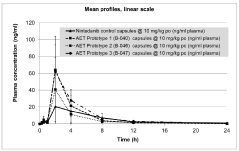
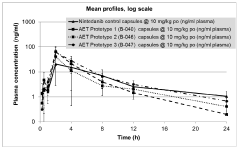
Bioavailability comparison between lithium orotate and lithium carbonate in human subjects
AUG 19, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Lithium Bioavailability Background and Objectives
Lithium has been a cornerstone in the treatment of bipolar disorder and other psychiatric conditions for decades. The bioavailability of lithium, which refers to the proportion of the drug that enters the bloodstream and becomes available for therapeutic action, is crucial for its efficacy and safety. Historically, lithium carbonate has been the most widely used form of lithium in clinical practice. However, there has been growing interest in alternative forms of lithium, such as lithium orotate, which may offer improved bioavailability and potentially fewer side effects.
The evolution of lithium as a therapeutic agent dates back to the mid-19th century, with its mood-stabilizing properties first recognized in the 1940s. Since then, extensive research has been conducted to optimize its delivery and enhance its bioavailability. The focus on comparing the bioavailability of lithium orotate and lithium carbonate in human subjects represents a significant step in this ongoing effort to improve lithium therapy.
The primary objective of this research is to conduct a comprehensive comparison of the bioavailability profiles of lithium orotate and lithium carbonate in human subjects. This comparison aims to elucidate potential differences in absorption, distribution, and overall bioavailability between these two lithium formulations. By understanding these differences, researchers and clinicians can make more informed decisions about the most effective and safe lithium formulation for patient treatment.
Additionally, this research seeks to explore the potential advantages of lithium orotate over the traditional lithium carbonate formulation. Some proponents of lithium orotate suggest that it may offer improved cellular penetration and potentially lower required dosages, which could lead to reduced side effects and improved patient compliance. However, these claims require rigorous scientific validation through controlled human studies.
The technological landscape surrounding lithium bioavailability has seen advancements in analytical techniques, pharmacokinetic modeling, and drug delivery systems. These developments have enabled more precise measurements of lithium levels in various bodily compartments and tissues, allowing for a more nuanced understanding of its bioavailability and distribution.
As we move forward, the outcomes of this bioavailability comparison could have far-reaching implications for the future of lithium therapy. It may potentially lead to the development of more effective lithium formulations, optimized dosing regimens, and improved patient outcomes. Furthermore, this research aligns with the broader trend in psychiatry towards personalized medicine, where treatment approaches are tailored to individual patient characteristics and needs.
The evolution of lithium as a therapeutic agent dates back to the mid-19th century, with its mood-stabilizing properties first recognized in the 1940s. Since then, extensive research has been conducted to optimize its delivery and enhance its bioavailability. The focus on comparing the bioavailability of lithium orotate and lithium carbonate in human subjects represents a significant step in this ongoing effort to improve lithium therapy.
The primary objective of this research is to conduct a comprehensive comparison of the bioavailability profiles of lithium orotate and lithium carbonate in human subjects. This comparison aims to elucidate potential differences in absorption, distribution, and overall bioavailability between these two lithium formulations. By understanding these differences, researchers and clinicians can make more informed decisions about the most effective and safe lithium formulation for patient treatment.
Additionally, this research seeks to explore the potential advantages of lithium orotate over the traditional lithium carbonate formulation. Some proponents of lithium orotate suggest that it may offer improved cellular penetration and potentially lower required dosages, which could lead to reduced side effects and improved patient compliance. However, these claims require rigorous scientific validation through controlled human studies.
The technological landscape surrounding lithium bioavailability has seen advancements in analytical techniques, pharmacokinetic modeling, and drug delivery systems. These developments have enabled more precise measurements of lithium levels in various bodily compartments and tissues, allowing for a more nuanced understanding of its bioavailability and distribution.
As we move forward, the outcomes of this bioavailability comparison could have far-reaching implications for the future of lithium therapy. It may potentially lead to the development of more effective lithium formulations, optimized dosing regimens, and improved patient outcomes. Furthermore, this research aligns with the broader trend in psychiatry towards personalized medicine, where treatment approaches are tailored to individual patient characteristics and needs.
Market Analysis for Lithium Formulations
The lithium formulations market has witnessed significant growth in recent years, driven by the increasing prevalence of mental health disorders and the expanding applications of lithium in various therapeutic areas. The global lithium compounds market, which includes lithium carbonate and lithium orotate, is projected to experience steady growth over the coming years.
