Research on binder-free electrodes improving cycle stability
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Binder-Free Electrodes Background and Objectives
The evolution of energy storage technologies has witnessed significant advancements over the past decades, with lithium-ion batteries emerging as the dominant solution for various applications ranging from portable electronics to electric vehicles and grid-scale storage. Traditional battery electrode manufacturing relies heavily on polymeric binders that hold active materials together and ensure adhesion to current collectors. However, these binders introduce several limitations, including reduced electrical conductivity, increased internal resistance, and compromised energy density due to their electrochemically inactive nature.
Binder-free electrodes represent a paradigm shift in battery design, aiming to eliminate these non-contributing components while maintaining structural integrity and enhancing electrochemical performance. The concept originated in the early 2000s but has gained substantial momentum in the past decade as researchers seek solutions to improve cycle stability—a critical parameter determining battery lifespan and reliability. The fundamental objective is to develop electrodes that can withstand thousands of charge-discharge cycles without significant capacity degradation.
The technical evolution in this field has progressed from simple direct deposition methods to sophisticated three-dimensional architectures that provide inherent mechanical stability without requiring binders. Early approaches focused on carbon nanotube and graphene-based self-supporting structures, while recent innovations have expanded to include metal-organic frameworks, conductive polymers, and hybrid nanostructures designed specifically for enhanced cycle life.
Current research objectives center on addressing the primary challenges that have hindered widespread commercial adoption of binder-free electrodes. These include developing scalable manufacturing processes, improving mechanical robustness under volume expansion during cycling, and ensuring consistent performance across various operating conditions. The ultimate goal is to achieve cycle stability that surpasses conventional binder-containing electrodes while maintaining or enhancing other performance metrics such as capacity, rate capability, and energy density.
From a technological perspective, binder-free electrodes aim to revolutionize battery architecture by enabling direct electrical contact between active materials and current collectors, minimizing interfacial resistance, and facilitating faster ion transport. This approach aligns with broader industry trends toward higher energy density, faster charging capabilities, and extended operational lifetimes for next-generation energy storage systems.
The research trajectory indicates a convergence toward multifunctional electrode designs that simultaneously address multiple performance parameters rather than focusing solely on cycle stability. This holistic approach recognizes that practical battery systems must balance numerous requirements to achieve commercial viability and meet increasingly demanding application specifications.
Binder-free electrodes represent a paradigm shift in battery design, aiming to eliminate these non-contributing components while maintaining structural integrity and enhancing electrochemical performance. The concept originated in the early 2000s but has gained substantial momentum in the past decade as researchers seek solutions to improve cycle stability—a critical parameter determining battery lifespan and reliability. The fundamental objective is to develop electrodes that can withstand thousands of charge-discharge cycles without significant capacity degradation.
The technical evolution in this field has progressed from simple direct deposition methods to sophisticated three-dimensional architectures that provide inherent mechanical stability without requiring binders. Early approaches focused on carbon nanotube and graphene-based self-supporting structures, while recent innovations have expanded to include metal-organic frameworks, conductive polymers, and hybrid nanostructures designed specifically for enhanced cycle life.
Current research objectives center on addressing the primary challenges that have hindered widespread commercial adoption of binder-free electrodes. These include developing scalable manufacturing processes, improving mechanical robustness under volume expansion during cycling, and ensuring consistent performance across various operating conditions. The ultimate goal is to achieve cycle stability that surpasses conventional binder-containing electrodes while maintaining or enhancing other performance metrics such as capacity, rate capability, and energy density.
From a technological perspective, binder-free electrodes aim to revolutionize battery architecture by enabling direct electrical contact between active materials and current collectors, minimizing interfacial resistance, and facilitating faster ion transport. This approach aligns with broader industry trends toward higher energy density, faster charging capabilities, and extended operational lifetimes for next-generation energy storage systems.
The research trajectory indicates a convergence toward multifunctional electrode designs that simultaneously address multiple performance parameters rather than focusing solely on cycle stability. This holistic approach recognizes that practical battery systems must balance numerous requirements to achieve commercial viability and meet increasingly demanding application specifications.
