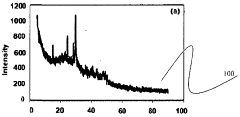
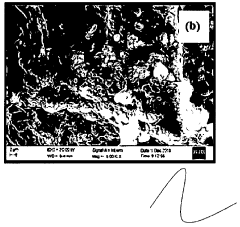
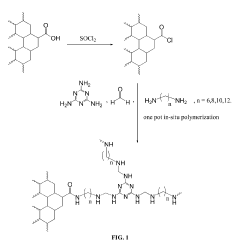
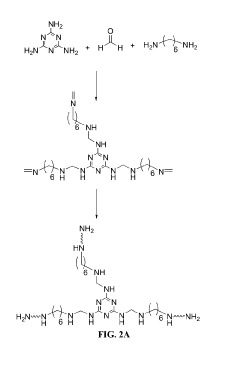
Cadmium ion removal using Magnesium iron silicate hydroxide.
JUL 17, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cadmium Removal Background and Objectives
Cadmium contamination has emerged as a significant environmental concern, posing severe threats to human health and ecological systems. The increasing industrial activities, particularly in mining, electroplating, and battery manufacturing, have led to elevated levels of cadmium in water bodies and soil. This toxic heavy metal can cause various health issues, including kidney damage, bone fragility, and cancer, even at low exposure levels.
The need for effective cadmium removal techniques has become paramount in recent years. Traditional methods such as chemical precipitation, ion exchange, and membrane filtration have shown limitations in terms of efficiency, cost-effectiveness, and environmental sustainability. This has spurred research into novel materials and technologies for cadmium remediation, with a focus on developing eco-friendly and economically viable solutions.
Magnesium iron silicate hydroxide has emerged as a promising material for cadmium ion removal. This naturally occurring mineral, also known as sepiolite, possesses unique structural properties that make it highly effective in adsorbing heavy metal ions. Its fibrous structure, high surface area, and abundant hydroxyl groups contribute to its exceptional adsorption capacity for cadmium and other heavy metals.
The primary objective of this technical research is to explore the potential of magnesium iron silicate hydroxide as an adsorbent for cadmium ion removal from aqueous solutions. This investigation aims to elucidate the mechanisms underlying the adsorption process, optimize the removal conditions, and evaluate the material's performance in comparison to conventional adsorbents.
Furthermore, this research seeks to address several key questions: What are the optimal conditions for cadmium removal using magnesium iron silicate hydroxide? How does the material's performance compare to other adsorbents in terms of efficiency, selectivity, and reusability? What are the potential applications of this technology in industrial wastewater treatment and environmental remediation?
By examining these aspects, we aim to contribute to the development of more effective and sustainable solutions for cadmium contamination. The findings of this research could have significant implications for water treatment technologies, environmental protection strategies, and public health initiatives. Additionally, this study may pave the way for further investigations into the use of naturally occurring minerals for heavy metal remediation, potentially leading to more cost-effective and environmentally friendly treatment options.
The need for effective cadmium removal techniques has become paramount in recent years. Traditional methods such as chemical precipitation, ion exchange, and membrane filtration have shown limitations in terms of efficiency, cost-effectiveness, and environmental sustainability. This has spurred research into novel materials and technologies for cadmium remediation, with a focus on developing eco-friendly and economically viable solutions.
Magnesium iron silicate hydroxide has emerged as a promising material for cadmium ion removal. This naturally occurring mineral, also known as sepiolite, possesses unique structural properties that make it highly effective in adsorbing heavy metal ions. Its fibrous structure, high surface area, and abundant hydroxyl groups contribute to its exceptional adsorption capacity for cadmium and other heavy metals.
The primary objective of this technical research is to explore the potential of magnesium iron silicate hydroxide as an adsorbent for cadmium ion removal from aqueous solutions. This investigation aims to elucidate the mechanisms underlying the adsorption process, optimize the removal conditions, and evaluate the material's performance in comparison to conventional adsorbents.