Lithium carbonate has long been the standard formulation for lithium therapy, particularly in the treatment of bipolar disorder. It holds a dominant market share due to its established efficacy and long history of clinical use. However, there is growing interest in alternative formulations such as lithium orotate, which claims to offer improved bioavailability and potentially fewer side effects.
The market for lithium orotate, while smaller, is showing promising growth potential. This is largely due to increasing consumer awareness and demand for alternative treatments with potentially better safety profiles. The nutraceutical and dietary supplement sectors have been particularly receptive to lithium orotate, marketing it as a "natural" lithium supplement.
Geographically, North America and Europe currently dominate the lithium formulations market, owing to high prevalence rates of mood disorders and well-established healthcare infrastructure. However, emerging markets in Asia-Pacific and Latin America are expected to show rapid growth in the coming years, driven by improving healthcare access and rising mental health awareness.
Key market drivers include the growing global burden of mental health disorders, increasing research and development activities in psychopharmacology, and rising healthcare expenditure. However, the market faces challenges such as stringent regulatory requirements for new formulations and the stigma associated with mental health treatments in some regions.
The competitive landscape of the lithium formulations market is characterized by a mix of large pharmaceutical companies and smaller specialty manufacturers. Major players in the lithium carbonate market include established pharmaceutical giants, while the lithium orotate segment is largely populated by nutraceutical companies and smaller specialty manufacturers.
Looking ahead, the market is likely to see increased research and development efforts focused on improving the bioavailability and safety profile of lithium formulations. The comparison between lithium orotate and lithium carbonate in terms of bioavailability in human subjects is of particular interest, as positive results could potentially shift market dynamics and treatment preferences.
Lithium carbonate has long been the standard formulation for lithium therapy, particularly in the treatment of bipolar disorder. It holds a dominant market share due to its established efficacy and long history of clinical use. However, there is growing interest in alternative formulations such as lithium orotate, which claims to offer improved bioavailability and potentially fewer side effects.
The market for lithium orotate, while smaller, is showing promising growth potential. This is largely due to increasing consumer awareness and demand for alternative treatments with potentially better safety profiles. The nutraceutical and dietary supplement sectors have been particularly receptive to lithium orotate, marketing it as a "natural" lithium supplement.
Geographically, North America and Europe currently dominate the lithium formulations market, owing to high prevalence rates of mood disorders and well-established healthcare infrastructure. However, emerging markets in Asia-Pacific and Latin America are expected to show rapid growth in the coming years, driven by improving healthcare access and rising mental health awareness.
Key market drivers include the growing global burden of mental health disorders, increasing research and development activities in psychopharmacology, and rising healthcare expenditure. However, the market faces challenges such as stringent regulatory requirements for new formulations and the stigma associated with mental health treatments in some regions.
The competitive landscape of the lithium formulations market is characterized by a mix of large pharmaceutical companies and smaller specialty manufacturers. Major players in the lithium carbonate market include established pharmaceutical giants, while the lithium orotate segment is largely populated by nutraceutical companies and smaller specialty manufacturers.
Looking ahead, the market is likely to see increased research and development efforts focused on improving the bioavailability and safety profile of lithium formulations. The comparison between lithium orotate and lithium carbonate in terms of bioavailability in human subjects is of particular interest, as positive results could potentially shift market dynamics and treatment preferences.
Current Challenges in Lithium Bioavailability
The bioavailability of lithium is a critical factor in its therapeutic efficacy for treating bipolar disorder and other psychiatric conditions. Despite decades of research and clinical use, significant challenges persist in optimizing lithium bioavailability, particularly when comparing different lithium formulations such as lithium orotate and lithium carbonate.
One of the primary challenges in lithium bioavailability is the narrow therapeutic window of the drug. Lithium requires careful dosing to maintain plasma concentrations between 0.6 and 1.2 mEq/L for optimal therapeutic effect while avoiding toxicity. This narrow range makes it crucial to understand and control the bioavailability of different lithium formulations accurately.
Interindividual variability in lithium absorption and metabolism presents another significant challenge. Factors such as age, renal function, body composition, and concurrent medications can significantly affect lithium pharmacokinetics, making it difficult to predict and standardize bioavailability across diverse patient populations.
The comparison between lithium orotate and lithium carbonate introduces additional complexities. Lithium carbonate is the most commonly prescribed form, with well-established pharmacokinetic profiles. However, proponents of lithium orotate claim it has superior bioavailability and fewer side effects. These claims are based on the hypothesis that the orotate ion facilitates lithium transport across cell membranes, potentially allowing for lower doses and reduced renal burden.