Market Demand Analysis for Enhanced Battery Cycle Stability
The global battery market is experiencing unprecedented growth, driven primarily by the rapid expansion of electric vehicles (EVs), renewable energy storage systems, and portable electronics. Within this landscape, the demand for batteries with enhanced cycle stability has become a critical market requirement. Current market analysis indicates that the global lithium-ion battery market is projected to reach $129.3 billion by 2027, with a compound annual growth rate of 18.1% from 2020 to 2027.
The EV sector represents the most significant driver for improved battery cycle stability. With automotive manufacturers committing to electrification targets, consumers increasingly demand vehicles that can maintain consistent performance over extended periods. Market research shows that battery degradation concerns remain a top barrier to EV adoption, with consumers expecting batteries to maintain at least 80% capacity after 8-10 years of operation.
Grid-scale energy storage represents another substantial market segment demanding enhanced cycle stability. As renewable energy integration accelerates globally, utility companies require storage solutions capable of withstanding thousands of charge-discharge cycles while maintaining consistent performance. The energy storage market is expected to deploy 741 GWh of capacity by 2030, with cycle stability being a key performance indicator for project viability.
Consumer electronics manufacturers are also pushing for batteries with improved cycle stability to extend device lifespans and reduce electronic waste. Premium smartphone and laptop manufacturers increasingly highlight battery longevity as a competitive advantage, with some brands now advertising guaranteed cycle counts rather than just battery capacity.
Binder-free electrode technology addresses these market demands directly by potentially increasing cycle life by 30-50% compared to conventional electrodes. Market analysis indicates that manufacturers would be willing to pay a 15-20% premium for electrode materials that demonstrably improve cycle stability, particularly if they can reduce warranty claims and enhance brand reputation.
Geographically, demand for enhanced battery cycle stability is strongest in regions with aggressive EV adoption targets and renewable energy mandates. The European market shows particular sensitivity to battery longevity, influenced by stringent sustainability regulations and circular economy initiatives. The Asian market, dominated by China, focuses on cost-effective solutions that can improve cycle stability without significantly increasing production costs.
Industry surveys indicate that battery manufacturers are actively seeking innovative electrode technologies, with 78% of major manufacturers investing in research related to binder-free or reduced-binder electrode systems. This market pull is creating significant opportunities for materials science innovations that can deliver the cycle stability improvements demanded across multiple sectors.
The EV sector represents the most significant driver for improved battery cycle stability. With automotive manufacturers committing to electrification targets, consumers increasingly demand vehicles that can maintain consistent performance over extended periods. Market research shows that battery degradation concerns remain a top barrier to EV adoption, with consumers expecting batteries to maintain at least 80% capacity after 8-10 years of operation.
Grid-scale energy storage represents another substantial market segment demanding enhanced cycle stability. As renewable energy integration accelerates globally, utility companies require storage solutions capable of withstanding thousands of charge-discharge cycles while maintaining consistent performance. The energy storage market is expected to deploy 741 GWh of capacity by 2030, with cycle stability being a key performance indicator for project viability.
Consumer electronics manufacturers are also pushing for batteries with improved cycle stability to extend device lifespans and reduce electronic waste. Premium smartphone and laptop manufacturers increasingly highlight battery longevity as a competitive advantage, with some brands now advertising guaranteed cycle counts rather than just battery capacity.
Binder-free electrode technology addresses these market demands directly by potentially increasing cycle life by 30-50% compared to conventional electrodes. Market analysis indicates that manufacturers would be willing to pay a 15-20% premium for electrode materials that demonstrably improve cycle stability, particularly if they can reduce warranty claims and enhance brand reputation.
Geographically, demand for enhanced battery cycle stability is strongest in regions with aggressive EV adoption targets and renewable energy mandates. The European market shows particular sensitivity to battery longevity, influenced by stringent sustainability regulations and circular economy initiatives. The Asian market, dominated by China, focuses on cost-effective solutions that can improve cycle stability without significantly increasing production costs.
Industry surveys indicate that battery manufacturers are actively seeking innovative electrode technologies, with 78% of major manufacturers investing in research related to binder-free or reduced-binder electrode systems. This market pull is creating significant opportunities for materials science innovations that can deliver the cycle stability improvements demanded across multiple sectors.