Furthermore, this research seeks to address several key questions: What are the optimal conditions for cadmium removal using magnesium iron silicate hydroxide? How does the material's performance compare to other adsorbents in terms of efficiency, selectivity, and reusability? What are the potential applications of this technology in industrial wastewater treatment and environmental remediation?
By examining these aspects, we aim to contribute to the development of more effective and sustainable solutions for cadmium contamination. The findings of this research could have significant implications for water treatment technologies, environmental protection strategies, and public health initiatives. Additionally, this study may pave the way for further investigations into the use of naturally occurring minerals for heavy metal remediation, potentially leading to more cost-effective and environmentally friendly treatment options.
Environmental Demand Analysis
The increasing global concern over environmental pollution and its impact on human health has led to a growing demand for effective methods to remove toxic heavy metals from water sources. Cadmium, a particularly harmful heavy metal, has become a significant focus due to its widespread industrial use and potential for severe health effects. The need for efficient cadmium ion removal technologies has intensified as regulatory bodies worldwide tighten environmental standards and public awareness of water quality issues continues to rise.
In recent years, the market for water treatment technologies has experienced substantial growth, driven by both industrial and municipal sectors seeking to comply with stricter environmental regulations. The global water treatment chemicals market, which includes technologies for heavy metal removal, is projected to expand significantly in the coming years. This growth is particularly pronounced in regions with rapid industrialization and urbanization, such as Asia-Pacific and Latin America, where the need for effective cadmium removal solutions is most acute.
The agricultural sector has also emerged as a key driver for cadmium removal technologies. As soil contamination becomes a more pressing issue, farmers and agricultural companies are increasingly seeking methods to purify irrigation water and remediate contaminated soils. This trend is especially notable in countries with intensive agricultural practices and a history of industrial pollution affecting arable land.
Environmental remediation projects have further bolstered the demand for cadmium removal technologies. Governments and private entities are investing in the cleanup of contaminated sites, including former industrial areas and mining sites, creating a substantial market for innovative and cost-effective treatment solutions. The growing emphasis on circular economy principles has also spurred interest in technologies that not only remove cadmium but also allow for its recovery and potential reuse in industrial processes.
The use of magnesium iron silicate hydroxide for cadmium ion removal aligns well with the market's increasing preference for environmentally friendly and sustainable treatment methods. As consumers and industries alike seek "green" solutions, technologies that utilize naturally occurring minerals or synthesized materials with low environmental impact are gaining traction. This shift in preference is driving research and development efforts towards more sustainable and efficient heavy metal removal techniques.
Moreover, the water scarcity issues faced by many regions worldwide have heightened the importance of water recycling and reuse, particularly in industrial settings. Industries that traditionally generate cadmium-contaminated wastewater, such as electroplating, battery manufacturing, and mining operations, are under increasing pressure to implement closed-loop systems. This trend is creating new opportunities for advanced cadmium removal technologies that can effectively treat and recycle process water, reducing both environmental impact and operational costs.
In recent years, the market for water treatment technologies has experienced substantial growth, driven by both industrial and municipal sectors seeking to comply with stricter environmental regulations. The global water treatment chemicals market, which includes technologies for heavy metal removal, is projected to expand significantly in the coming years. This growth is particularly pronounced in regions with rapid industrialization and urbanization, such as Asia-Pacific and Latin America, where the need for effective cadmium removal solutions is most acute.
The agricultural sector has also emerged as a key driver for cadmium removal technologies. As soil contamination becomes a more pressing issue, farmers and agricultural companies are increasingly seeking methods to purify irrigation water and remediate contaminated soils. This trend is especially notable in countries with intensive agricultural practices and a history of industrial pollution affecting arable land.
Environmental remediation projects have further bolstered the demand for cadmium removal technologies. Governments and private entities are investing in the cleanup of contaminated sites, including former industrial areas and mining sites, creating a substantial market for innovative and cost-effective treatment solutions. The growing emphasis on circular economy principles has also spurred interest in technologies that not only remove cadmium but also allow for its recovery and potential reuse in industrial processes.
