Comparative testing of electroplated versus hot-dip zinc coatings
OCT 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Zinc Coating Technology Background and Objectives
Zinc coating technology has evolved significantly over the past century as a primary method for protecting steel and iron from corrosion. The fundamental principle behind zinc coatings relies on zinc's sacrificial protection mechanism, where zinc corrodes preferentially to protect the underlying steel substrate. This galvanic protection has made zinc coatings indispensable across numerous industries including automotive, construction, infrastructure, and consumer goods manufacturing.
The historical development of zinc coating technologies can be traced back to the early 19th century with the introduction of hot-dip galvanizing in France. This process has remained relatively unchanged in principle, though significant improvements in process control and efficiency have been implemented. Electroplating emerged as an alternative zinc application method in the early 20th century, offering different characteristics and application advantages.
Current technological trends in zinc coating are focused on enhancing corrosion resistance while minimizing environmental impact and reducing costs. The industry has witnessed a shift toward thinner coatings with improved performance characteristics, achieved through alloying additions and post-treatment processes. Additionally, there is growing interest in developing zinc coating technologies that eliminate hazardous chemicals and reduce energy consumption.
The comparative analysis of electroplated versus hot-dip zinc coatings represents a critical area of research as each method offers distinct advantages and limitations. Hot-dip galvanizing typically provides thicker coatings with excellent adhesion and impact resistance, while electroplating offers superior surface finish and dimensional precision for complex components. Understanding these differences is essential for optimizing coating selection across various applications.
The primary objectives of this technical research report are to comprehensively evaluate the performance characteristics of electroplated and hot-dip zinc coatings under standardized testing conditions. Specifically, the research aims to quantify differences in corrosion resistance, coating adhesion, thickness uniformity, and mechanical properties between these two predominant zinc coating technologies.
Furthermore, this investigation seeks to establish correlations between coating microstructure, application parameters, and performance outcomes to provide evidence-based guidance for coating selection across different industrial applications. The findings will contribute to the development of improved testing methodologies and performance standards that more accurately reflect real-world service conditions for zinc-coated components.
The ultimate goal is to provide manufacturers and engineers with definitive data to optimize coating selection decisions based on specific application requirements, environmental exposure conditions, and economic considerations, thereby enhancing product durability while minimizing unnecessary costs.
The historical development of zinc coating technologies can be traced back to the early 19th century with the introduction of hot-dip galvanizing in France. This process has remained relatively unchanged in principle, though significant improvements in process control and efficiency have been implemented. Electroplating emerged as an alternative zinc application method in the early 20th century, offering different characteristics and application advantages.
Current technological trends in zinc coating are focused on enhancing corrosion resistance while minimizing environmental impact and reducing costs. The industry has witnessed a shift toward thinner coatings with improved performance characteristics, achieved through alloying additions and post-treatment processes. Additionally, there is growing interest in developing zinc coating technologies that eliminate hazardous chemicals and reduce energy consumption.
The comparative analysis of electroplated versus hot-dip zinc coatings represents a critical area of research as each method offers distinct advantages and limitations. Hot-dip galvanizing typically provides thicker coatings with excellent adhesion and impact resistance, while electroplating offers superior surface finish and dimensional precision for complex components. Understanding these differences is essential for optimizing coating selection across various applications.
The primary objectives of this technical research report are to comprehensively evaluate the performance characteristics of electroplated and hot-dip zinc coatings under standardized testing conditions. Specifically, the research aims to quantify differences in corrosion resistance, coating adhesion, thickness uniformity, and mechanical properties between these two predominant zinc coating technologies.
Furthermore, this investigation seeks to establish correlations between coating microstructure, application parameters, and performance outcomes to provide evidence-based guidance for coating selection across different industrial applications. The findings will contribute to the development of improved testing methodologies and performance standards that more accurately reflect real-world service conditions for zinc-coated components.
The ultimate goal is to provide manufacturers and engineers with definitive data to optimize coating selection decisions based on specific application requirements, environmental exposure conditions, and economic considerations, thereby enhancing product durability while minimizing unnecessary costs.
