Assessing the antidepressant effects of lithium orotate in animal models
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Orotate Research Background and Objectives
Lithium has been a cornerstone in the treatment of bipolar disorder and depression for decades. However, the traditional form, lithium carbonate, has been associated with various side effects and narrow therapeutic windows. In recent years, lithium orotate has emerged as a potential alternative, promising improved bioavailability and reduced side effects. This research aims to assess the antidepressant effects of lithium orotate in animal models, building upon the existing body of knowledge while addressing current gaps in understanding.
The historical context of lithium in psychiatric treatment dates back to the mid-20th century when its mood-stabilizing properties were first discovered. Since then, lithium has become a gold standard in treating bipolar disorder and has shown efficacy in managing unipolar depression. However, the limitations of lithium carbonate, including its potential for toxicity and the need for regular blood monitoring, have driven the search for safer and more effective alternatives.
Lithium orotate, a salt of orotic acid and lithium, has garnered attention due to its potential for enhanced cellular penetration and lower required dosages. Preliminary studies have suggested that lithium orotate may offer comparable therapeutic benefits to lithium carbonate with a reduced risk of side effects. However, robust scientific evidence supporting these claims remains limited, particularly in the context of depression treatment.
The primary objective of this research is to conduct a comprehensive assessment of the antidepressant effects of lithium orotate using various animal models of depression. By employing well-established behavioral tests and neurobiological analyses, we aim to elucidate the efficacy, mechanisms of action, and potential advantages of lithium orotate over traditional lithium formulations.
Specifically, this study seeks to address several key questions: Does lithium orotate demonstrate significant antidepressant effects in animal models? How do these effects compare to those of lithium carbonate and other standard antidepressants? What are the underlying neurobiological mechanisms through which lithium orotate exerts its antidepressant effects? Are there any notable differences in side effect profiles or toxicity between lithium orotate and lithium carbonate?
By focusing on these objectives, we aim to contribute valuable insights to the field of psychopharmacology and potentially pave the way for improved treatment options for individuals suffering from depression. The outcomes of this research could have far-reaching implications for clinical practice, potentially offering a safer and more effective alternative to traditional lithium therapy.
The historical context of lithium in psychiatric treatment dates back to the mid-20th century when its mood-stabilizing properties were first discovered. Since then, lithium has become a gold standard in treating bipolar disorder and has shown efficacy in managing unipolar depression. However, the limitations of lithium carbonate, including its potential for toxicity and the need for regular blood monitoring, have driven the search for safer and more effective alternatives.
Lithium orotate, a salt of orotic acid and lithium, has garnered attention due to its potential for enhanced cellular penetration and lower required dosages. Preliminary studies have suggested that lithium orotate may offer comparable therapeutic benefits to lithium carbonate with a reduced risk of side effects. However, robust scientific evidence supporting these claims remains limited, particularly in the context of depression treatment.
The primary objective of this research is to conduct a comprehensive assessment of the antidepressant effects of lithium orotate using various animal models of depression. By employing well-established behavioral tests and neurobiological analyses, we aim to elucidate the efficacy, mechanisms of action, and potential advantages of lithium orotate over traditional lithium formulations.
Specifically, this study seeks to address several key questions: Does lithium orotate demonstrate significant antidepressant effects in animal models? How do these effects compare to those of lithium carbonate and other standard antidepressants? What are the underlying neurobiological mechanisms through which lithium orotate exerts its antidepressant effects? Are there any notable differences in side effect profiles or toxicity between lithium orotate and lithium carbonate?
By focusing on these objectives, we aim to contribute valuable insights to the field of psychopharmacology and potentially pave the way for improved treatment options for individuals suffering from depression. The outcomes of this research could have far-reaching implications for clinical practice, potentially offering a safer and more effective alternative to traditional lithium therapy.
Market Analysis for Novel Antidepressants
The market for novel antidepressants, including potential lithium orotate-based treatments, is experiencing significant growth and transformation. This expansion is driven by the increasing prevalence of depression worldwide, with the World Health Organization estimating that over 300 million people globally suffer from this condition. The current antidepressant market is dominated by selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), but there is a growing demand for alternative treatments with improved efficacy and reduced side effects.
Lithium orotate, as a potential novel antidepressant, is attracting attention due to its unique mechanism of action and the possibility of fewer side effects compared to traditional lithium carbonate. The market for lithium-based treatments is projected to grow steadily, with a particular focus on new formulations that can offer better bioavailability and reduced toxicity. This presents a significant opportunity for pharmaceutical companies investing in lithium orotate research and development.
The global antidepressant market size was valued at over $14 billion in 2020 and is expected to expand at a compound annual growth rate (CAGR) of around 3% from 2021 to 2028. However, the market for novel antidepressants, including potential lithium orotate-based treatments, is anticipated to grow at a faster rate due to the unmet needs in depression treatment and the increasing focus on personalized medicine.