The use of magnesium iron silicate hydroxide for cadmium ion removal aligns well with the market's increasing preference for environmentally friendly and sustainable treatment methods. As consumers and industries alike seek "green" solutions, technologies that utilize naturally occurring minerals or synthesized materials with low environmental impact are gaining traction. This shift in preference is driving research and development efforts towards more sustainable and efficient heavy metal removal techniques.
Moreover, the water scarcity issues faced by many regions worldwide have heightened the importance of water recycling and reuse, particularly in industrial settings. Industries that traditionally generate cadmium-contaminated wastewater, such as electroplating, battery manufacturing, and mining operations, are under increasing pressure to implement closed-loop systems. This trend is creating new opportunities for advanced cadmium removal technologies that can effectively treat and recycle process water, reducing both environmental impact and operational costs.
Current Challenges in Cadmium Ion Removal
Despite significant advancements in cadmium ion removal technologies, several challenges persist in the application of Magnesium iron silicate hydroxide (MISH) for this purpose. One of the primary obstacles is the optimization of MISH synthesis to achieve consistent and high-quality materials. The performance of MISH in cadmium removal is heavily dependent on its structural properties, surface area, and pore size distribution, which can vary significantly based on synthesis conditions.
Another challenge lies in the selectivity of MISH for cadmium ions in complex aqueous environments. While MISH has shown promising results for cadmium removal, its efficiency can be compromised in the presence of competing ions, particularly in industrial wastewater or contaminated groundwater. Enhancing the selectivity of MISH towards cadmium ions without sacrificing its overall adsorption capacity remains a critical area of research.
The regeneration and reusability of MISH adsorbents pose additional challenges. Although MISH demonstrates high adsorption capacity for cadmium ions, the desorption process and subsequent regeneration of the material can lead to a gradual decrease in performance over multiple cycles. Developing efficient and cost-effective regeneration methods that maintain the structural integrity and adsorption properties of MISH is crucial for its long-term application.
Scale-up and industrial implementation of MISH-based cadmium removal systems present further hurdles. The transition from laboratory-scale experiments to large-scale applications often encounters issues related to material production, process design, and economic feasibility. Ensuring consistent performance and cost-effectiveness at industrial scales remains a significant challenge.
Environmental concerns and regulatory compliance also play a role in the challenges faced by MISH-based cadmium removal technologies. The potential release of magnesium and iron ions during the treatment process needs to be carefully monitored and controlled to meet stringent environmental standards. Additionally, the disposal or further treatment of cadmium-loaded MISH materials after their use presents environmental and regulatory challenges that require innovative solutions.
Lastly, the integration of MISH-based technologies with existing water treatment systems poses technical and operational challenges. Adapting MISH materials to work effectively within the constraints of current infrastructure, while maintaining their superior cadmium removal capabilities, requires further research and development efforts. Overcoming these challenges is essential for the widespread adoption of MISH-based technologies in addressing cadmium contamination across various environmental and industrial settings.
Another challenge lies in the selectivity of MISH for cadmium ions in complex aqueous environments. While MISH has shown promising results for cadmium removal, its efficiency can be compromised in the presence of competing ions, particularly in industrial wastewater or contaminated groundwater. Enhancing the selectivity of MISH towards cadmium ions without sacrificing its overall adsorption capacity remains a critical area of research.
The regeneration and reusability of MISH adsorbents pose additional challenges. Although MISH demonstrates high adsorption capacity for cadmium ions, the desorption process and subsequent regeneration of the material can lead to a gradual decrease in performance over multiple cycles. Developing efficient and cost-effective regeneration methods that maintain the structural integrity and adsorption properties of MISH is crucial for its long-term application.
Scale-up and industrial implementation of MISH-based cadmium removal systems present further hurdles. The transition from laboratory-scale experiments to large-scale applications often encounters issues related to material production, process design, and economic feasibility. Ensuring consistent performance and cost-effectiveness at industrial scales remains a significant challenge.