Market Demand Analysis for Corrosion Protection Solutions
The global market for corrosion protection solutions continues to expand significantly, driven by increasing infrastructure development and growing awareness of the economic impact of corrosion. Current estimates value the global corrosion protection market at over 66 billion USD, with projections indicating a compound annual growth rate of approximately 5.2% through 2028. Within this broader market, zinc coating technologies represent a substantial segment, accounting for nearly 40% of metal protection applications across industries.
Demand for zinc coatings is particularly strong in automotive manufacturing, construction, and infrastructure development sectors. The automotive industry alone consumes approximately 15% of all zinc coating applications, with stringent requirements for both performance and aesthetic qualities. Construction and infrastructure projects account for an additional 35%, where long-term durability under harsh environmental conditions is paramount.
Regional analysis reveals varying market dynamics. North America and Europe demonstrate mature markets with emphasis on high-performance, environmentally compliant solutions, while Asia-Pacific represents the fastest-growing region with 7.3% annual growth, driven by rapid industrialization in China and India. Latin America and Middle East regions show increasing adoption rates as infrastructure investments accelerate.
Customer requirements are evolving beyond basic corrosion protection. End-users increasingly demand solutions that offer extended service life, reduced maintenance costs, and environmental sustainability. This shift has intensified interest in comparative performance between electroplated and hot-dip zinc coatings, with particular focus on thickness consistency, adhesion properties, and corrosion resistance in specific environments.
Regulatory factors significantly influence market demand patterns. Stringent environmental regulations in Europe and North America have accelerated the transition toward more sustainable coating technologies with reduced environmental impact. Restrictions on hexavalent chromium and other hazardous substances have particularly affected electroplating processes, creating market opportunities for alternative technologies.
Cost considerations remain a critical factor in market adoption. While hot-dip galvanizing typically offers lower per-unit costs for large-scale applications, electroplating provides advantages in precision applications requiring tight dimensional tolerances. The total cost of ownership, including initial application costs, maintenance requirements, and service life, increasingly drives purchasing decisions rather than upfront costs alone.
Market research indicates growing demand for specialized testing and certification services that can validate comparative performance claims between different zinc coating technologies, particularly in high-value applications where failure costs are substantial.
Demand for zinc coatings is particularly strong in automotive manufacturing, construction, and infrastructure development sectors. The automotive industry alone consumes approximately 15% of all zinc coating applications, with stringent requirements for both performance and aesthetic qualities. Construction and infrastructure projects account for an additional 35%, where long-term durability under harsh environmental conditions is paramount.
Regional analysis reveals varying market dynamics. North America and Europe demonstrate mature markets with emphasis on high-performance, environmentally compliant solutions, while Asia-Pacific represents the fastest-growing region with 7.3% annual growth, driven by rapid industrialization in China and India. Latin America and Middle East regions show increasing adoption rates as infrastructure investments accelerate.
Customer requirements are evolving beyond basic corrosion protection. End-users increasingly demand solutions that offer extended service life, reduced maintenance costs, and environmental sustainability. This shift has intensified interest in comparative performance between electroplated and hot-dip zinc coatings, with particular focus on thickness consistency, adhesion properties, and corrosion resistance in specific environments.
Regulatory factors significantly influence market demand patterns. Stringent environmental regulations in Europe and North America have accelerated the transition toward more sustainable coating technologies with reduced environmental impact. Restrictions on hexavalent chromium and other hazardous substances have particularly affected electroplating processes, creating market opportunities for alternative technologies.
Cost considerations remain a critical factor in market adoption. While hot-dip galvanizing typically offers lower per-unit costs for large-scale applications, electroplating provides advantages in precision applications requiring tight dimensional tolerances. The total cost of ownership, including initial application costs, maintenance requirements, and service life, increasingly drives purchasing decisions rather than upfront costs alone.
Market research indicates growing demand for specialized testing and certification services that can validate comparative performance claims between different zinc coating technologies, particularly in high-value applications where failure costs are substantial.
Current Status and Challenges in Zinc Coating Technologies
Zinc coating technologies have evolved significantly over the past decades, with electroplating and hot-dip galvanizing emerging as the two dominant methods in corrosion protection for steel and iron products. Currently, these technologies are widely implemented across various industries including automotive, construction, and infrastructure development. The global zinc coating market was valued at approximately $20 billion in 2022, with projections indicating continued growth at a CAGR of 5.2% through 2030.
