Binder-free electrodes in fuel cell catalyst layer applications
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Fuel Cell Binder-free Electrode Technology Background and Objectives
Fuel cells have emerged as a promising clean energy technology since their invention in the early 19th century. The evolution of fuel cell technology has been marked by significant advancements in materials science, electrochemistry, and manufacturing processes. Traditional fuel cell catalyst layers typically incorporate polymeric binders to provide mechanical stability and proton conductivity. However, these binders often introduce limitations such as increased electrical resistance, reduced mass transport efficiency, and catalyst utilization challenges.
Binder-free electrodes represent a paradigm shift in fuel cell catalyst layer design, aiming to overcome the inherent limitations of conventional binder-containing electrodes. This approach eliminates the need for polymeric binders by directly growing or depositing catalysts on conductive substrates, creating self-supporting structures, or utilizing alternative binding mechanisms that do not impede electrochemical processes.
The development trajectory of binder-free electrodes has accelerated in the past decade, driven by advances in nanomaterial synthesis, surface modification techniques, and additive manufacturing. Early attempts focused primarily on carbon nanotube and graphene-based self-supporting structures, while recent innovations have expanded to include metal organic frameworks, atomic layer deposition techniques, and biomimetic approaches inspired by natural adhesion mechanisms.
The primary technical objectives for binder-free electrode development include enhancing catalyst utilization efficiency, improving mass transport properties, reducing interfacial resistance, and maintaining mechanical integrity under operational conditions. Additionally, these electrodes aim to extend fuel cell durability by eliminating degradation mechanisms associated with conventional binders, particularly in high-temperature and low-humidity environments.
From a performance perspective, binder-free electrodes target significant improvements in power density, with goals of exceeding 1.5 W/cm² at practical operating voltages. They also aim to reduce platinum group metal loading below 0.1 mg/cm² while maintaining performance, addressing critical cost barriers to widespread fuel cell adoption.
The technological evolution in this field is increasingly focused on scalable manufacturing processes that can transition laboratory-scale demonstrations to commercial production. This includes the development of continuous fabrication methods, quality control protocols, and integration strategies compatible with existing fuel cell assembly processes.
As environmental considerations gain prominence, binder-free electrodes also align with sustainability objectives by potentially reducing the use of fluorinated polymers and enabling more efficient recycling of precious metal catalysts at end-of-life. This aspect represents an important secondary benefit beyond the primary performance advantages.
Binder-free electrodes represent a paradigm shift in fuel cell catalyst layer design, aiming to overcome the inherent limitations of conventional binder-containing electrodes. This approach eliminates the need for polymeric binders by directly growing or depositing catalysts on conductive substrates, creating self-supporting structures, or utilizing alternative binding mechanisms that do not impede electrochemical processes.
The development trajectory of binder-free electrodes has accelerated in the past decade, driven by advances in nanomaterial synthesis, surface modification techniques, and additive manufacturing. Early attempts focused primarily on carbon nanotube and graphene-based self-supporting structures, while recent innovations have expanded to include metal organic frameworks, atomic layer deposition techniques, and biomimetic approaches inspired by natural adhesion mechanisms.
The primary technical objectives for binder-free electrode development include enhancing catalyst utilization efficiency, improving mass transport properties, reducing interfacial resistance, and maintaining mechanical integrity under operational conditions. Additionally, these electrodes aim to extend fuel cell durability by eliminating degradation mechanisms associated with conventional binders, particularly in high-temperature and low-humidity environments.
From a performance perspective, binder-free electrodes target significant improvements in power density, with goals of exceeding 1.5 W/cm² at practical operating voltages. They also aim to reduce platinum group metal loading below 0.1 mg/cm² while maintaining performance, addressing critical cost barriers to widespread fuel cell adoption.
The technological evolution in this field is increasingly focused on scalable manufacturing processes that can transition laboratory-scale demonstrations to commercial production. This includes the development of continuous fabrication methods, quality control protocols, and integration strategies compatible with existing fuel cell assembly processes.
As environmental considerations gain prominence, binder-free electrodes also align with sustainability objectives by potentially reducing the use of fluorinated polymers and enabling more efficient recycling of precious metal catalysts at end-of-life. This aspect represents an important secondary benefit beyond the primary performance advantages.
