proper waste management protocols should be established, including the segregation of chemical waste and its disposal through approved channels.
In the context of bioassay development, the interaction between luminol and biological samples must be carefully evaluated. Although luminol is not known to interfere significantly with most biological processes, its potential effects on living cells or organisms used in bioassays should be thoroughly investigated. This is particularly important when developing in vivo or ex vivo assays where luminol may come into direct contact with living tissues.
Biosafety considerations also extend to the handling of biological samples used in luminol-based bioassays. Proper containment measures should be implemented to prevent contamination and ensure the integrity of both the samples and the assay results. This may include the use of biosafety cabinets for handling potentially infectious materials and strict adherence to aseptic techniques.
Researchers should be trained in the safe handling of luminol and associated chemicals, as well as in emergency procedures in case of accidental exposure or spills. Regular safety audits and updates to laboratory protocols are essential to maintain a safe working environment. Additionally, any new applications of luminol in bioassay development should undergo a thorough risk assessment to identify and mitigate potential biosafety concerns before implementation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!