Electroplated zinc coatings, characterized by their thin and uniform application (typically 5-25 μm), have achieved significant advancements in recent years. Modern electroplating processes now incorporate pulse plating techniques and advanced brightening agents, resulting in more consistent coating distribution and enhanced adhesion properties. However, this technology continues to face challenges related to hydrogen embrittlement, particularly when applied to high-strength steels, and environmental concerns regarding the disposal of plating chemicals.
Hot-dip galvanizing, offering thicker coatings (typically 45-200 μm), has seen improvements in process control and alloy development. The introduction of automated dipping systems has enhanced coating uniformity, while advancements in flux technology have improved surface preparation efficiency. Nevertheless, challenges persist in controlling coating thickness on complex geometries and managing zinc ash formation during the galvanizing process.
Geographically, Asia-Pacific dominates the zinc coating market, accounting for approximately 45% of global production, with China being the largest contributor. Europe and North America follow with sophisticated applications focusing on high-performance coatings for specialized industries. Emerging markets in South America and Africa are showing increased adoption of zinc coating technologies, albeit with less advanced process controls.
A significant technical challenge facing both technologies is the balance between coating thickness and functional performance. While thicker coatings generally provide longer corrosion protection, they may compromise dimensional tolerances and surface finish quality. Additionally, the industry faces increasing pressure to reduce environmental impact, particularly regarding zinc runoff and waste management.
Recent comparative testing has revealed that electroplated coatings offer superior aesthetic qualities and dimensional precision, while hot-dip galvanized coatings provide enhanced long-term corrosion resistance in aggressive environments. However, standardized testing methodologies remain inconsistent across different regions, complicating direct performance comparisons.
The development of hybrid coating systems, combining the advantages of both technologies, represents a promising but technically challenging frontier. Current research focuses on optimizing zinc-nickel alloy compositions for electroplating and developing more environmentally friendly flux systems for hot-dip processes, while exploring nanotechnology applications to enhance coating performance without increasing thickness.
Electroplated zinc coatings, characterized by their thin and uniform application (typically 5-25 μm), have achieved significant advancements in recent years. Modern electroplating processes now incorporate pulse plating techniques and advanced brightening agents, resulting in more consistent coating distribution and enhanced adhesion properties. However, this technology continues to face challenges related to hydrogen embrittlement, particularly when applied to high-strength steels, and environmental concerns regarding the disposal of plating chemicals.
Hot-dip galvanizing, offering thicker coatings (typically 45-200 μm), has seen improvements in process control and alloy development. The introduction of automated dipping systems has enhanced coating uniformity, while advancements in flux technology have improved surface preparation efficiency. Nevertheless, challenges persist in controlling coating thickness on complex geometries and managing zinc ash formation during the galvanizing process.
Geographically, Asia-Pacific dominates the zinc coating market, accounting for approximately 45% of global production, with China being the largest contributor. Europe and North America follow with sophisticated applications focusing on high-performance coatings for specialized industries. Emerging markets in South America and Africa are showing increased adoption of zinc coating technologies, albeit with less advanced process controls.
A significant technical challenge facing both technologies is the balance between coating thickness and functional performance. While thicker coatings generally provide longer corrosion protection, they may compromise dimensional tolerances and surface finish quality. Additionally, the industry faces increasing pressure to reduce environmental impact, particularly regarding zinc runoff and waste management.
Recent comparative testing has revealed that electroplated coatings offer superior aesthetic qualities and dimensional precision, while hot-dip galvanized coatings provide enhanced long-term corrosion resistance in aggressive environments. However, standardized testing methodologies remain inconsistent across different regions, complicating direct performance comparisons.
The development of hybrid coating systems, combining the advantages of both technologies, represents a promising but technically challenging frontier. Current research focuses on optimizing zinc-nickel alloy compositions for electroplating and developing more environmentally friendly flux systems for hot-dip processes, while exploring nanotechnology applications to enhance coating performance without increasing thickness.