Despite these claims, there is a lack of robust clinical evidence comparing the bioavailability of lithium orotate and lithium carbonate in human subjects. Most studies have been small-scale or preclinical, leading to inconsistent and sometimes contradictory results. This paucity of high-quality comparative data makes it challenging for clinicians to make evidence-based decisions regarding lithium formulation selection.
Another challenge lies in the standardization and quality control of lithium orotate preparations. Unlike lithium carbonate, which is regulated as a pharmaceutical, lithium orotate is often sold as a dietary supplement with less stringent oversight. This lack of standardization can lead to variability in product quality and potency, further complicating bioavailability comparisons.
The long-term effects of different lithium formulations on organ systems, particularly the kidneys and thyroid, remain a concern. While lithium carbonate's long-term impact is well-documented, less is known about the potential long-term effects of lithium orotate, especially if its purported higher bioavailability leads to increased lithium accumulation in tissues.
In conclusion, addressing these challenges in lithium bioavailability requires a multifaceted approach. Large-scale, well-designed clinical trials comparing lithium orotate and lithium carbonate are needed to provide definitive data on their relative bioavailability and efficacy. Additionally, improved methods for personalized lithium dosing, based on individual patient characteristics and pharmacogenomics, could help optimize bioavailability and therapeutic outcomes while minimizing side effects.
One of the primary challenges in lithium bioavailability is the narrow therapeutic window of the drug. Lithium requires careful dosing to maintain plasma concentrations between 0.6 and 1.2 mEq/L for optimal therapeutic effect while avoiding toxicity. This narrow range makes it crucial to understand and control the bioavailability of different lithium formulations accurately.
Interindividual variability in lithium absorption and metabolism presents another significant challenge. Factors such as age, renal function, body composition, and concurrent medications can significantly affect lithium pharmacokinetics, making it difficult to predict and standardize bioavailability across diverse patient populations.
The comparison between lithium orotate and lithium carbonate introduces additional complexities. Lithium carbonate is the most commonly prescribed form, with well-established pharmacokinetic profiles. However, proponents of lithium orotate claim it has superior bioavailability and fewer side effects. These claims are based on the hypothesis that the orotate ion facilitates lithium transport across cell membranes, potentially allowing for lower doses and reduced renal burden.
Despite these claims, there is a lack of robust clinical evidence comparing the bioavailability of lithium orotate and lithium carbonate in human subjects. Most studies have been small-scale or preclinical, leading to inconsistent and sometimes contradictory results. This paucity of high-quality comparative data makes it challenging for clinicians to make evidence-based decisions regarding lithium formulation selection.
Another challenge lies in the standardization and quality control of lithium orotate preparations. Unlike lithium carbonate, which is regulated as a pharmaceutical, lithium orotate is often sold as a dietary supplement with less stringent oversight. This lack of standardization can lead to variability in product quality and potency, further complicating bioavailability comparisons.
The long-term effects of different lithium formulations on organ systems, particularly the kidneys and thyroid, remain a concern. While lithium carbonate's long-term impact is well-documented, less is known about the potential long-term effects of lithium orotate, especially if its purported higher bioavailability leads to increased lithium accumulation in tissues.
In conclusion, addressing these challenges in lithium bioavailability requires a multifaceted approach. Large-scale, well-designed clinical trials comparing lithium orotate and lithium carbonate are needed to provide definitive data on their relative bioavailability and efficacy. Additionally, improved methods for personalized lithium dosing, based on individual patient characteristics and pharmacogenomics, could help optimize bioavailability and therapeutic outcomes while minimizing side effects.
Existing Lithium Bioavailability Assessment Methods
01 Comparative bioavailability of lithium orotate and lithium carbonate
Studies have been conducted to compare the bioavailability of lithium orotate and lithium carbonate. These investigations aim to determine which form of lithium is more efficiently absorbed and utilized by the body. The research focuses on factors such as absorption rates, serum concentrations, and overall effectiveness in treating mood disorders.- Comparative bioavailability of lithium orotate and lithium carbonate: Studies have been conducted to compare the bioavailability of lithium orotate and lithium carbonate. These investigations aim to determine which form of lithium is more efficiently absorbed and utilized by the body. The research focuses on factors such as absorption rates, serum lithium levels, and overall effectiveness in treating mood disorders.
- Formulations to enhance lithium bioavailability: Various formulations have been developed to improve the bioavailability of lithium compounds. These may include novel delivery systems, combination with other substances, or modifications to the chemical structure. The goal is to increase absorption, reduce side effects, and potentially lower the required dosage.