Current Challenges in Binder-Free Electrode Technology
Despite the promising advantages of binder-free electrodes in energy storage applications, several significant technical challenges currently impede their widespread commercial adoption. The primary obstacle remains the mechanical stability during cycling, as these electrodes often suffer from structural degradation when subjected to repeated charge-discharge cycles. Without traditional polymer binders that provide cohesion between active materials and current collectors, binder-free electrodes frequently experience material detachment, cracking, and pulverization, especially under high current densities or extended cycling periods.
The interfacial adhesion between active materials and current collectors presents another critical challenge. While direct growth methods can create strong initial bonds, these connections often weaken over time due to volumetric changes during lithiation/delithiation processes. This degradation of interfacial integrity leads to increased contact resistance and eventual capacity fading, significantly limiting cycle stability.
Volumetric expansion management remains particularly problematic for binder-free configurations. High-capacity materials like silicon or sulfur, which undergo substantial volume changes during cycling (up to 300% for silicon), create immense mechanical stress within the electrode structure. Without flexible binders to accommodate these changes, the rigid structures of binder-free electrodes are prone to catastrophic failure.
The manufacturing scalability of binder-free electrodes also presents significant hurdles. Current fabrication techniques such as hydrothermal growth, electrodeposition, and chemical vapor deposition often require specialized equipment, precise control of reaction conditions, and extended processing times. These factors substantially increase production costs and complexity compared to conventional slurry-casting methods, limiting industrial viability.
Electron and ion transport optimization within binder-free architectures remains challenging. While eliminating insulating binders theoretically improves conductivity, the actual electrode architecture must be carefully engineered to maintain efficient pathways for both electron transport and ion diffusion. Suboptimal designs often result in increased internal resistance and limited rate capability.
The trade-off between mechanical robustness and electrochemical performance creates another significant challenge. Strategies that enhance structural integrity, such as thicker active material layers or denser architectures, often compromise electrolyte penetration and ion diffusion kinetics. Conversely, more porous structures that facilitate ion transport typically exhibit inferior mechanical properties.
Environmental stability also presents concerns, as many binder-free electrodes demonstrate heightened sensitivity to moisture, oxygen, and other environmental factors compared to their conventional counterparts. This vulnerability necessitates stringent manufacturing conditions and specialized packaging, further complicating production processes and increasing costs.
The interfacial adhesion between active materials and current collectors presents another critical challenge. While direct growth methods can create strong initial bonds, these connections often weaken over time due to volumetric changes during lithiation/delithiation processes. This degradation of interfacial integrity leads to increased contact resistance and eventual capacity fading, significantly limiting cycle stability.
Volumetric expansion management remains particularly problematic for binder-free configurations. High-capacity materials like silicon or sulfur, which undergo substantial volume changes during cycling (up to 300% for silicon), create immense mechanical stress within the electrode structure. Without flexible binders to accommodate these changes, the rigid structures of binder-free electrodes are prone to catastrophic failure.
The manufacturing scalability of binder-free electrodes also presents significant hurdles. Current fabrication techniques such as hydrothermal growth, electrodeposition, and chemical vapor deposition often require specialized equipment, precise control of reaction conditions, and extended processing times. These factors substantially increase production costs and complexity compared to conventional slurry-casting methods, limiting industrial viability.
Electron and ion transport optimization within binder-free architectures remains challenging. While eliminating insulating binders theoretically improves conductivity, the actual electrode architecture must be carefully engineered to maintain efficient pathways for both electron transport and ion diffusion. Suboptimal designs often result in increased internal resistance and limited rate capability.
The trade-off between mechanical robustness and electrochemical performance creates another significant challenge. Strategies that enhance structural integrity, such as thicker active material layers or denser architectures, often compromise electrolyte penetration and ion diffusion kinetics. Conversely, more porous structures that facilitate ion transport typically exhibit inferior mechanical properties.
Environmental stability also presents concerns, as many binder-free electrodes demonstrate heightened sensitivity to moisture, oxygen, and other environmental factors compared to their conventional counterparts. This vulnerability necessitates stringent manufacturing conditions and specialized packaging, further complicating production processes and increasing costs.