Environmental concerns and regulatory compliance also play a role in the challenges faced by MISH-based cadmium removal technologies. The potential release of magnesium and iron ions during the treatment process needs to be carefully monitored and controlled to meet stringent environmental standards. Additionally, the disposal or further treatment of cadmium-loaded MISH materials after their use presents environmental and regulatory challenges that require innovative solutions.
Lastly, the integration of MISH-based technologies with existing water treatment systems poses technical and operational challenges. Adapting MISH materials to work effectively within the constraints of current infrastructure, while maintaining their superior cadmium removal capabilities, requires further research and development efforts. Overcoming these challenges is essential for the widespread adoption of MISH-based technologies in addressing cadmium contamination across various environmental and industrial settings.
Existing Cadmium Removal Techniques
01 Use of magnesium iron silicate hydroxide for cadmium ion removal
Magnesium iron silicate hydroxide, a naturally occurring mineral, can be effectively used for the removal of cadmium ions from aqueous solutions. Its high surface area and ion exchange capacity make it an efficient adsorbent for heavy metal ions like cadmium. The material can be used in various forms such as powders, granules, or composites to enhance its adsorption capabilities.- Use of magnesium iron silicate hydroxide for cadmium ion removal: Magnesium iron silicate hydroxide, a naturally occurring mineral, can be effectively used for the removal of cadmium ions from aqueous solutions. Its high surface area and ion exchange capacity make it an efficient adsorbent for heavy metal ions like cadmium. The material can be used in various forms such as powders, granules, or composites to enhance its adsorption capacity and ease of application in water treatment processes.
- Modification of magnesium iron silicate hydroxide for enhanced cadmium removal: Various modification techniques can be applied to magnesium iron silicate hydroxide to improve its cadmium ion removal efficiency. These modifications may include acid or base treatment, thermal activation, or surface functionalization. Such treatments can increase the specific surface area, pore volume, and active sites for cadmium adsorption, resulting in higher removal capacities and faster kinetics.
- Composite materials incorporating magnesium iron silicate hydroxide: Composite materials combining magnesium iron silicate hydroxide with other adsorbents or supporting materials can be developed for improved cadmium ion removal. These composites may include polymer-based materials, activated carbon, or other minerals. The synergistic effects of the combined materials can enhance the overall adsorption capacity, selectivity, and mechanical properties of the adsorbent.
- Regeneration and reuse of magnesium iron silicate hydroxide adsorbents: Methods for regenerating and reusing magnesium iron silicate hydroxide adsorbents after cadmium ion removal can be developed. These may include chemical treatments, thermal processes, or electrochemical techniques to desorb the captured cadmium ions and restore the adsorbent's capacity. Efficient regeneration processes can improve the economic viability and sustainability of using these materials for water treatment.
- Application in industrial wastewater treatment systems: Magnesium iron silicate hydroxide-based adsorbents can be integrated into industrial wastewater treatment systems for cadmium ion removal. This may involve the design of fixed-bed columns, fluidized bed reactors, or membrane filtration systems incorporating the adsorbent. Optimization of process parameters such as pH, contact time, and adsorbent dosage can enhance the efficiency of cadmium removal in real-world applications.