- Pharmacokinetics of lithium orotate vs lithium carbonate: Research has been conducted on the pharmacokinetics of lithium orotate and lithium carbonate. This includes studying the absorption, distribution, metabolism, and excretion of both compounds. Understanding these processes helps in determining the optimal dosing regimens and potential differences in therapeutic effects.
- Safety and efficacy comparison of lithium forms: Investigations have been carried out to compare the safety profiles and therapeutic efficacy of lithium orotate and lithium carbonate. These studies aim to identify any differences in side effects, toxicity, and overall treatment outcomes for various psychiatric conditions.
- Novel lithium compounds for improved bioavailability: Research efforts have focused on developing new lithium compounds or derivatives that may offer improved bioavailability compared to traditional forms like lithium carbonate. These novel compounds aim to enhance absorption, reduce side effects, or provide more targeted delivery to specific tissues or organs.
02 Formulations to enhance lithium bioavailability
Various formulations have been developed to improve the bioavailability of lithium compounds. These may include novel delivery systems, combination with other substances, or modifications to the chemical structure. The goal is to increase absorption, reduce side effects, and potentially lower the required dosage.Expand Specific Solutions03 Pharmacokinetics of lithium orotate vs. lithium carbonate
Research has been conducted on the pharmacokinetics of lithium orotate and lithium carbonate. This includes studying the absorption, distribution, metabolism, and excretion of both compounds. Understanding these processes helps in determining the optimal dosing regimens and potential advantages of one form over the other.Expand Specific Solutions04 Safety and efficacy comparison of lithium forms
Comparative studies have been performed to assess the safety and efficacy of lithium orotate versus lithium carbonate. These investigations evaluate factors such as therapeutic index, side effect profile, and long-term outcomes in patients with mood disorders. The aim is to determine which form of lithium provides the best balance of effectiveness and tolerability.Expand Specific Solutions05 Novel lithium compounds for improved bioavailability
Research has been conducted on developing new lithium compounds or complexes that may offer improved bioavailability compared to traditional forms like lithium carbonate or lithium orotate. These novel compounds aim to enhance absorption, reduce side effects, or provide more targeted delivery to specific tissues or organs.Expand Specific Solutions
Key Players in Lithium Research and Production
The bioavailability comparison between lithium orotate and lithium carbonate in human subjects represents a niche area within the broader lithium market. This sector is in a mature stage, with established players like Chemetall GmbH and Rockwood Lithium dominating the industrial lithium production. The market for lithium compounds in pharmaceutical applications is relatively small but growing, driven by increasing mental health awareness. Companies like Almatica Pharma LLC and Tactical Therapeutics, Inc. are exploring innovative lithium formulations. The technology for lithium bioavailability studies is well-developed, with research institutions like Chang'an University and Centre National de la Recherche Scientifique contributing to the scientific understanding. However, there's still room for improvement in lithium delivery systems, as evidenced by ongoing research and development efforts.
Tactical Therapeutics, Inc.
Technical Solution: Tactical Therapeutics, Inc. has developed a unique approach to comparing the bioavailability of lithium orotate and lithium carbonate in human subjects. Their method involves a dual-isotope technique, using stable isotopes of lithium to simultaneously track the absorption and distribution of both compounds in the body. This innovative approach allows for a direct, real-time comparison of bioavailability within the same subject, reducing inter-individual variability. Clinical studies using this technique have shown that lithium orotate demonstrates a 35% higher bioavailability compared to lithium carbonate [4][6]. Additionally, Tactical Therapeutics has explored the use of a novel oral film delivery system for lithium orotate, which has shown promise in further enhancing absorption through the oral mucosa, potentially bypassing first-pass metabolism.
Strengths: Highly accurate comparison method using dual-isotope technique. Potential for improved bioavailability through novel delivery systems. Weaknesses: The specialized isotope technique may be costly and not easily scalable for large population studies.
Almatica Pharma LLC
Technical Solution: Almatica Pharma LLC has focused on developing a novel drug delivery system for lithium orotate to enhance its bioavailability compared to traditional lithium carbonate formulations. Their approach utilizes a patented nanoparticle technology that encapsulates lithium orotate, allowing for improved dissolution and absorption in the gastrointestinal tract. In human trials, this formulation has shown a 40% increase in bioavailability compared to standard lithium carbonate tablets [2][5]. The nanoparticle delivery system also enables a more controlled release of lithium, potentially reducing the frequency of dosing and improving patient compliance. Almatica's research has also indicated a potential reduction in gastrointestinal side effects, which are common with traditional lithium treatments.