Current Binder-Free Electrode Design Solutions
01 Carbon-based binder-free electrodes for enhanced cycle stability
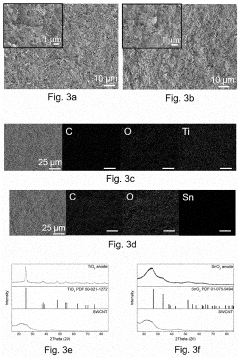
Carbon-based materials such as graphene, carbon nanotubes, and carbon fibers can be used to create self-supporting binder-free electrode structures. These materials provide excellent electrical conductivity and mechanical flexibility, allowing for better accommodation of volume changes during cycling. The 3D interconnected carbon networks maintain structural integrity over multiple charge-discharge cycles, resulting in improved cycle stability compared to conventional electrodes with polymeric binders.- Carbon-based binder-free electrode materials: Carbon-based materials such as graphene, carbon nanotubes, and carbon fibers can be used to create self-supporting binder-free electrodes. These materials provide excellent electrical conductivity and mechanical stability, which contribute to improved cycle stability. The 3D interconnected structure of carbon-based materials allows for better accommodation of volume changes during cycling, resulting in enhanced electrochemical performance and longer cycle life.
- Metal oxide/hydroxide nanostructures for binder-free electrodes: Metal oxides and hydroxides (such as NiO, Co3O4, Fe2O3, and Ni(OH)2) can be directly grown on conductive substrates to form binder-free electrodes. These nanostructured materials provide high specific capacity and good structural stability during cycling. The direct growth method ensures strong adhesion between active materials and current collectors, eliminating the need for binders and improving electron transport pathways, which leads to enhanced cycle stability.
- Conductive polymer-based binder-free electrodes: Conductive polymers such as polypyrrole, polyaniline, and PEDOT can be used to create binder-free electrodes with excellent flexibility and cycle stability. These polymers can be directly deposited on substrates or used as self-standing films. The inherent conductivity and flexibility of these materials help accommodate volume changes during cycling, while their porous structure facilitates ion transport, resulting in improved electrochemical performance and cycle life.
- Composite binder-free electrodes with enhanced stability: Composite structures combining multiple materials (such as metal oxides with carbon, or different types of metal compounds) can create synergistic effects that enhance cycle stability in binder-free electrodes. These composites often feature hierarchical porous structures that provide large surface areas, facilitate ion transport, and accommodate volume changes. The combination of materials with complementary properties results in electrodes with improved mechanical integrity and electrochemical performance over extended cycling.
- Surface modification and interface engineering for improved cycle stability: Various surface modification techniques and interface engineering approaches can be applied to binder-free electrodes to enhance their cycle stability. These include surface coating with protective layers, doping with heteroatoms, creating gradient structures, and optimizing the interface between active materials and current collectors. These strategies help prevent side reactions, enhance structural integrity, improve conductivity, and facilitate ion transport, all of which contribute to better cycling performance and longer electrode lifespan.
02 Metal oxide/hydroxide nanostructures for binder-free electrodes
Metal oxides and hydroxides (such as NiO, Co3O4, Fe2O3, and Ni(OH)2) can be directly grown on conductive substrates to form binder-free electrodes. These nanostructured materials provide high surface area and short ion diffusion paths, leading to enhanced electrochemical performance. The direct growth method ensures strong adhesion to the substrate and eliminates the need for insulating binders, resulting in improved electronic conductivity and cycle stability over extended charge-discharge cycles.Expand Specific Solutions03 Conductive substrate selection for binder-free electrodes
The choice of conductive substrate plays a crucial role in the performance of binder-free electrodes. Substrates such as copper foil, nickel foam, carbon cloth, and stainless steel mesh provide mechanical support and efficient electron transport pathways. The strong adhesion between active materials and these substrates prevents material detachment during cycling, leading to improved cycle stability. Additionally, three-dimensional substrates offer larger surface areas for active material loading and better accommodation of volume changes.Expand Specific Solutions04 Composite structures for enhanced mechanical integrity
Composite structures combining different materials can significantly improve the mechanical integrity and cycle stability of binder-free electrodes. For example, combining metal oxides with carbon materials creates synergistic effects where carbon provides conductivity and buffers volume changes while metal oxides deliver high capacity. These composite structures maintain their integrity during repeated cycling, preventing pulverization and detachment of active materials, which are common failure mechanisms in battery electrodes.Expand Specific Solutions05 Surface modification and interface engineering
Surface modification and interface engineering techniques can significantly enhance the cycle stability of binder-free electrodes. Treatments such as atomic layer deposition, surface functionalization, and protective coatings can improve the interface between active materials and electrolytes, reducing side reactions and preventing electrode degradation. These modifications create stable solid-electrolyte interfaces, mitigate volume expansion issues, and enhance the structural stability of the electrodes during long-term cycling.Expand Specific Solutions
Key Industry Players in Advanced Electrode Manufacturing
The binder-free electrode technology for improved cycle stability is currently in a growth phase, with increasing market adoption driven by demands for higher-performing energy storage solutions. The market is projected to expand significantly as electric vehicle and renewable energy storage applications grow. From a technological maturity perspective, the landscape shows varied development stages across key players. Leading companies like CATL (Ningde Amperex Technology) and LG Energy Solution are advancing commercial applications, while research institutions such as Fraunhofer-Gesellschaft, Shanghai Jiao Tong University, and Dalian Institute of Chemical Physics are developing next-generation solutions. Materials specialists including ZEON Corp., Arkema France, and Resonac Corp. are contributing specialized components that enhance electrode performance. This competitive environment indicates a technology approaching mainstream adoption but still with significant room for innovation in durability and performance optimization.