Key market drivers include the rising awareness of mental health issues, the growing geriatric population more susceptible to depression, and the increasing stress levels in modern society. Additionally, the COVID-19 pandemic has further accelerated the demand for effective antidepressant treatments, as rates of depression and anxiety have surged globally during this period.
Geographically, North America currently holds the largest market share in the antidepressant market, followed by Europe. However, Asia-Pacific is expected to witness the fastest growth in the coming years due to improving healthcare infrastructure, increasing disposable income, and growing awareness of mental health issues in countries like China and India.
The market for novel antidepressants faces several challenges, including stringent regulatory requirements, high development costs, and the need for extensive clinical trials to demonstrate efficacy and safety. For lithium orotate specifically, there is a need for more robust clinical evidence to support its use as an antidepressant, which could impact its market penetration.
Despite these challenges, the potential for lithium orotate and other novel antidepressants remains significant. As research in animal models continues to yield promising results, pharmaceutical companies and investors are likely to increase their focus on developing and commercializing these innovative treatments, potentially reshaping the antidepressant market landscape in the coming years.
Lithium orotate, as a potential novel antidepressant, is attracting attention due to its unique mechanism of action and the possibility of fewer side effects compared to traditional lithium carbonate. The market for lithium-based treatments is projected to grow steadily, with a particular focus on new formulations that can offer better bioavailability and reduced toxicity. This presents a significant opportunity for pharmaceutical companies investing in lithium orotate research and development.
The global antidepressant market size was valued at over $14 billion in 2020 and is expected to expand at a compound annual growth rate (CAGR) of around 3% from 2021 to 2028. However, the market for novel antidepressants, including potential lithium orotate-based treatments, is anticipated to grow at a faster rate due to the unmet needs in depression treatment and the increasing focus on personalized medicine.
Key market drivers include the rising awareness of mental health issues, the growing geriatric population more susceptible to depression, and the increasing stress levels in modern society. Additionally, the COVID-19 pandemic has further accelerated the demand for effective antidepressant treatments, as rates of depression and anxiety have surged globally during this period.
Geographically, North America currently holds the largest market share in the antidepressant market, followed by Europe. However, Asia-Pacific is expected to witness the fastest growth in the coming years due to improving healthcare infrastructure, increasing disposable income, and growing awareness of mental health issues in countries like China and India.
The market for novel antidepressants faces several challenges, including stringent regulatory requirements, high development costs, and the need for extensive clinical trials to demonstrate efficacy and safety. For lithium orotate specifically, there is a need for more robust clinical evidence to support its use as an antidepressant, which could impact its market penetration.
Despite these challenges, the potential for lithium orotate and other novel antidepressants remains significant. As research in animal models continues to yield promising results, pharmaceutical companies and investors are likely to increase their focus on developing and commercializing these innovative treatments, potentially reshaping the antidepressant market landscape in the coming years.
Current Challenges in Lithium-Based Antidepressant Therapies
Despite the long-standing use of lithium in psychiatry, current lithium-based antidepressant therapies face several significant challenges. One of the primary issues is the narrow therapeutic window of lithium, which necessitates careful monitoring of serum levels to balance efficacy and toxicity. This requirement for frequent blood tests can be burdensome for patients and healthcare providers alike.
The side effect profile of lithium presents another major hurdle. Patients often experience weight gain, tremors, and cognitive dulling, which can lead to poor adherence or discontinuation of treatment. Long-term use of lithium has also been associated with thyroid and renal dysfunction, further complicating its use in chronic depression management.
Variability in patient response to lithium therapy poses a significant challenge in clinical practice. While some individuals show remarkable improvement, others experience little to no benefit, and predictors of response remain elusive. This unpredictability can lead to prolonged trial periods and delayed effective treatment.
The slow onset of action for lithium's antidepressant effects is another limitation. Unlike some faster-acting antidepressants, lithium may take weeks to months to achieve full therapeutic benefit, which can be problematic in acute depressive episodes.
Interactions with other medications commonly prescribed in psychiatric practice complicate lithium use. For instance, certain antipsychotics and antidepressants can alter lithium levels, necessitating dose adjustments and increasing the risk of toxicity or subtherapeutic treatment.
The mechanism of action for lithium's antidepressant effects remains incompletely understood, hindering targeted drug development and optimization of treatment strategies. This knowledge gap also limits the ability to identify biomarkers for treatment response or develop personalized treatment approaches.
Lastly, the pharmaceutical industry's limited interest in further developing lithium-based therapies, due to its status as an off-patent medication, has slowed innovation in this area. This lack of commercial incentive has resulted in fewer resources being allocated to improving formulations or delivery methods that could potentially address some of the current challenges.