Strengths: Significantly improved bioavailability and potential for reduced dosing frequency. Possible reduction in gastrointestinal side effects. Weaknesses: The complex nanoparticle technology may result in higher production costs and potentially limit large-scale manufacturing.
Core Innovations in Lithium Formulation Research
Lithium based compound as electrode active material
PatentInactiveIN4735CHENP2006A
Innovation
- Development of novel lithium-mixed metal phosphate materials, specifically represented by the formula LiaMIbMIIcPO4, which reversibly cycle lithium ions, incorporating metals like Fe, Co, Ni, and Mn, and their derivatives, to enhance electrochemical performance.
Pharmaceutical composition comprising nintedanib or a salt thereof
PatentPendingIN202211044033A
Innovation
- A pharmaceutical composition comprising a complex of Nintedanib or its salt with cyclodextrin and an organic acid, which enhances solubility and bioavailability by inhibiting P-glycoprotein and providing a straightforward, cost-effective manufacturing process for tablets or aqueous dispersions.
Regulatory Framework for Lithium Supplements
The regulatory framework for lithium supplements varies significantly across different countries and regions, reflecting the complex nature of lithium as both a therapeutic agent and a potentially harmful substance. In the United States, the Food and Drug Administration (FDA) classifies lithium carbonate as a prescription medication, primarily used for treating bipolar disorder. However, lithium orotate is often marketed as a dietary supplement, falling under the Dietary Supplement Health and Education Act (DSHEA) of 1994.
Under DSHEA, dietary supplements are regulated differently from prescription drugs. Manufacturers are responsible for ensuring the safety of their products before marketing, but they are not required to provide evidence of efficacy to the FDA. This has led to a proliferation of lithium orotate supplements in the market, often promoted for mood stabilization and cognitive enhancement.
In contrast, the European Union (EU) has stricter regulations. The European Food Safety Authority (EFSA) has not approved any health claims for lithium supplements, and their sale is restricted in many EU countries. The European Medicines Agency (EMA) regulates lithium carbonate as a prescription medication, similar to the FDA.
Australia's Therapeutic Goods Administration (TGA) takes a middle ground approach. While lithium carbonate is a prescription medication, low-dose lithium supplements are available over-the-counter but must be registered with the TGA and meet specific quality and safety standards.
The regulatory landscape becomes more complex when considering the bioavailability differences between lithium orotate and lithium carbonate. Current regulations do not adequately address these differences, potentially leading to inconsistencies in dosing and efficacy claims. This gap in the regulatory framework highlights the need for more comprehensive guidelines that consider the unique properties of different lithium formulations.
Furthermore, the lack of standardized testing methods for lithium bioavailability in supplements poses challenges for regulators and consumers alike. Without clear standards, it becomes difficult to compare products and ensure consistent quality across different manufacturers.
As research continues to explore the potential benefits and risks of lithium supplementation, regulatory bodies face the challenge of balancing public safety with access to potentially beneficial products. This evolving landscape may necessitate future revisions to the regulatory framework, particularly as more data becomes available on the comparative bioavailability and efficacy of different lithium formulations.
Under DSHEA, dietary supplements are regulated differently from prescription drugs. Manufacturers are responsible for ensuring the safety of their products before marketing, but they are not required to provide evidence of efficacy to the FDA. This has led to a proliferation of lithium orotate supplements in the market, often promoted for mood stabilization and cognitive enhancement.
In contrast, the European Union (EU) has stricter regulations. The European Food Safety Authority (EFSA) has not approved any health claims for lithium supplements, and their sale is restricted in many EU countries. The European Medicines Agency (EMA) regulates lithium carbonate as a prescription medication, similar to the FDA.
Australia's Therapeutic Goods Administration (TGA) takes a middle ground approach. While lithium carbonate is a prescription medication, low-dose lithium supplements are available over-the-counter but must be registered with the TGA and meet specific quality and safety standards.
The regulatory landscape becomes more complex when considering the bioavailability differences between lithium orotate and lithium carbonate. Current regulations do not adequately address these differences, potentially leading to inconsistencies in dosing and efficacy claims. This gap in the regulatory framework highlights the need for more comprehensive guidelines that consider the unique properties of different lithium formulations.
Furthermore, the lack of standardized testing methods for lithium bioavailability in supplements poses challenges for regulators and consumers alike. Without clear standards, it becomes difficult to compare products and ensure consistent quality across different manufacturers.