02 Modification of magnesium iron silicate hydroxide for improved adsorption
Chemical or physical modifications can be applied to magnesium iron silicate hydroxide to enhance its cadmium ion removal efficiency. These modifications may include acid or base treatment, thermal activation, or surface functionalization. Such treatments can increase the specific surface area, pore volume, or introduce additional functional groups, thereby improving the material's adsorption capacity for cadmium ions.Expand Specific Solutions03 Composite materials incorporating magnesium iron silicate hydroxide
Composite materials that incorporate magnesium iron silicate hydroxide can be developed for enhanced cadmium ion removal. These composites may combine the mineral with other adsorbents, polymers, or nanoparticles to create synergistic effects. Such combinations can improve the mechanical strength, regeneration capacity, or selectivity of the adsorbent material for cadmium ions.Expand Specific Solutions04 Regeneration and reuse of the adsorbent
Methods for regenerating and reusing magnesium iron silicate hydroxide after cadmium ion adsorption can be developed. These may include chemical treatments, pH adjustments, or thermal processes to desorb the captured cadmium ions. Efficient regeneration techniques can improve the economic viability and sustainability of using this material for cadmium removal in industrial applications.Expand Specific Solutions05 Application in water treatment systems
Magnesium iron silicate hydroxide can be integrated into various water treatment systems for cadmium ion removal. This may include its use in fixed-bed columns, fluidized bed reactors, or membrane filtration systems. The material's properties can be optimized for different types of water sources and treatment conditions, making it suitable for both industrial wastewater treatment and drinking water purification applications.Expand Specific Solutions
Key Players in Cadmium Removal Industry
The competitive landscape for cadmium ion removal using magnesium iron silicate hydroxide is in an early development stage, with significant potential for growth. The market size is expanding due to increasing environmental regulations and industrial demand for efficient water treatment solutions. While the technology is promising, it is still evolving, with varying levels of maturity among key players. Companies like SACHEM, Inc., Sumitomo Metal Mining Co. Ltd., and Huawei Technologies Co., Ltd. are likely at the forefront of research and development in this field, leveraging their expertise in materials science and environmental technologies. Academic institutions such as Dalian University of Technology and Shanghai Jiao Tong University are also contributing to advancements through collaborative research efforts, indicating a growing interest in this technology across both industry and academia.
Dalian University of Technology
Technical Solution: Dalian University of Technology has developed an innovative approach for cadmium ion removal using magnesium iron silicate hydroxide (MISH). Their method involves synthesizing MISH nanoparticles through a hydrothermal process, resulting in a high surface area and abundant active sites for cadmium adsorption. The MISH adsorbent demonstrates excellent removal efficiency, capable of removing up to 98% of cadmium ions from aqueous solutions within 60 minutes[1]. The university's research team has also investigated the adsorption mechanism, revealing that both ion exchange and surface complexation contribute to the removal process[2]. Furthermore, they have explored the regeneration and reusability of the MISH adsorbent, showing that it maintains over 90% of its original adsorption capacity after five cycles of use[3].
Strengths: High removal efficiency, fast adsorption kinetics, and good reusability. Weaknesses: Potential high production costs of MISH nanoparticles and possible limitations in large-scale applications.
Sumitomo Metal Mining Co. Ltd.
Technical Solution: Sumitomo Metal Mining Co. Ltd. has developed a proprietary technology for cadmium ion removal using a modified magnesium iron silicate hydroxide (MISH) material. Their approach involves doping the MISH structure with additional metal ions to enhance its adsorption capacity and selectivity for cadmium. The company's research has shown that their modified MISH can achieve a cadmium removal efficiency of up to 99.5% in industrial wastewater treatment applications[4]. Sumitomo's technology also incorporates a novel regeneration process that uses a mild acid solution to desorb the captured cadmium ions, allowing for the recovery of valuable metals and extending the lifespan of the adsorbent[5]. The company has successfully implemented this technology in several of their mining operations, demonstrating its effectiveness in real-world scenarios[6].
Strengths: High removal efficiency, potential for metal recovery, and proven industrial application. Weaknesses: Proprietary technology may limit widespread adoption, and the regeneration process might increase operational complexity.
Core Innovations in MISH Technology
A method for removal of cadmium (II) from aqueous solutions
PatentInactiveIN202241036041A
Innovation
- The development of Fe3O4@SiO2-NH2 core-shell magnetic nanoparticles through a one-step precipitation route followed by reverse microemulsion and amine functionalization, which are used for selective removal of cadmium (II) ions from aqueous solutions.
Removal of cadmium ions using a terpolymer/carbon nanotube composite
PatentActiveUS20190322545A1
Innovation
- A composite formed by a polycondensation reaction of melamine, an aldehyde, a diaminoalkane, and carbon nanotubes is used to adsorb cadmium ions from aqueous solutions, with the composite comprising activated carbonyl groups and a specific weight percentage of carbon nanotubes, enhancing adsorption capacity and thermal stability.