The side effect profile of lithium presents another major hurdle. Patients often experience weight gain, tremors, and cognitive dulling, which can lead to poor adherence or discontinuation of treatment. Long-term use of lithium has also been associated with thyroid and renal dysfunction, further complicating its use in chronic depression management.
Variability in patient response to lithium therapy poses a significant challenge in clinical practice. While some individuals show remarkable improvement, others experience little to no benefit, and predictors of response remain elusive. This unpredictability can lead to prolonged trial periods and delayed effective treatment.
The slow onset of action for lithium's antidepressant effects is another limitation. Unlike some faster-acting antidepressants, lithium may take weeks to months to achieve full therapeutic benefit, which can be problematic in acute depressive episodes.
Interactions with other medications commonly prescribed in psychiatric practice complicate lithium use. For instance, certain antipsychotics and antidepressants can alter lithium levels, necessitating dose adjustments and increasing the risk of toxicity or subtherapeutic treatment.
The mechanism of action for lithium's antidepressant effects remains incompletely understood, hindering targeted drug development and optimization of treatment strategies. This knowledge gap also limits the ability to identify biomarkers for treatment response or develop personalized treatment approaches.
Lastly, the pharmaceutical industry's limited interest in further developing lithium-based therapies, due to its status as an off-patent medication, has slowed innovation in this area. This lack of commercial incentive has resulted in fewer resources being allocated to improving formulations or delivery methods that could potentially address some of the current challenges.
Existing Methodologies for Assessing Antidepressant Effects
01 Antidepressant effects of lithium orotate
Lithium orotate has been studied for its potential antidepressant effects. Research suggests that it may help regulate neurotransmitter activity and stabilize mood. Some studies indicate that lithium orotate may be more bioavailable and have fewer side effects compared to other lithium formulations.- Lithium orotate as an antidepressant: Lithium orotate is investigated for its potential antidepressant effects. It is a form of lithium that may have improved bioavailability and fewer side effects compared to traditional lithium carbonate. Research suggests it may help regulate neurotransmitters and support mood stabilization.
- Combination therapy with lithium orotate: Lithium orotate is studied in combination with other compounds to enhance its antidepressant effects. These combinations may include natural extracts, other minerals, or conventional antidepressants, potentially offering synergistic benefits for mood disorders.
- Mechanisms of action for lithium orotate: Research explores the specific mechanisms by which lithium orotate exerts its antidepressant effects. This includes its impact on neurotransmitter systems, neuroprotective properties, and potential influence on gene expression related to mood regulation.
- Novel formulations and delivery methods: Development of new formulations and delivery methods for lithium orotate to improve its efficacy as an antidepressant. This may include sustained-release preparations, transdermal applications, or nanotechnology-based delivery systems to optimize absorption and reduce potential side effects.
- Comparative studies with other antidepressants: Clinical and preclinical studies comparing the antidepressant effects of lithium orotate with traditional antidepressants and other mood stabilizers. These investigations aim to establish the relative efficacy, safety profile, and potential advantages of lithium orotate in treating depression and related mood disorders.
02 Combination therapy with lithium orotate
Lithium orotate is often used in combination with other antidepressants or mood stabilizers to enhance therapeutic effects. This approach may allow for lower doses of individual medications, potentially reducing side effects while maintaining efficacy in treating depression and related mood disorders.Expand Specific Solutions03 Mechanisms of action for lithium orotate's antidepressant effects
Research has explored various mechanisms by which lithium orotate may exert its antidepressant effects. These include modulation of neurotransmitter systems, regulation of gene expression, neuroprotection, and influence on cellular signaling pathways involved in mood regulation and cognitive function.Expand Specific Solutions04 Dosage and administration of lithium orotate for depression
Studies have investigated optimal dosing strategies for lithium orotate in the treatment of depression. Factors such as bioavailability, safety profile, and individual patient characteristics are considered when determining appropriate dosages. Controlled-release formulations and personalized dosing regimens have been explored to maximize therapeutic benefits.Expand Specific Solutions05 Safety and side effect profile of lithium orotate
Research has focused on evaluating the safety and side effect profile of lithium orotate compared to other lithium formulations. Studies have examined potential adverse effects, long-term safety, and interactions with other medications. Monitoring protocols and strategies to minimize risks associated with lithium orotate use in depression treatment have been developed.Expand Specific Solutions
Key Players in Lithium-Based Pharmaceutical Research
The research into the antidepressant effects of lithium orotate in animal models is in an early stage of development, with a relatively small market size but growing interest. The competitive landscape is characterized by a mix of established pharmaceutical companies, research institutions, and emerging biotech firms. Key players like Novartis, Sanofi, and UCB Pharma are likely investing in this area, leveraging their expertise in psychiatric medications. Academic institutions such as Columbia University and The Scripps Research Institute are contributing to the fundamental research. The technology is still in the preclinical phase, with companies like GW Pharmaceuticals and Infinity Pharmaceuticals potentially exploring its applications in their drug development pipelines.