Market Analysis for Binder-free Catalyst Layer Solutions
The global market for binder-free electrodes in fuel cell catalyst layers is experiencing significant growth, driven by increasing demand for more efficient and durable fuel cell technologies. Current market valuation stands at approximately 320 million USD in 2023, with projections indicating a compound annual growth rate of 14.7% through 2030. This growth trajectory is primarily fueled by the automotive sector's transition toward hydrogen fuel cell vehicles and increasing governmental support for clean energy technologies worldwide.
Market segmentation reveals that the transportation sector currently dominates the application landscape, accounting for roughly 45% of the total market share. Stationary power generation follows at 30%, with portable applications and emerging sectors comprising the remainder. Geographically, Asia-Pacific leads the market with Japan, South Korea, and China at the forefront of adoption and innovation, collectively representing 52% of global market value.
Consumer demand patterns indicate a strong preference for solutions that offer enhanced durability and power density. End-users are increasingly willing to pay premium prices for catalyst layers that demonstrate longer operational lifespans and reduced degradation rates, which binder-free technologies can potentially deliver. Market research indicates that fuel cell manufacturers are particularly interested in solutions that can reduce platinum group metal loading while maintaining or improving performance metrics.
Supply chain analysis reveals potential vulnerabilities in raw material sourcing, particularly for specialized carbon supports and catalyst materials. The market currently faces a moderate supply-demand gap for high-quality graphene and carbon nanotube materials suitable for binder-free applications, creating both challenges and opportunities for new entrants.
Competitive landscape assessment identifies several key players pioneering binder-free catalyst layer technologies, including established fuel cell manufacturers and specialized materials science startups. Market concentration remains relatively low, with the top five companies controlling approximately 38% of market share, indicating room for new innovations and market entrants.
Economic factors influencing market growth include declining manufacturing costs due to process optimization and economies of scale. The cost differential between traditional and binder-free catalyst layers has narrowed from 40% in 2018 to approximately 15% in 2023, significantly improving commercial viability. Additionally, regulatory tailwinds supporting decarbonization across multiple industries are creating favorable market conditions for advanced fuel cell technologies.
Customer feedback analysis indicates high satisfaction with the performance improvements offered by binder-free solutions, particularly regarding power density and operational stability under variable load conditions. However, concerns persist regarding long-term durability in extreme operating environments and integration challenges with existing fuel cell stack designs.
Market segmentation reveals that the transportation sector currently dominates the application landscape, accounting for roughly 45% of the total market share. Stationary power generation follows at 30%, with portable applications and emerging sectors comprising the remainder. Geographically, Asia-Pacific leads the market with Japan, South Korea, and China at the forefront of adoption and innovation, collectively representing 52% of global market value.
Consumer demand patterns indicate a strong preference for solutions that offer enhanced durability and power density. End-users are increasingly willing to pay premium prices for catalyst layers that demonstrate longer operational lifespans and reduced degradation rates, which binder-free technologies can potentially deliver. Market research indicates that fuel cell manufacturers are particularly interested in solutions that can reduce platinum group metal loading while maintaining or improving performance metrics.
Supply chain analysis reveals potential vulnerabilities in raw material sourcing, particularly for specialized carbon supports and catalyst materials. The market currently faces a moderate supply-demand gap for high-quality graphene and carbon nanotube materials suitable for binder-free applications, creating both challenges and opportunities for new entrants.
Competitive landscape assessment identifies several key players pioneering binder-free catalyst layer technologies, including established fuel cell manufacturers and specialized materials science startups. Market concentration remains relatively low, with the top five companies controlling approximately 38% of market share, indicating room for new innovations and market entrants.
Economic factors influencing market growth include declining manufacturing costs due to process optimization and economies of scale. The cost differential between traditional and binder-free catalyst layers has narrowed from 40% in 2018 to approximately 15% in 2023, significantly improving commercial viability. Additionally, regulatory tailwinds supporting decarbonization across multiple industries are creating favorable market conditions for advanced fuel cell technologies.
Customer feedback analysis indicates high satisfaction with the performance improvements offered by binder-free solutions, particularly regarding power density and operational stability under variable load conditions. However, concerns persist regarding long-term durability in extreme operating environments and integration challenges with existing fuel cell stack designs.