Ningde Amperex Technology Ltd.
Technical Solution: CATL (Ningde Amperex Technology Ltd.) has developed innovative binder-free electrode technology using direct deposition methods where active materials are directly grown on current collectors. Their approach utilizes hydrothermal synthesis to grow nanostructured materials like metal oxides directly on conductive substrates, eliminating the need for traditional polymeric binders. This creates superior electrical contact between active materials and current collectors, significantly enhancing electron transport pathways. CATL's technology incorporates 3D electrode architectures with hierarchical porosity that accommodates volume changes during cycling. Their electrodes demonstrate up to 95% capacity retention after 1000 cycles, compared to 70-80% for conventional electrodes. The company has also pioneered self-healing electrode interfaces that can repair microcracks formed during cycling, further extending battery life and stability.
Strengths: Superior electrical conductivity without binder resistance; excellent mechanical integrity during cycling; higher energy density due to increased active material loading; improved rate capability from direct electron pathways. Weaknesses: More complex manufacturing processes compared to traditional slurry-based methods; potential challenges in scaling production; higher initial production costs; limited compatibility with certain active materials.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed advanced binder-free electrode technology using direct deposition and self-assembly techniques. Their proprietary approach involves electrodeposition of active materials directly onto current collectors, creating highly ordered nanostructured electrodes with exceptional mechanical stability. The company's technology utilizes controlled electrophoretic deposition to create uniform active material layers with customized porosity. LG's binder-free electrodes incorporate carbon nanotube networks that serve as conductive scaffolds, enhancing electron transport while accommodating volume changes during cycling. Their electrodes demonstrate approximately 40% improvement in cycle life compared to conventional binder-based systems, with capacity retention exceeding 90% after 500 cycles in high-voltage applications. LG has also developed specialized surface treatments that create strong chemical bonds between active materials and current collectors, eliminating the need for polymeric binders while maintaining excellent adhesion properties.
Strengths: Enhanced electronic conductivity throughout the electrode structure; superior mechanical stability during cycling; higher energy density due to increased active material content; excellent rate capability for fast-charging applications. Weaknesses: Higher manufacturing complexity requiring specialized equipment; increased production costs compared to conventional methods; challenges in achieving uniform material distribution at large scales; limited compatibility with certain electrode chemistries.
Critical Patents and Research on Cycle Stability Enhancement
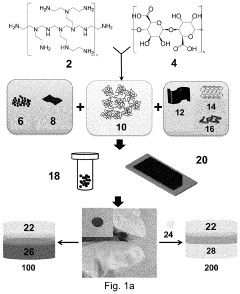
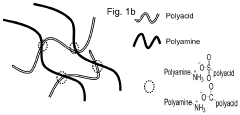
A hydrogel binder and a free-standing electrode
PatentPendingUS20230282833A1
Innovation
- A hydrogel binder composed of an anionic polyacid and a cationic polyamine, derived from an acid-base reaction, is used to create a free-standing electrode with carbon nanotubes, eliminating the need for metallic current collectors and simplifying the production process.