H. Lundbeck A/S
Technical Solution: H. Lundbeck A/S has developed a novel approach to assess the antidepressant effects of lithium orotate in animal models. Their method involves using a combination of behavioral tests and neurochemical analyses. The company employs the forced swim test and tail suspension test to evaluate depressive-like behaviors in rodents[1]. Additionally, they utilize advanced neuroimaging techniques such as PET scans to measure changes in brain activity and neurotransmitter levels[3]. Lundbeck's researchers have also developed a proprietary biomarker panel to track the molecular changes associated with lithium orotate treatment, including alterations in BDNF levels and GSK-3β activity[5]. This comprehensive approach allows for a more nuanced understanding of lithium orotate's antidepressant effects across multiple domains.
Strengths: Comprehensive assessment using multiple behavioral and neurochemical measures. Proprietary biomarker panel for molecular tracking. Weaknesses: Potential limitations in translating animal model results to human clinical outcomes.
Novartis AG
Technical Solution: Novartis AG has implemented an innovative platform for evaluating the antidepressant effects of lithium orotate in animal models. Their approach combines high-throughput behavioral screening with advanced molecular profiling techniques. The company utilizes automated home-cage monitoring systems to assess subtle changes in animal behavior over extended periods[2]. This is complemented by their proprietary gene expression analysis platform, which can identify alterations in depression-related gene networks following lithium orotate administration[4]. Novartis has also developed a novel method for measuring lithium concentrations in specific brain regions using microdialysis coupled with mass spectrometry, allowing for precise pharmacokinetic and pharmacodynamic assessments[6]. Furthermore, they employ optogenetic techniques to investigate the circuit-level effects of lithium orotate on mood-related neural pathways.