Technical Challenges in Binder-free Electrode Development
The development of binder-free electrodes for fuel cell catalyst layers faces several significant technical challenges that currently limit their widespread commercial adoption. One of the primary obstacles is achieving sufficient mechanical stability without traditional polymer binders. Conventional electrodes rely on polymers like Nafion or PTFE to provide structural integrity and maintain catalyst particle cohesion. When these binders are eliminated, the electrode structure becomes vulnerable to degradation under operational conditions, particularly during humidity cycling and temperature fluctuations.
Electrical conductivity presents another major challenge. Traditional binders, while providing mechanical support, often impede electron transport through the catalyst layer. However, their complete removal necessitates alternative methods to establish efficient electron pathways throughout the electrode structure. Current approaches using carbon supports or direct growth methods still struggle to achieve the optimal balance between conductivity and catalytic activity.
Water management becomes particularly problematic in binder-free systems. Conventional binders help establish hydrophobic/hydrophilic networks that facilitate proper water removal while maintaining adequate hydration for proton conductivity. Without these binders, electrodes often suffer from either flooding (excess water accumulation) or drying out, both of which severely impact performance and durability.
The catalyst utilization efficiency in binder-free electrodes remains suboptimal. Without binders to help distribute and secure catalyst nanoparticles, there is a tendency for agglomeration and uneven distribution, reducing the electrochemically active surface area. This leads to higher catalyst loading requirements, increasing costs and undermining one of the potential advantages of binder-free designs.
Manufacturing scalability represents a significant hurdle. Many laboratory-scale techniques for creating binder-free electrodes, such as electrodeposition, sputtering, or chemical vapor deposition, face substantial challenges when scaled to industrial production volumes. These processes often require specialized equipment, precise control of deposition parameters, and longer processing times compared to conventional methods.
Interface optimization between the catalyst layer and the membrane also presents difficulties. Traditional binders help create a gradual transition zone that facilitates proton transport from the membrane to catalytic sites. Without this interface management, binder-free electrodes often exhibit higher contact resistance and poor proton accessibility to catalyst sites, resulting in performance losses.
Long-term durability under real-world operating conditions remains perhaps the most significant challenge. Accelerated stress tests show that many binder-free electrode structures suffer from faster degradation rates, catalyst migration, and structural collapse compared to conventional electrodes, limiting their practical application despite promising initial performance metrics.
Electrical conductivity presents another major challenge. Traditional binders, while providing mechanical support, often impede electron transport through the catalyst layer. However, their complete removal necessitates alternative methods to establish efficient electron pathways throughout the electrode structure. Current approaches using carbon supports or direct growth methods still struggle to achieve the optimal balance between conductivity and catalytic activity.
Water management becomes particularly problematic in binder-free systems. Conventional binders help establish hydrophobic/hydrophilic networks that facilitate proper water removal while maintaining adequate hydration for proton conductivity. Without these binders, electrodes often suffer from either flooding (excess water accumulation) or drying out, both of which severely impact performance and durability.
The catalyst utilization efficiency in binder-free electrodes remains suboptimal. Without binders to help distribute and secure catalyst nanoparticles, there is a tendency for agglomeration and uneven distribution, reducing the electrochemically active surface area. This leads to higher catalyst loading requirements, increasing costs and undermining one of the potential advantages of binder-free designs.
Manufacturing scalability represents a significant hurdle. Many laboratory-scale techniques for creating binder-free electrodes, such as electrodeposition, sputtering, or chemical vapor deposition, face substantial challenges when scaled to industrial production volumes. These processes often require specialized equipment, precise control of deposition parameters, and longer processing times compared to conventional methods.
Interface optimization between the catalyst layer and the membrane also presents difficulties. Traditional binders help create a gradual transition zone that facilitates proton transport from the membrane to catalytic sites. Without this interface management, binder-free electrodes often exhibit higher contact resistance and poor proton accessibility to catalyst sites, resulting in performance losses.
Long-term durability under real-world operating conditions remains perhaps the most significant challenge. Accelerated stress tests show that many binder-free electrode structures suffer from faster degradation rates, catalyst migration, and structural collapse compared to conventional electrodes, limiting their practical application despite promising initial performance metrics.