Current Technical Solutions for Zinc Coating Applications
01 Electroplated zinc coating compositions and processes
Electroplating processes for applying zinc coatings to metal substrates involve specific bath compositions and electrical parameters. These processes typically use zinc salts in acidic or alkaline solutions with various additives to control deposition characteristics. Electroplated zinc coatings provide corrosion protection while allowing for precise thickness control and are suitable for complex geometries. The coatings can be further enhanced with post-treatments to improve corrosion resistance and appearance.- Hot-dip galvanizing process and composition: Hot-dip galvanizing involves immersing steel or iron components in molten zinc to form a protective coating. The process typically includes surface preparation, fluxing, dipping in a zinc bath at temperatures around 450°C, and cooling. Various additives such as aluminum, nickel, or magnesium can be incorporated into the zinc bath to enhance coating properties like corrosion resistance, adhesion, and appearance. The resulting coating consists of zinc-iron alloy layers topped with pure zinc, providing long-lasting protection against corrosion.
- Electroplating zinc coating methods: Electroplating processes for zinc coatings involve the deposition of zinc onto metal substrates using an electric current in an electrolyte solution. These processes can be controlled to achieve specific coating thicknesses, appearance, and properties. Various electrolyte compositions, current densities, and additives can be used to optimize the plating process and enhance coating characteristics. Electroplated zinc coatings typically provide good corrosion protection while allowing for precise control over coating thickness and uniformity.
- Zinc alloy coatings for enhanced corrosion resistance: Zinc alloy coatings incorporate additional elements such as nickel, iron, cobalt, or aluminum to enhance corrosion protection beyond what pure zinc can provide. These alloys can be applied through either electroplating or hot-dip processes. The addition of alloying elements can significantly improve coating properties including corrosion resistance, hardness, ductility, and wear resistance. Different alloy compositions are tailored for specific environmental conditions and applications, providing optimized protection for various exposure scenarios.
- Post-treatment and passivation of zinc coatings: Post-treatment processes for zinc coatings include chromate conversion coatings, phosphating, and application of organic sealers to enhance corrosion resistance and adhesion properties. These treatments create protective layers that prevent white rust formation and extend the service life of zinc coatings. Modern environmentally friendly alternatives to traditional chromate treatments include trivalent chromium, silicate-based, and organic passivation systems. Post-treatments can also improve the coating's appearance and provide a suitable surface for subsequent painting or powder coating applications.
- Specialized zinc coating applications and innovations: Advanced zinc coating technologies address specific industrial needs through innovations in application methods and composition. These include duplex systems combining zinc coatings with organic topcoats, specialized zinc flake coatings for high-temperature applications, and zinc-rich primers for maintenance coatings. Recent developments focus on improving coating uniformity on complex geometries, reducing environmental impact, enhancing coating performance in extreme environments, and developing self-healing zinc coating systems. These specialized applications extend the use of zinc coatings to challenging environments and demanding applications.
02 Hot-dip galvanizing methods and equipment
Hot-dip galvanizing involves immersing steel components in molten zinc at temperatures around 450°C. The process creates a metallurgical bond between the zinc and steel substrate, forming zinc-iron alloy layers that provide superior corrosion protection. The equipment used includes zinc baths, flux systems, and cooling mechanisms. Process parameters such as immersion time, withdrawal rate, and bath temperature significantly influence coating quality, thickness, and appearance.Expand Specific Solutions03 Zinc alloy coatings for enhanced performance
Zinc alloy coatings incorporate elements such as aluminum, magnesium, nickel, or iron to enhance specific properties. These alloying elements can improve corrosion resistance, hardness, ductility, or appearance compared to pure zinc coatings. The composition of these alloys can be tailored for specific applications and environments. Both electroplating and hot-dip processes can be modified to produce zinc alloy coatings with optimized performance characteristics.Expand Specific Solutions04 Surface treatments and post-processing of zinc coatings
Various post-processing treatments can be applied to zinc coatings to enhance their properties. These include chromate conversion coatings, phosphating, passivation treatments, and organic topcoats. Such treatments improve corrosion resistance, adhesion for subsequent painting, and aesthetic appearance. Post-processing can also include heat treatments to modify the microstructure of the zinc coating or to promote specific zinc-iron alloy formation, thereby enhancing durability and performance in aggressive environments.Expand Specific Solutions05 Corrosion resistance mechanisms and testing of zinc coatings
Zinc coatings protect steel substrates through both barrier protection and sacrificial cathodic protection. The effectiveness of these mechanisms depends on coating thickness, composition, and microstructure. Various accelerated testing methods are used to evaluate corrosion performance, including salt spray tests, electrochemical impedance spectroscopy, and cyclic corrosion testing. These tests help in predicting service life and optimizing coating formulations for specific environmental conditions and applications.Expand Specific Solutions
Major Industry Players in Zinc Coating Market
The zinc coating market is currently in a mature growth phase, characterized by established technologies and steady demand across automotive, construction, and infrastructure sectors. The global market size for zinc coatings is estimated at $4-5 billion annually, with projected growth of 3-4% CAGR through 2028. In terms of technical maturity, electroplated zinc coatings offer superior thickness control and aesthetic finish, while hot-dip galvanizing provides better corrosion resistance for harsh environments. Leading players include Tata Steel and POSCO Holdings in integrated steel production with coating capabilities; MacDermid and Atotech Deutschland as chemical specialists; and JFE Steel, NIPPON STEEL, and Dipsol Chemicals as technology innovators focusing on enhanced coating performance and environmental compliance. Regional competition is intensifying with Asian manufacturers like Yuken Industry expanding market share through cost advantages.