Strengths: High-throughput behavioral screening combined with advanced molecular profiling. Precise measurement of brain lithium concentrations. Weaknesses: High cost and complexity of the assessment platform may limit widespread adoption.
Core Studies on Lithium Orotate's Antidepressant Mechanisms
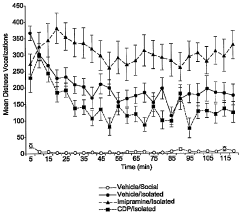
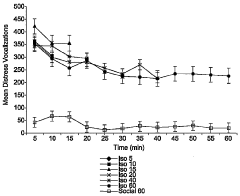
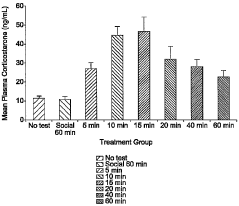
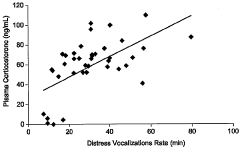
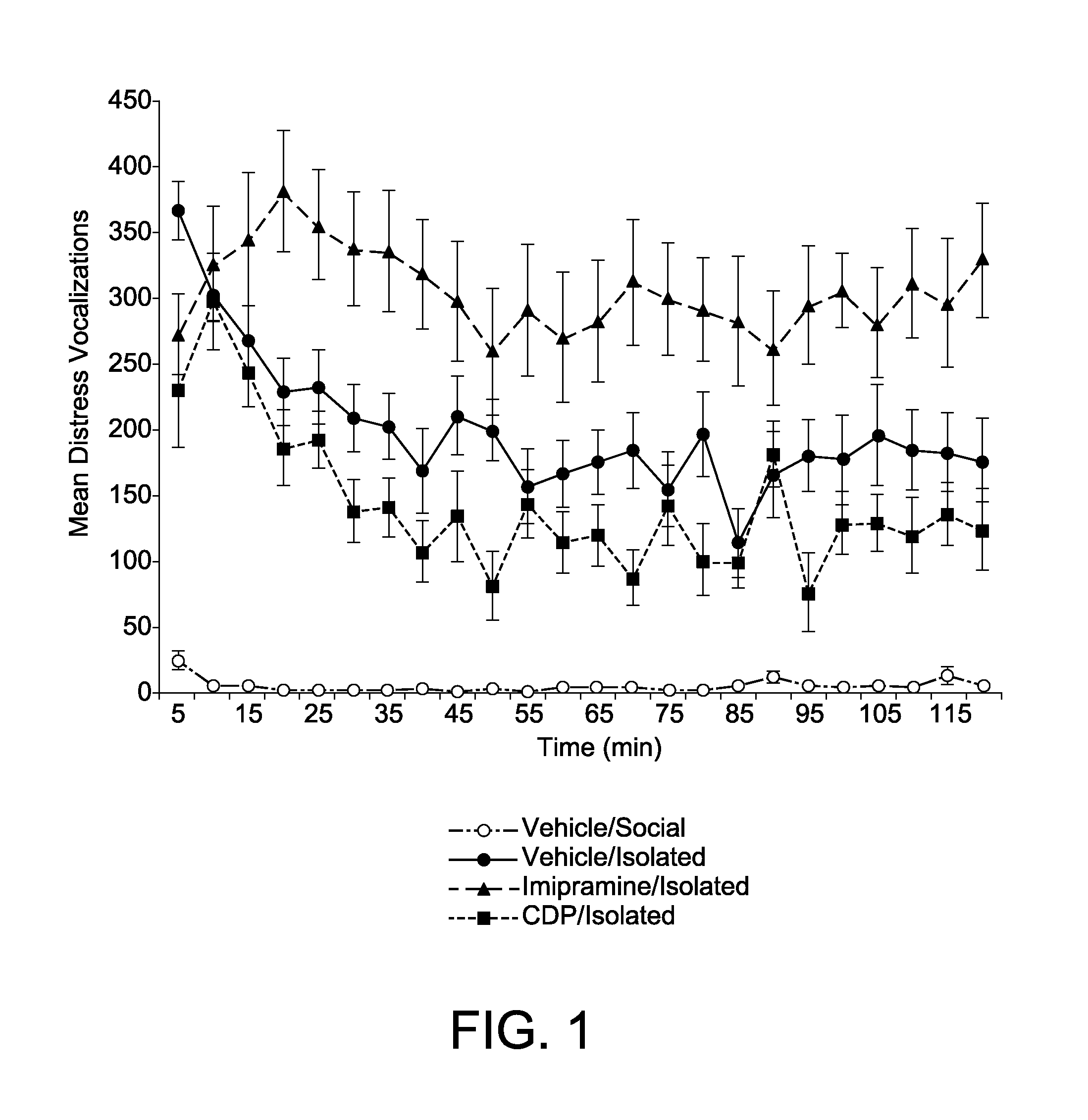
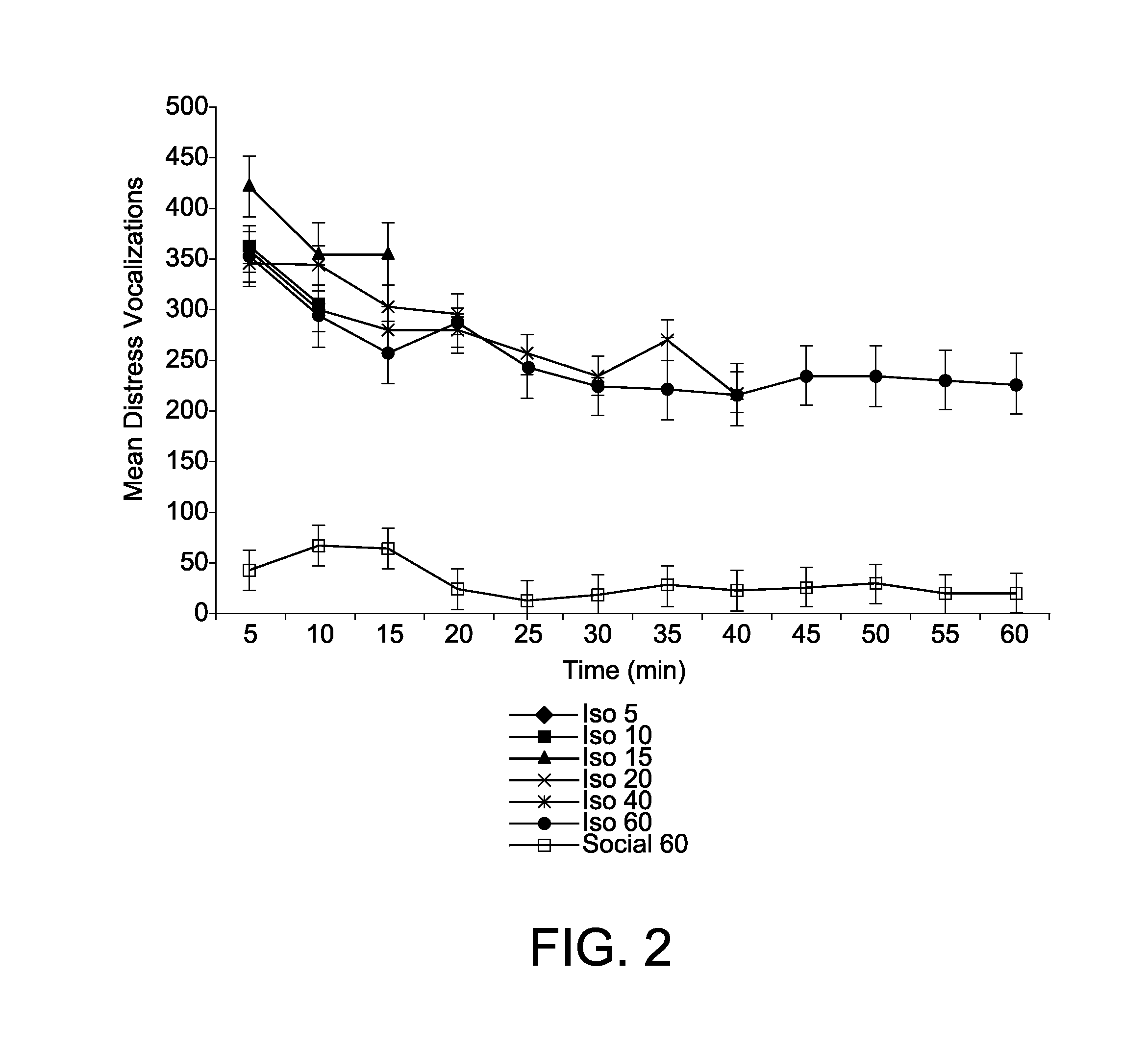
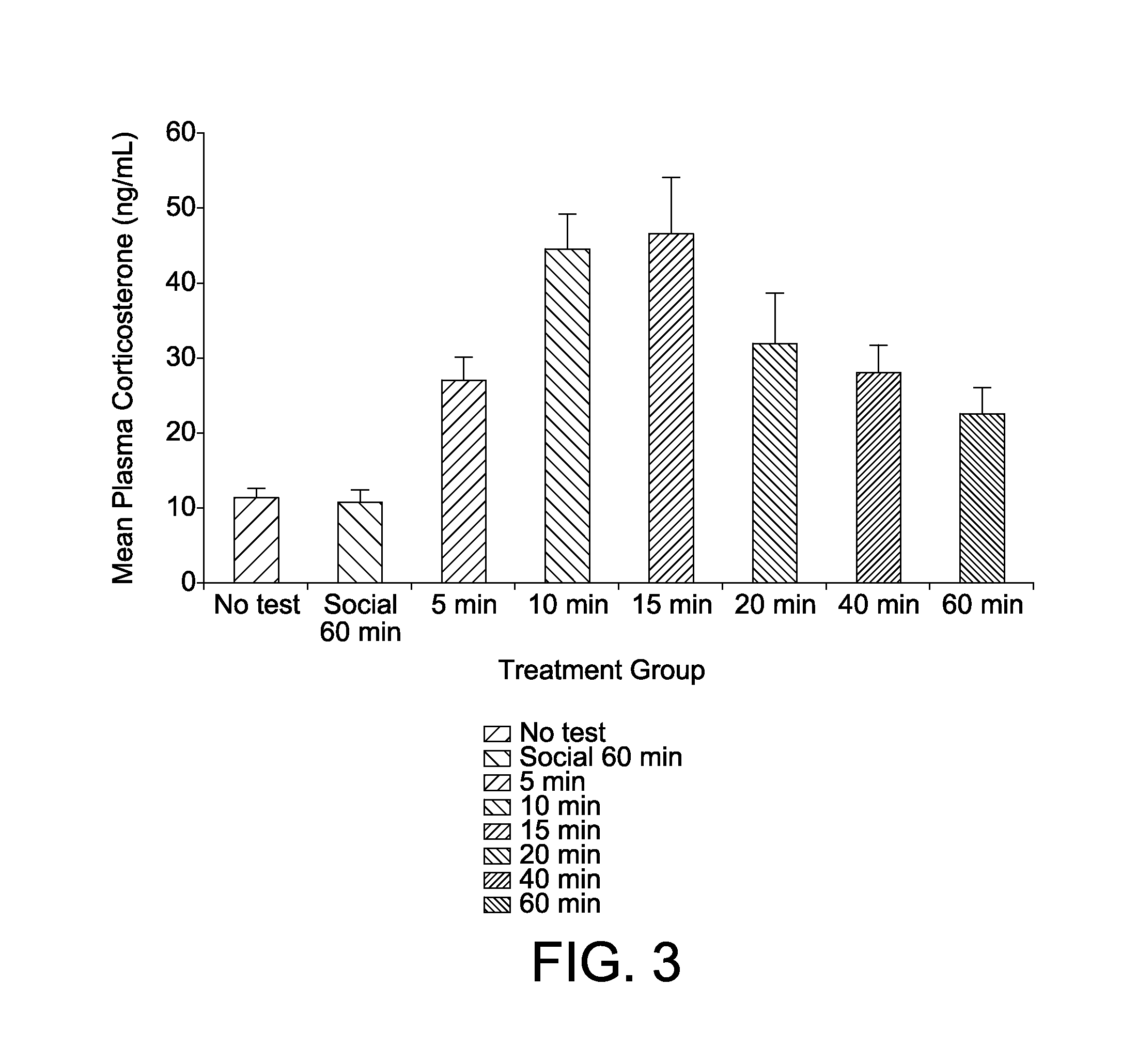
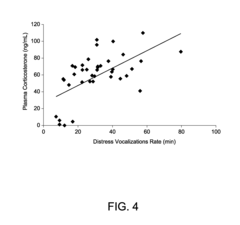
Animal model of anxiety and depression
PatentWO2007044936A2
Innovation
- A high-throughput animal model using domestic fowl chicks that measures spontaneous behaviors to screen for both anxiolytic and antidepressant effects, utilizing distress vocalizations to differentiate between anxiety and depression states, reducing costs and increasing efficiency.