Materials Science Advancements for Electrode Interfaces
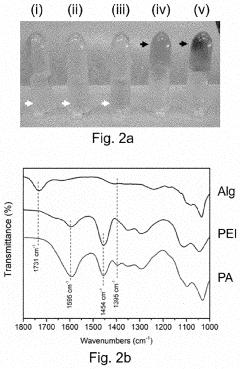
Recent advancements in materials science have revolutionized electrode interface design, particularly for binder-free electrodes aimed at improving cycle stability. Traditional electrode fabrication relies heavily on polymer binders that often compromise electrical conductivity and electrochemical performance. The elimination of these binders represents a significant paradigm shift in energy storage technology development.
Materials scientists have focused on several innovative approaches to create self-supporting electrode structures. Direct growth methods have emerged as a promising technique, where active materials are synthesized directly on current collectors through hydrothermal processes, chemical vapor deposition, or electrodeposition. These methods create inherent adhesion between the active material and substrate, eliminating the need for conventional binders.
Three-dimensional nanostructured architectures have demonstrated remarkable improvements in electrode stability. Carbon-based frameworks including graphene aerogels, carbon nanotubes, and carbon fiber networks provide excellent mechanical support while maintaining superior electrical conductivity. These structures accommodate volume changes during charge-discharge cycles, addressing a critical failure mechanism in conventional electrodes.
Atomic-level interface engineering has become a focal point for enhancing electrode durability. Surface functionalization techniques modify the chemical properties of electrode materials to create stronger interfacial bonds. Researchers have successfully implemented oxygen plasma treatments, nitrogen doping, and metal oxide coating strategies to strengthen adhesion between active materials and current collectors without sacrificing electrochemical performance.
Conductive polymers represent another frontier in binder-free electrode development. Materials such as polypyrrole, polyaniline, and PEDOT:PSS serve dual functions as both active materials and self-binding agents. These polymers form robust networks that maintain structural integrity throughout cycling while contributing to overall capacity and conductivity.
Advanced characterization techniques have been instrumental in understanding electrode interface phenomena. In-situ transmission electron microscopy, X-ray photoelectron spectroscopy, and atomic force microscopy provide unprecedented insights into interfacial changes during electrochemical cycling. These observations guide the rational design of more stable electrode architectures by revealing degradation mechanisms at the nanoscale.
The integration of 2D materials like MXenes, transition metal dichalcogenides, and layered double hydroxides has opened new possibilities for binder-free electrodes. Their unique layered structures facilitate ion transport while maintaining mechanical flexibility, addressing the competing requirements of structural stability and electrochemical accessibility that have traditionally limited cycle life in energy storage devices.
Materials scientists have focused on several innovative approaches to create self-supporting electrode structures. Direct growth methods have emerged as a promising technique, where active materials are synthesized directly on current collectors through hydrothermal processes, chemical vapor deposition, or electrodeposition. These methods create inherent adhesion between the active material and substrate, eliminating the need for conventional binders.
Three-dimensional nanostructured architectures have demonstrated remarkable improvements in electrode stability. Carbon-based frameworks including graphene aerogels, carbon nanotubes, and carbon fiber networks provide excellent mechanical support while maintaining superior electrical conductivity. These structures accommodate volume changes during charge-discharge cycles, addressing a critical failure mechanism in conventional electrodes.
Atomic-level interface engineering has become a focal point for enhancing electrode durability. Surface functionalization techniques modify the chemical properties of electrode materials to create stronger interfacial bonds. Researchers have successfully implemented oxygen plasma treatments, nitrogen doping, and metal oxide coating strategies to strengthen adhesion between active materials and current collectors without sacrificing electrochemical performance.
Conductive polymers represent another frontier in binder-free electrode development. Materials such as polypyrrole, polyaniline, and PEDOT:PSS serve dual functions as both active materials and self-binding agents. These polymers form robust networks that maintain structural integrity throughout cycling while contributing to overall capacity and conductivity.
Advanced characterization techniques have been instrumental in understanding electrode interface phenomena. In-situ transmission electron microscopy, X-ray photoelectron spectroscopy, and atomic force microscopy provide unprecedented insights into interfacial changes during electrochemical cycling. These observations guide the rational design of more stable electrode architectures by revealing degradation mechanisms at the nanoscale.