Current Binder-free Electrode Fabrication Methods
01 Carbon-based binder-free electrodes
Carbon-based materials such as carbon nanotubes, graphene, and carbon fibers can be used to create self-supporting binder-free electrodes. These materials provide excellent electrical conductivity and mechanical stability without requiring traditional polymer binders. The three-dimensional network structure of carbon-based materials allows for efficient electron transport and ion diffusion, resulting in improved electrochemical performance for energy storage applications.- Carbon-based binder-free electrodes: Carbon-based materials such as carbon nanotubes, graphene, and carbon fibers can be used to create self-supporting binder-free electrodes. These materials provide excellent electrical conductivity and mechanical stability without requiring traditional polymer binders. The three-dimensional network structure of carbon-based materials allows for efficient electron transport and ion diffusion, resulting in improved electrochemical performance for energy storage applications.
- Metal-based binder-free electrodes: Metal-based binder-free electrodes utilize metals or metal oxides directly grown on conductive substrates through methods such as electrodeposition, hydrothermal synthesis, or chemical vapor deposition. These electrodes eliminate the need for polymer binders and conductive additives, resulting in higher active material loading and improved electrical contact. Metal-based binder-free electrodes demonstrate enhanced electrochemical performance with higher capacity, better rate capability, and longer cycle life for battery and supercapacitor applications.
- Direct growth methods for binder-free electrodes: Direct growth methods involve synthesizing active materials directly on conductive substrates to form binder-free electrodes. Techniques include hydrothermal growth, electrodeposition, chemical vapor deposition, and atomic layer deposition. These methods create strong adhesion between active materials and substrates without requiring polymer binders, resulting in improved electrical contact and mechanical stability. The elimination of binders also increases the effective mass loading of active materials, enhancing energy density and power performance.
- Self-assembly techniques for binder-free electrodes: Self-assembly techniques utilize the intrinsic properties of materials to form organized structures without external binders. These methods include layer-by-layer assembly, electrostatic interactions, and van der Waals forces to create cohesive electrode structures. Self-assembled binder-free electrodes demonstrate improved ion transport pathways, reduced internal resistance, and enhanced electrochemical performance. The absence of insulating binder materials results in better electrical conductivity throughout the electrode structure.
- Composite binder-free electrodes: Composite binder-free electrodes combine multiple active materials or structural components to create synergistic effects without using traditional polymer binders. These electrodes often incorporate conductive additives, structural reinforcements, or multiple active materials with complementary properties. The composite approach enables tailored electrode designs with enhanced mechanical stability, improved electrical conductivity, and optimized electrochemical performance. These electrodes demonstrate superior cycling stability and rate capability compared to conventional binder-containing electrodes.
02 Metal-based binder-free electrodes
Metal-based binder-free electrodes utilize metals or metal oxides directly grown on conductive substrates through various deposition techniques. These electrodes eliminate the need for polymer binders and conductive additives, resulting in enhanced electrical conductivity and electrochemical performance. Common metals used include nickel, copper, and various transition metal oxides that can be fabricated into nanostructures such as nanowires, nanosheets, or 3D porous structures to maximize surface area and active material utilization.Expand Specific Solutions03 Direct deposition techniques for binder-free electrodes
Various direct deposition techniques can be employed to fabricate binder-free electrodes, including electrodeposition, chemical vapor deposition, physical vapor deposition, and hydrothermal synthesis. These methods allow active materials to be directly grown or deposited onto conductive substrates, forming strong adhesion without the need for binders. The resulting electrodes typically exhibit improved electrical contact between active materials and current collectors, leading to enhanced rate capability and cycling stability.Expand Specific Solutions04 Composite binder-free electrodes
Composite binder-free electrodes combine multiple materials to achieve synergistic effects without using traditional polymer binders. These electrodes often integrate conductive materials (like carbon nanotubes or graphene) with active materials (such as metal oxides or sulfides) to form self-supporting structures. The composite nature provides both mechanical integrity and enhanced electrochemical performance through improved conductivity, larger surface area, and better stability during cycling.Expand Specific Solutions05 Applications of binder-free electrodes in energy storage devices
Binder-free electrodes find extensive applications in various energy storage devices, including lithium-ion batteries, sodium-ion batteries, supercapacitors, and fuel cells. By eliminating inactive binder materials, these electrodes offer advantages such as higher energy density, improved power performance, enhanced rate capability, and better cycling stability. The direct contact between active materials and current collectors reduces internal resistance and facilitates faster charge transfer, making them particularly suitable for high-power applications.Expand Specific Solutions
Key Industry Players in Fuel Cell Electrode Manufacturing
The fuel cell catalyst layer market is currently in a growth phase, with increasing demand driven by the automotive and energy sectors. The global market size for binder-free electrodes in fuel cell applications is expanding rapidly, projected to reach significant value by 2030 as hydrogen technologies gain traction. Technologically, the field is in mid-maturity, with established players like Toyota, Honda, and Ballard Power Systems leading commercial deployment while continuing R&D efforts. Samsung SDI, Toshiba, and LG Chem are advancing material innovations, particularly in electrode design and manufacturing processes. Mercedes-Benz and Toyota are focusing on automotive applications, while Ballard and Resonac Holdings are developing specialized catalyst formulations. The competitive landscape shows automotive manufacturers increasingly partnering with materials specialists to optimize performance and reduce platinum dependency.