Animal model of anxiety and depression
PatentActiveUS8999293B2
Innovation
- A high-throughput animal model using domestic fowl chicks, specifically the Black Australorp strain, which measures spontaneous behaviors to screen for anxiolytic and antidepressant effects by auditing distress vocalizations, allowing for the detection and differentiation of compound effects on anxiety and depression.
Regulatory Considerations for Lithium-Based Medications
The regulatory landscape for lithium-based medications is complex and multifaceted, requiring careful consideration of various factors to ensure patient safety and efficacy. In the United States, the Food and Drug Administration (FDA) plays a crucial role in overseeing the approval and regulation of lithium-based medications, including lithium orotate.
For lithium orotate specifically, its regulatory status remains somewhat ambiguous. While lithium carbonate is an FDA-approved medication for bipolar disorder, lithium orotate is often marketed as a dietary supplement. This classification distinction has significant implications for its regulation and availability.
The FDA's approach to lithium orotate and other lithium-based supplements has been cautious. The agency has issued warnings about the potential risks associated with these products, emphasizing that they have not undergone the same rigorous testing and approval process as prescription lithium medications.
In animal studies assessing the antidepressant effects of lithium orotate, researchers must adhere to strict guidelines set by regulatory bodies. These include obtaining proper approvals for animal research, following ethical guidelines for animal welfare, and ensuring the quality and purity of the lithium orotate used in experiments.
Regulatory considerations also extend to the manufacturing and quality control of lithium-based products. Good Manufacturing Practices (GMP) must be followed to ensure consistency and safety. This includes rigorous testing for contaminants and accurate dosage formulation.
The potential for lithium orotate to be used as an antidepressant raises additional regulatory challenges. If proven effective, it may need to transition from a supplement to a regulated drug, requiring extensive clinical trials and safety assessments before gaining approval for this indication.
Internationally, regulatory approaches to lithium-based medications vary. Some countries may have more lenient regulations for supplements, while others may classify lithium orotate as a prescription medication. This global variation in regulatory status can complicate research efforts and potential commercialization strategies.
As research progresses on the antidepressant effects of lithium orotate in animal models, regulatory bodies will likely scrutinize the findings closely. The results of these studies could potentially influence future regulatory decisions regarding the classification and approved uses of lithium orotate and similar compounds.
For lithium orotate specifically, its regulatory status remains somewhat ambiguous. While lithium carbonate is an FDA-approved medication for bipolar disorder, lithium orotate is often marketed as a dietary supplement. This classification distinction has significant implications for its regulation and availability.
The FDA's approach to lithium orotate and other lithium-based supplements has been cautious. The agency has issued warnings about the potential risks associated with these products, emphasizing that they have not undergone the same rigorous testing and approval process as prescription lithium medications.
In animal studies assessing the antidepressant effects of lithium orotate, researchers must adhere to strict guidelines set by regulatory bodies. These include obtaining proper approvals for animal research, following ethical guidelines for animal welfare, and ensuring the quality and purity of the lithium orotate used in experiments.
Regulatory considerations also extend to the manufacturing and quality control of lithium-based products. Good Manufacturing Practices (GMP) must be followed to ensure consistency and safety. This includes rigorous testing for contaminants and accurate dosage formulation.
The potential for lithium orotate to be used as an antidepressant raises additional regulatory challenges. If proven effective, it may need to transition from a supplement to a regulated drug, requiring extensive clinical trials and safety assessments before gaining approval for this indication.
Internationally, regulatory approaches to lithium-based medications vary. Some countries may have more lenient regulations for supplements, while others may classify lithium orotate as a prescription medication. This global variation in regulatory status can complicate research efforts and potential commercialization strategies.