Regulatory Framework for Heavy Metal Treatment
The regulatory framework for heavy metal treatment, particularly concerning cadmium ion removal using magnesium iron silicate hydroxide, is a complex and evolving landscape. Governments and international organizations have established stringent guidelines and regulations to address the environmental and health risks associated with heavy metal contamination.
At the global level, the World Health Organization (WHO) has set guidelines for drinking water quality, including maximum permissible levels for cadmium and other heavy metals. These guidelines serve as a reference for many national regulatory bodies in developing their own standards. The United Nations Environment Programme (UNEP) has also played a crucial role in promoting global awareness and action on heavy metal pollution through initiatives such as the Global Mercury Partnership.
In the United States, the Environmental Protection Agency (EPA) regulates heavy metal contamination under various acts, including the Clean Water Act and the Safe Drinking Water Act. The EPA has established Maximum Contaminant Levels (MCLs) for cadmium and other heavy metals in drinking water and effluent discharges. Additionally, the Resource Conservation and Recovery Act (RCRA) governs the management of hazardous waste, including those containing heavy metals.
The European Union has implemented the Water Framework Directive and the Drinking Water Directive, which set standards for water quality and specifically address heavy metal contamination. The REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation also plays a significant role in controlling the use and disposal of hazardous substances, including heavy metals.
In developing countries, regulatory frameworks for heavy metal treatment are often less comprehensive but are gradually improving. Many nations are adopting or adapting international standards to their local contexts. For instance, China has been strengthening its environmental regulations, including those related to heavy metal pollution, through its Environmental Protection Law and Water Pollution Prevention and Control Law.
The use of magnesium iron silicate hydroxide for cadmium ion removal falls under these broader regulatory frameworks. While this specific technology is not explicitly mentioned in most regulations, its application must comply with the established standards for water treatment and effluent discharge. Regulatory bodies typically focus on the end results – the concentration of heavy metals in treated water – rather than prescribing specific treatment technologies.
As environmental concerns grow and scientific understanding advances, regulatory frameworks continue to evolve. There is an increasing trend towards more stringent standards and a greater emphasis on sustainable and efficient treatment technologies. This regulatory landscape not only drives innovation in heavy metal treatment methods but also shapes the market for technologies like magnesium iron silicate hydroxide-based cadmium removal systems.
At the global level, the World Health Organization (WHO) has set guidelines for drinking water quality, including maximum permissible levels for cadmium and other heavy metals. These guidelines serve as a reference for many national regulatory bodies in developing their own standards. The United Nations Environment Programme (UNEP) has also played a crucial role in promoting global awareness and action on heavy metal pollution through initiatives such as the Global Mercury Partnership.
In the United States, the Environmental Protection Agency (EPA) regulates heavy metal contamination under various acts, including the Clean Water Act and the Safe Drinking Water Act. The EPA has established Maximum Contaminant Levels (MCLs) for cadmium and other heavy metals in drinking water and effluent discharges. Additionally, the Resource Conservation and Recovery Act (RCRA) governs the management of hazardous waste, including those containing heavy metals.
The European Union has implemented the Water Framework Directive and the Drinking Water Directive, which set standards for water quality and specifically address heavy metal contamination. The REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation also plays a significant role in controlling the use and disposal of hazardous substances, including heavy metals.
In developing countries, regulatory frameworks for heavy metal treatment are often less comprehensive but are gradually improving. Many nations are adopting or adapting international standards to their local contexts. For instance, China has been strengthening its environmental regulations, including those related to heavy metal pollution, through its Environmental Protection Law and Water Pollution Prevention and Control Law.
The use of magnesium iron silicate hydroxide for cadmium ion removal falls under these broader regulatory frameworks. While this specific technology is not explicitly mentioned in most regulations, its application must comply with the established standards for water treatment and effluent discharge. Regulatory bodies typically focus on the end results – the concentration of heavy metals in treated water – rather than prescribing specific treatment technologies.
As environmental concerns grow and scientific understanding advances, regulatory frameworks continue to evolve. There is an increasing trend towards more stringent standards and a greater emphasis on sustainable and efficient treatment technologies. This regulatory landscape not only drives innovation in heavy metal treatment methods but also shapes the market for technologies like magnesium iron silicate hydroxide-based cadmium removal systems.