Tata Steel Ltd.
Technical Solution: Tata Steel has developed advanced comparative testing methodologies for zinc coatings that evaluate both electroplated and hot-dip galvanized products across multiple parameters. Their approach includes accelerated corrosion testing in salt spray chambers that simulate years of environmental exposure in weeks, electrochemical impedance spectroscopy to measure coating barrier properties, and adhesion testing using cross-cut and pull-off methods. Tata's testing protocol incorporates thickness uniformity measurements using magnetic gauges and microscopic cross-section analysis, allowing for precise comparison of coating distribution between the two processes. Their research has demonstrated that while hot-dip coatings typically provide 2-3 times longer corrosion protection in industrial environments, electroplated coatings offer superior surface finish with roughness values approximately 40% lower.
Strengths: Comprehensive testing infrastructure with advanced analytical equipment and established industry standards integration. Their large-scale production capabilities allow for industrial-scale validation of laboratory findings. Weaknesses: Testing methodologies may be optimized for their specific production processes, potentially limiting applicability to other manufacturing environments.
POSCO Holdings, Inc.
Technical Solution: POSCO has pioneered a multi-phase comparative testing framework for zinc coatings that evaluates performance across diverse environmental conditions. Their methodology includes cyclic corrosion testing that alternates between salt spray, humidity, and dry conditions to simulate real-world exposure scenarios more accurately than traditional single-environment tests. POSCO's approach incorporates microstructural analysis using scanning electron microscopy to examine zinc-iron intermetallic layers, which are critical to understanding coating durability differences. Their research has quantified that hot-dip galvanized coatings typically exhibit 30-50% better edge protection than electroplated alternatives due to the naturally thicker coating at edges and corners. POSCO has also developed specialized impact resistance testing that demonstrates hot-dip coatings' superior performance under mechanical stress, while acknowledging electroplated coatings' advantages in dimensional precision for threaded components.
Strengths: Industry-leading metallurgical expertise and advanced testing facilities that can simulate diverse environmental conditions. Their testing protocols are widely recognized in the automotive and construction sectors. Weaknesses: Testing may emphasize applications relevant to their primary markets (automotive, construction), potentially overlooking niche applications where electroplated coatings might excel.
Key Technical Innovations in Coating Performance Testing
Corrosion-resistant valve disc
PatentWO2016110840A1
Innovation
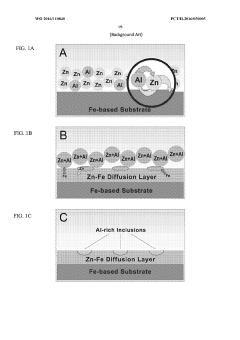
- A combination of a zinc and iron diffusion layer with discrete aluminum-rich inclusions, followed by a polymeric coating, specifically using polymers like nylon or ECTFE, to enhance corrosion resistance and adhesion, thereby creating a synergistic anti-corrosion effect.
Environmental Impact Assessment of Zinc Coating Processes
The environmental impact of zinc coating processes represents a critical consideration in the selection between electroplating and hot-dip galvanizing methods. Both processes serve the essential function of providing corrosion protection to steel substrates, yet their environmental footprints differ significantly across multiple dimensions.