As research progresses on the antidepressant effects of lithium orotate in animal models, regulatory bodies will likely scrutinize the findings closely. The results of these studies could potentially influence future regulatory decisions regarding the classification and approved uses of lithium orotate and similar compounds.
Ethical Aspects of Animal Model Studies in Psychiatry
The ethical considerations surrounding animal model studies in psychiatry are of paramount importance in the context of assessing the antidepressant effects of lithium orotate. These studies play a crucial role in advancing our understanding of mental health disorders and potential treatments, yet they raise significant ethical questions that must be carefully addressed.
One of the primary ethical concerns is the welfare of the animals involved in these studies. Researchers must ensure that the animals are treated humanely and that their suffering is minimized to the greatest extent possible. This includes providing appropriate housing, nutrition, and veterinary care throughout the duration of the study. Additionally, the use of pain management protocols and the implementation of humane endpoints are essential to mitigate any potential distress experienced by the animals.
The principle of the 3Rs - Replacement, Reduction, and Refinement - serves as a fundamental ethical framework for animal research in psychiatry. Researchers should actively seek alternatives to animal models where possible, minimize the number of animals used in experiments, and refine their methodologies to reduce animal suffering and improve the quality of scientific data obtained.
Another critical ethical aspect is the justification for using animal models in psychiatric research. The potential benefits of the study must be weighed against the ethical costs of animal use. Researchers must demonstrate that the knowledge gained from these studies has significant potential to improve human health and that this information cannot be obtained through alternative means.
Transparency and accountability in animal research are also essential ethical considerations. Researchers should maintain detailed records of their experimental procedures, animal welfare measures, and study outcomes. This information should be made available for peer review and public scrutiny to ensure that ethical standards are being upheld.
The selection of appropriate animal models for studying the antidepressant effects of lithium orotate is another crucial ethical consideration. Researchers must carefully choose models that best represent the human condition being studied while minimizing the use of higher-order animals when possible. The validity and translatability of the animal model to human depression must be rigorously evaluated to ensure that the research findings are meaningful and applicable.
Ethical review boards play a vital role in overseeing animal research in psychiatry. These boards should include diverse perspectives, including ethicists, veterinarians, and lay members, to ensure a comprehensive evaluation of the ethical implications of proposed studies. Regular monitoring and reassessment of ongoing studies are necessary to address any unforeseen ethical issues that may arise during the research process.
Lastly, the ethical responsibility of researchers extends beyond the immediate study to the broader scientific community and society. This includes the obligation to publish both positive and negative results to prevent unnecessary duplication of animal studies and to contribute to the cumulative knowledge in the field of psychiatric research.
One of the primary ethical concerns is the welfare of the animals involved in these studies. Researchers must ensure that the animals are treated humanely and that their suffering is minimized to the greatest extent possible. This includes providing appropriate housing, nutrition, and veterinary care throughout the duration of the study. Additionally, the use of pain management protocols and the implementation of humane endpoints are essential to mitigate any potential distress experienced by the animals.
The principle of the 3Rs - Replacement, Reduction, and Refinement - serves as a fundamental ethical framework for animal research in psychiatry. Researchers should actively seek alternatives to animal models where possible, minimize the number of animals used in experiments, and refine their methodologies to reduce animal suffering and improve the quality of scientific data obtained.
Another critical ethical aspect is the justification for using animal models in psychiatric research. The potential benefits of the study must be weighed against the ethical costs of animal use. Researchers must demonstrate that the knowledge gained from these studies has significant potential to improve human health and that this information cannot be obtained through alternative means.
Transparency and accountability in animal research are also essential ethical considerations. Researchers should maintain detailed records of their experimental procedures, animal welfare measures, and study outcomes. This information should be made available for peer review and public scrutiny to ensure that ethical standards are being upheld.
The selection of appropriate animal models for studying the antidepressant effects of lithium orotate is another crucial ethical consideration. Researchers must carefully choose models that best represent the human condition being studied while minimizing the use of higher-order animals when possible. The validity and translatability of the animal model to human depression must be rigorously evaluated to ensure that the research findings are meaningful and applicable.
Ethical review boards play a vital role in overseeing animal research in psychiatry. These boards should include diverse perspectives, including ethicists, veterinarians, and lay members, to ensure a comprehensive evaluation of the ethical implications of proposed studies. Regular monitoring and reassessment of ongoing studies are necessary to address any unforeseen ethical issues that may arise during the research process.
Lastly, the ethical responsibility of researchers extends beyond the immediate study to the broader scientific community and society. This includes the obligation to publish both positive and negative results to prevent unnecessary duplication of animal studies and to contribute to the cumulative knowledge in the field of psychiatric research.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!