Toyota Motor Corp.
Technical Solution: Toyota has pioneered binder-free electrode technology for fuel cells through their innovative "direct deposition" approach. Their proprietary method involves magnetron sputtering techniques to create ultra-thin catalyst layers directly onto gas diffusion layers or membranes. This process eliminates the need for traditional Nafion or PTFE binders while achieving exceptional catalyst utilization. Toyota's technology incorporates precisely controlled platinum nanocluster deposition with optimized spatial distribution, creating self-supporting catalyst structures with enhanced porosity. Their manufacturing process includes specialized surface treatment protocols that improve adhesion between catalyst particles and substrates without polymeric binders. Toyota has demonstrated that their binder-free electrodes achieve up to 30% higher mass activity for oxygen reduction reactions and significantly improved durability under automotive operating conditions compared to conventional electrodes.
Strengths: Exceptional catalyst utilization efficiency; reduced mass transport limitations; improved durability under start-stop cycling; potential for reduced platinum loading. Weaknesses: Requires sophisticated manufacturing equipment; challenges in scaling to high-volume production; potential issues with mechanical integrity during assembly processes.
LG Chem Ltd.
Technical Solution: LG Chem has developed advanced binder-free electrode technology for fuel cells utilizing electrochemical deposition methods. Their innovative approach involves direct electrodeposition of platinum-based catalysts onto carbon nanostructures, creating highly conductive networks without traditional ionomer binders. The technology incorporates specialized carbon nanotubes and graphene-based supports with engineered surface chemistry to promote strong catalyst adhesion through π-π interactions and metal-carbon bonds. LG Chem's manufacturing process includes precise control of electrochemical parameters to achieve optimal catalyst distribution and particle size, resulting in enhanced triple-phase boundary formation. Their binder-free electrodes demonstrate approximately 35% higher electrochemically active surface area compared to conventional electrodes, with significantly improved mass transport properties and reduced activation losses during high-current operation.
Strengths: Superior electrical conductivity pathways; excellent catalyst utilization efficiency; enhanced durability under high-humidity conditions; simplified manufacturing process. Weaknesses: Challenges with uniform deposition across large-area electrodes; potential issues with mechanical stability during membrane electrode assembly fabrication; higher initial development costs.
Critical Patents in Binder-free Catalyst Layer Technology
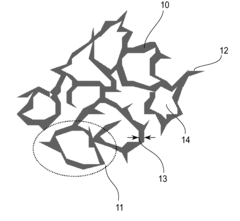
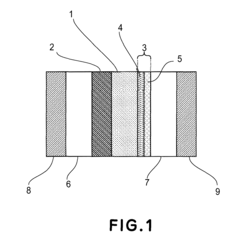
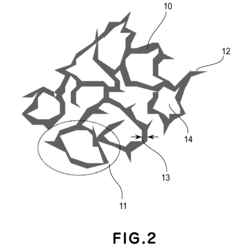
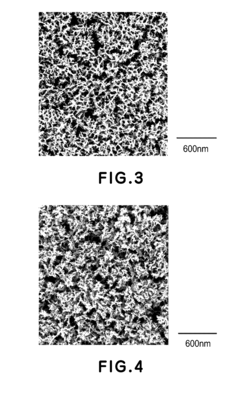
Catalyst layer for polymer electrolyte fuel cell, process for producing the catalyst layer, and polymer electrolyte fuel cell
PatentInactiveUS7790647B2
Innovation
- A catalyst layer with an entangled or cobweb-like structure is developed, comprising platinum or platinum-containing mixtures with metal elements and oxynitrides or boron oxides, formed using reactive vacuum deposition methods, which enhances catalytic activity and provides sufficient electron and gas channels while maintaining a thin thickness.