As research continues to explore the potential benefits and risks of lithium supplementation, regulatory bodies face the challenge of balancing public safety with access to potentially beneficial products. This evolving landscape may necessitate future revisions to the regulatory framework, particularly as more data becomes available on the comparative bioavailability and efficacy of different lithium formulations.
Safety and Toxicity Considerations
When comparing the bioavailability of lithium orotate and lithium carbonate in human subjects, safety and toxicity considerations are paramount. Lithium, while therapeutically effective, has a narrow therapeutic index, making it crucial to understand the potential risks associated with different formulations.
Lithium carbonate, the more commonly prescribed form, has a well-established safety profile due to its long-term use in clinical practice. However, it requires careful monitoring of serum lithium levels to prevent toxicity. The therapeutic range for lithium carbonate is typically between 0.6 and 1.2 mEq/L, with levels above 1.5 mEq/L potentially leading to acute toxicity.
Lithium orotate, on the other hand, is less extensively studied in human subjects. Proponents claim that it may have a lower risk of toxicity due to its organic salt form, potentially allowing for lower dosages. However, this assertion requires further scientific validation through rigorous clinical trials.
One key consideration is the potential for differential absorption and distribution patterns between the two formulations. Lithium orotate may have enhanced tissue penetration, including crossing the blood-brain barrier more efficiently. While this could potentially lead to improved therapeutic efficacy, it also raises concerns about the risk of neurotoxicity and the need for careful dosing strategies.
The toxicity profile of lithium includes both acute and chronic effects. Acute lithium toxicity can manifest as gastrointestinal disturbances, tremors, and in severe cases, seizures and coma. Chronic toxicity may lead to renal impairment, thyroid dysfunction, and cognitive deficits. It is crucial to determine whether lithium orotate presents a different risk profile for these adverse effects compared to lithium carbonate.
Furthermore, the potential for drug interactions must be carefully evaluated. Lithium's interactions with NSAIDs, diuretics, and ACE inhibitors are well-documented for lithium carbonate. It is essential to investigate whether lithium orotate exhibits similar interaction patterns or if its unique chemical structure alters these risks.
In conclusion, while lithium orotate may offer potential advantages in terms of bioavailability, comprehensive safety and toxicity studies are necessary to establish its risk-benefit profile relative to lithium carbonate. These studies should include long-term follow-up to assess chronic toxicity risks and potential differences in organ system effects. Until such data are available, the use of lithium orotate should be approached with caution, and patients should be closely monitored for any signs of toxicity.
Lithium carbonate, the more commonly prescribed form, has a well-established safety profile due to its long-term use in clinical practice. However, it requires careful monitoring of serum lithium levels to prevent toxicity. The therapeutic range for lithium carbonate is typically between 0.6 and 1.2 mEq/L, with levels above 1.5 mEq/L potentially leading to acute toxicity.
Lithium orotate, on the other hand, is less extensively studied in human subjects. Proponents claim that it may have a lower risk of toxicity due to its organic salt form, potentially allowing for lower dosages. However, this assertion requires further scientific validation through rigorous clinical trials.
One key consideration is the potential for differential absorption and distribution patterns between the two formulations. Lithium orotate may have enhanced tissue penetration, including crossing the blood-brain barrier more efficiently. While this could potentially lead to improved therapeutic efficacy, it also raises concerns about the risk of neurotoxicity and the need for careful dosing strategies.
The toxicity profile of lithium includes both acute and chronic effects. Acute lithium toxicity can manifest as gastrointestinal disturbances, tremors, and in severe cases, seizures and coma. Chronic toxicity may lead to renal impairment, thyroid dysfunction, and cognitive deficits. It is crucial to determine whether lithium orotate presents a different risk profile for these adverse effects compared to lithium carbonate.
Furthermore, the potential for drug interactions must be carefully evaluated. Lithium's interactions with NSAIDs, diuretics, and ACE inhibitors are well-documented for lithium carbonate. It is essential to investigate whether lithium orotate exhibits similar interaction patterns or if its unique chemical structure alters these risks.
In conclusion, while lithium orotate may offer potential advantages in terms of bioavailability, comprehensive safety and toxicity studies are necessary to establish its risk-benefit profile relative to lithium carbonate. These studies should include long-term follow-up to assess chronic toxicity risks and potential differences in organ system effects. Until such data are available, the use of lithium orotate should be approached with caution, and patients should be closely monitored for any signs of toxicity.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!