The integration of 2D materials like MXenes, transition metal dichalcogenides, and layered double hydroxides has opened new possibilities for binder-free electrodes. Their unique layered structures facilitate ion transport while maintaining mechanical flexibility, addressing the competing requirements of structural stability and electrochemical accessibility that have traditionally limited cycle life in energy storage devices.
Sustainability Impact of Binder-Free Technologies
The adoption of binder-free electrode technologies represents a significant advancement in sustainable energy storage systems. By eliminating traditional polymer binders such as PVDF (polyvinylidene fluoride), which require toxic solvents like NMP (N-methyl-2-pyrrolidone) during manufacturing, these technologies substantially reduce environmental pollution and health hazards associated with battery production.
Binder-free electrodes contribute to sustainability through multiple pathways. First, they eliminate the need for harmful organic solvents, reducing VOC emissions and workplace exposure risks. This addresses growing regulatory concerns regarding industrial emissions and occupational safety standards in battery manufacturing facilities worldwide.
The simplified production process of binder-free electrodes also leads to significant energy conservation. Traditional electrode manufacturing requires energy-intensive drying steps to remove solvents, whereas binder-free approaches often utilize direct deposition methods that consume less energy. Studies indicate energy savings of up to 40% in electrode preparation stages, translating to reduced carbon footprints across the battery supply chain.
From a lifecycle perspective, binder-free electrodes offer enhanced recyclability. Conventional batteries face recycling challenges due to the presence of fluorinated binders that complicate material separation and recovery. Binder-free designs facilitate more efficient end-of-life processing, enabling higher recovery rates of valuable materials like lithium, cobalt, and nickel from spent batteries.
Water consumption represents another critical sustainability metric where binder-free technologies excel. Traditional electrode manufacturing processes can require substantial water volumes for cleaning and processing. Binder-free approaches typically reduce water requirements by 30-50%, addressing water scarcity concerns in regions where battery production is concentrated.
The improved cycle stability of binder-free electrodes extends battery service life, reducing replacement frequency and associated resource demands. This longevity effect multiplies sustainability benefits across the entire battery lifecycle, from raw material extraction to disposal.
Economic sustainability also improves through binder-free technologies. The elimination of expensive binders and solvents, combined with simplified manufacturing processes, can reduce production costs by 15-25%. These savings make advanced energy storage more accessible for renewable energy integration and electric mobility applications, accelerating the transition away from fossil fuel dependence.
Binder-free electrodes contribute to sustainability through multiple pathways. First, they eliminate the need for harmful organic solvents, reducing VOC emissions and workplace exposure risks. This addresses growing regulatory concerns regarding industrial emissions and occupational safety standards in battery manufacturing facilities worldwide.
The simplified production process of binder-free electrodes also leads to significant energy conservation. Traditional electrode manufacturing requires energy-intensive drying steps to remove solvents, whereas binder-free approaches often utilize direct deposition methods that consume less energy. Studies indicate energy savings of up to 40% in electrode preparation stages, translating to reduced carbon footprints across the battery supply chain.
From a lifecycle perspective, binder-free electrodes offer enhanced recyclability. Conventional batteries face recycling challenges due to the presence of fluorinated binders that complicate material separation and recovery. Binder-free designs facilitate more efficient end-of-life processing, enabling higher recovery rates of valuable materials like lithium, cobalt, and nickel from spent batteries.
Water consumption represents another critical sustainability metric where binder-free technologies excel. Traditional electrode manufacturing processes can require substantial water volumes for cleaning and processing. Binder-free approaches typically reduce water requirements by 30-50%, addressing water scarcity concerns in regions where battery production is concentrated.
The improved cycle stability of binder-free electrodes extends battery service life, reducing replacement frequency and associated resource demands. This longevity effect multiplies sustainability benefits across the entire battery lifecycle, from raw material extraction to disposal.
Economic sustainability also improves through binder-free technologies. The elimination of expensive binders and solvents, combined with simplified manufacturing processes, can reduce production costs by 15-25%. These savings make advanced energy storage more accessible for renewable energy integration and electric mobility applications, accelerating the transition away from fossil fuel dependence.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!