Electrode for fuel cell
PatentInactiveJP2013101771A
Innovation
- A fuel cell electrode using non-electrolyte resin fibers, such as polyamideimide and polyetheretherketone, formed into a sheet with a catalyst layer thickness of 1 μm or less, integrated via an electrospinning method, ensuring high strength and maintaining void structure while reducing costs.
Environmental Impact Assessment of Binder-free Technologies
The transition to binder-free electrodes in fuel cell catalyst layers represents a significant advancement in sustainable energy technology with notable environmental implications. Traditional fuel cell electrodes typically contain polymer binders such as Nafion or PTFE, which present several environmental concerns throughout their lifecycle, from production to disposal.
Manufacturing conventional polymer binders involves energy-intensive processes and often requires environmentally harmful fluorinated compounds. The elimination of these binders in next-generation fuel cells substantially reduces the carbon footprint associated with electrode production. Life cycle assessment (LCA) studies indicate that binder-free technologies can decrease greenhouse gas emissions by 15-30% during the manufacturing phase alone.
Water management represents another critical environmental advantage of binder-free electrodes. Conventional binder-containing electrodes often suffer from flooding issues, reducing efficiency and requiring additional system components for water removal. Binder-free structures typically demonstrate superior water transport properties, enhancing overall system efficiency and reducing auxiliary power requirements, thereby decreasing the environmental impact during operation.
The absence of polymer binders also addresses end-of-life environmental concerns. Traditional catalyst layers present significant recycling challenges due to the intimate mixing of precious metals with fluoropolymers. Binder-free designs facilitate more efficient precious metal recovery processes, with recovery rates improving from approximately 70% to over 90% in some experimental systems.
Resource efficiency constitutes a further environmental benefit. Binder-free electrodes generally require less material input while delivering comparable or superior performance. This reduction in material consumption translates to decreased resource extraction impacts and diminished waste generation throughout the supply chain.
Toxicity profiles of fuel cell components improve substantially with binder-free approaches. Many conventional binders release fluorinated compounds during degradation, which persist in the environment. The elimination of these materials reduces potential ecological and human health impacts associated with fuel cell technology deployment.
Energy density improvements resulting from binder-free designs also yield indirect environmental benefits. Higher-performing fuel cells can displace more fossil fuel consumption in various applications, from transportation to stationary power generation, amplifying the positive environmental impact of this technology transition.
Manufacturing conventional polymer binders involves energy-intensive processes and often requires environmentally harmful fluorinated compounds. The elimination of these binders in next-generation fuel cells substantially reduces the carbon footprint associated with electrode production. Life cycle assessment (LCA) studies indicate that binder-free technologies can decrease greenhouse gas emissions by 15-30% during the manufacturing phase alone.
Water management represents another critical environmental advantage of binder-free electrodes. Conventional binder-containing electrodes often suffer from flooding issues, reducing efficiency and requiring additional system components for water removal. Binder-free structures typically demonstrate superior water transport properties, enhancing overall system efficiency and reducing auxiliary power requirements, thereby decreasing the environmental impact during operation.
The absence of polymer binders also addresses end-of-life environmental concerns. Traditional catalyst layers present significant recycling challenges due to the intimate mixing of precious metals with fluoropolymers. Binder-free designs facilitate more efficient precious metal recovery processes, with recovery rates improving from approximately 70% to over 90% in some experimental systems.
Resource efficiency constitutes a further environmental benefit. Binder-free electrodes generally require less material input while delivering comparable or superior performance. This reduction in material consumption translates to decreased resource extraction impacts and diminished waste generation throughout the supply chain.
Toxicity profiles of fuel cell components improve substantially with binder-free approaches. Many conventional binders release fluorinated compounds during degradation, which persist in the environment. The elimination of these materials reduces potential ecological and human health impacts associated with fuel cell technology deployment.