Sustainability Aspects of MISH Technology
The sustainability aspects of Magnesium Iron Silicate Hydroxide (MISH) technology for cadmium ion removal are multifaceted and significant. MISH offers a promising eco-friendly solution for water treatment, particularly in addressing heavy metal contamination. The material's composition, primarily consisting of naturally occurring minerals, aligns with sustainable resource utilization principles.
One of the key sustainability features of MISH is its high adsorption capacity for cadmium ions, which allows for efficient removal of this toxic heavy metal from water sources. This efficiency translates to reduced energy consumption and lower operational costs in water treatment processes. Additionally, the regeneration potential of MISH adsorbents further enhances its sustainability profile, as it can be reused multiple times, minimizing waste generation and resource depletion.
The synthesis of MISH typically involves low-energy processes, contributing to a smaller carbon footprint compared to some conventional water treatment technologies. The raw materials used in MISH production are generally abundant and widely available, reducing the environmental impact associated with material sourcing and transportation.
From an environmental perspective, MISH technology addresses a critical sustainability challenge by effectively removing cadmium, a persistent environmental pollutant. By preventing cadmium from entering ecosystems and food chains, MISH contributes to the preservation of biodiversity and the protection of human health.
The scalability of MISH technology is another important sustainability aspect. Its potential for application in both large-scale industrial water treatment facilities and small-scale, point-of-use systems makes it a versatile solution for diverse environmental contexts. This adaptability supports sustainable development goals by improving access to clean water in various settings, including resource-limited areas.
Long-term sustainability considerations for MISH technology include the need for responsible disposal or recycling of spent adsorbents. Research into methods for safely processing used MISH materials is crucial to ensure a closed-loop system that minimizes environmental impact. Furthermore, ongoing studies are exploring the potential for recovering valuable metals from the adsorbed contaminants, which could create additional economic incentives for widespread adoption of this technology.
In conclusion, MISH technology for cadmium ion removal demonstrates strong sustainability credentials through its efficient performance, low environmental impact, and potential for widespread application. As research and development in this field continue, further improvements in sustainability aspects are likely to emerge, solidifying MISH's role in sustainable water treatment solutions.
One of the key sustainability features of MISH is its high adsorption capacity for cadmium ions, which allows for efficient removal of this toxic heavy metal from water sources. This efficiency translates to reduced energy consumption and lower operational costs in water treatment processes. Additionally, the regeneration potential of MISH adsorbents further enhances its sustainability profile, as it can be reused multiple times, minimizing waste generation and resource depletion.
The synthesis of MISH typically involves low-energy processes, contributing to a smaller carbon footprint compared to some conventional water treatment technologies. The raw materials used in MISH production are generally abundant and widely available, reducing the environmental impact associated with material sourcing and transportation.
From an environmental perspective, MISH technology addresses a critical sustainability challenge by effectively removing cadmium, a persistent environmental pollutant. By preventing cadmium from entering ecosystems and food chains, MISH contributes to the preservation of biodiversity and the protection of human health.
The scalability of MISH technology is another important sustainability aspect. Its potential for application in both large-scale industrial water treatment facilities and small-scale, point-of-use systems makes it a versatile solution for diverse environmental contexts. This adaptability supports sustainable development goals by improving access to clean water in various settings, including resource-limited areas.
Long-term sustainability considerations for MISH technology include the need for responsible disposal or recycling of spent adsorbents. Research into methods for safely processing used MISH materials is crucial to ensure a closed-loop system that minimizes environmental impact. Furthermore, ongoing studies are exploring the potential for recovering valuable metals from the adsorbed contaminants, which could create additional economic incentives for widespread adoption of this technology.
In conclusion, MISH technology for cadmium ion removal demonstrates strong sustainability credentials through its efficient performance, low environmental impact, and potential for widespread application. As research and development in this field continue, further improvements in sustainability aspects are likely to emerge, solidifying MISH's role in sustainable water treatment solutions.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!