Energy density improvements resulting from binder-free designs also yield indirect environmental benefits. Higher-performing fuel cells can displace more fossil fuel consumption in various applications, from transportation to stationary power generation, amplifying the positive environmental impact of this technology transition.
Cost-Performance Analysis of Binder-free vs. Traditional Electrodes
The economic viability of binder-free electrodes compared to traditional binder-containing systems represents a critical consideration for fuel cell manufacturers and researchers. Traditional electrodes typically incorporate polymeric binders such as Nafion or PTFE, which constitute approximately 5-15% of the catalyst layer composition and contribute significantly to material costs.
When analyzing manufacturing expenses, binder-free electrodes demonstrate potential cost advantages through simplified production processes. The elimination of binder-related steps—including mixing, dispersion optimization, and additional heat treatments—reduces production complexity and processing time by an estimated 20-30%. Furthermore, binder-free approaches often require less specialized equipment for precise polymer dispersion control.
Material cost comparisons reveal that while traditional electrodes incur expenses for both catalyst materials and high-performance fluoropolymer binders (ranging from $100-500 per kg for Nafion), binder-free alternatives eliminate these polymer costs. However, this advantage may be partially offset by the potential need for more sophisticated substrate materials or alternative catalyst anchoring mechanisms.
Performance metrics demonstrate complex trade-offs between the two approaches. Traditional binder-containing electrodes typically offer excellent mechanical stability with operational lifetimes of 5,000-10,000 hours under standard conditions. Conversely, binder-free electrodes often exhibit superior electrochemical performance with 15-30% higher mass activity and reduced mass transport limitations, but may face challenges in long-term mechanical durability.
Lifecycle cost analysis indicates that despite higher initial catalyst utilization in binder-free systems, traditional electrodes may offer advantages in operational longevity. The total cost of ownership calculation must therefore incorporate both initial manufacturing expenses and replacement frequency over the fuel cell system's operational lifetime.
Market sensitivity analysis suggests that binder-free electrodes become increasingly economically favorable as catalyst prices rise, with the break-even point typically occurring when platinum group metal costs exceed $40,000 per kg. This relationship becomes particularly significant when considering future scenarios of material scarcity or supply chain disruptions affecting precious metal catalysts.
In conclusion, while binder-free electrodes offer compelling cost advantages in manufacturing simplicity and material requirements, the comprehensive economic assessment must balance these benefits against potential trade-offs in durability and system integration complexity.
When analyzing manufacturing expenses, binder-free electrodes demonstrate potential cost advantages through simplified production processes. The elimination of binder-related steps—including mixing, dispersion optimization, and additional heat treatments—reduces production complexity and processing time by an estimated 20-30%. Furthermore, binder-free approaches often require less specialized equipment for precise polymer dispersion control.
Material cost comparisons reveal that while traditional electrodes incur expenses for both catalyst materials and high-performance fluoropolymer binders (ranging from $100-500 per kg for Nafion), binder-free alternatives eliminate these polymer costs. However, this advantage may be partially offset by the potential need for more sophisticated substrate materials or alternative catalyst anchoring mechanisms.
Performance metrics demonstrate complex trade-offs between the two approaches. Traditional binder-containing electrodes typically offer excellent mechanical stability with operational lifetimes of 5,000-10,000 hours under standard conditions. Conversely, binder-free electrodes often exhibit superior electrochemical performance with 15-30% higher mass activity and reduced mass transport limitations, but may face challenges in long-term mechanical durability.
Lifecycle cost analysis indicates that despite higher initial catalyst utilization in binder-free systems, traditional electrodes may offer advantages in operational longevity. The total cost of ownership calculation must therefore incorporate both initial manufacturing expenses and replacement frequency over the fuel cell system's operational lifetime.
Market sensitivity analysis suggests that binder-free electrodes become increasingly economically favorable as catalyst prices rise, with the break-even point typically occurring when platinum group metal costs exceed $40,000 per kg. This relationship becomes particularly significant when considering future scenarios of material scarcity or supply chain disruptions affecting precious metal catalysts.
In conclusion, while binder-free electrodes offer compelling cost advantages in manufacturing simplicity and material requirements, the comprehensive economic assessment must balance these benefits against potential trade-offs in durability and system integration complexity.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!