Electroplating zinc coatings typically involve cyanide or acid-based electrolyte solutions that present substantial environmental hazards if not properly managed. These processes generate wastewater containing heavy metals, cyanides, and other toxic compounds that require extensive treatment before discharge. The energy consumption in electroplating is generally lower per unit area compared to hot-dip processes, but the environmental burden of chemical waste management often offsets this advantage.
Hot-dip galvanizing, while more energy-intensive due to the high temperatures required to maintain zinc in molten state (approximately 450°C), produces significantly less hazardous waste. The primary environmental concerns with hot-dip processes relate to atmospheric emissions, including zinc oxide particulates and flux fumes containing ammonium chloride. However, modern facilities have implemented efficient capture and filtration systems that substantially reduce these emissions.
Life cycle assessment (LCA) studies comparing these processes indicate that hot-dip galvanizing generally demonstrates superior environmental performance in terms of longevity and reduced maintenance requirements. The thicker zinc coating provided by hot-dip processes (typically 50-200 μm versus 5-25 μm for electroplating) translates to extended service life, reducing the environmental impact associated with reapplication and maintenance operations.
Water consumption represents another significant environmental factor. Electroplating processes typically require multiple rinse stages, consuming substantial volumes of water. Recent technological advancements have introduced closed-loop water recycling systems for electroplating operations, though implementation remains inconsistent across the industry. Hot-dip processes, by contrast, consume minimal water, primarily for cooling and quenching operations.
Waste zinc recovery efficiency also differs between these processes. Hot-dip operations can reclaim approximately 95% of zinc waste through dross recovery and recycling, while electroplating recovery rates typically range from 60-80%, depending on the sophistication of recovery systems employed. This disparity significantly affects the overall resource efficiency and sustainability profile of each process.
Regulatory compliance costs further differentiate these coating methods. Electroplating facilities face increasingly stringent regulations regarding chemical handling, wastewater discharge, and worker safety, driving up operational costs and environmental management requirements. Hot-dip operations, while subject to air quality regulations, generally encounter fewer compliance challenges related to hazardous waste management.
Electroplating zinc coatings typically involve cyanide or acid-based electrolyte solutions that present substantial environmental hazards if not properly managed. These processes generate wastewater containing heavy metals, cyanides, and other toxic compounds that require extensive treatment before discharge. The energy consumption in electroplating is generally lower per unit area compared to hot-dip processes, but the environmental burden of chemical waste management often offsets this advantage.
Hot-dip galvanizing, while more energy-intensive due to the high temperatures required to maintain zinc in molten state (approximately 450°C), produces significantly less hazardous waste. The primary environmental concerns with hot-dip processes relate to atmospheric emissions, including zinc oxide particulates and flux fumes containing ammonium chloride. However, modern facilities have implemented efficient capture and filtration systems that substantially reduce these emissions.
Life cycle assessment (LCA) studies comparing these processes indicate that hot-dip galvanizing generally demonstrates superior environmental performance in terms of longevity and reduced maintenance requirements. The thicker zinc coating provided by hot-dip processes (typically 50-200 μm versus 5-25 μm for electroplating) translates to extended service life, reducing the environmental impact associated with reapplication and maintenance operations.
Water consumption represents another significant environmental factor. Electroplating processes typically require multiple rinse stages, consuming substantial volumes of water. Recent technological advancements have introduced closed-loop water recycling systems for electroplating operations, though implementation remains inconsistent across the industry. Hot-dip processes, by contrast, consume minimal water, primarily for cooling and quenching operations.
Waste zinc recovery efficiency also differs between these processes. Hot-dip operations can reclaim approximately 95% of zinc waste through dross recovery and recycling, while electroplating recovery rates typically range from 60-80%, depending on the sophistication of recovery systems employed. This disparity significantly affects the overall resource efficiency and sustainability profile of each process.
Regulatory compliance costs further differentiate these coating methods. Electroplating facilities face increasingly stringent regulations regarding chemical handling, wastewater discharge, and worker safety, driving up operational costs and environmental management requirements. Hot-dip operations, while subject to air quality regulations, generally encounter fewer compliance challenges related to hazardous waste management.
Cost-Benefit Analysis of Different Zinc Coating Methods
When evaluating zinc coating methods for corrosion protection, cost-benefit analysis provides critical insights for decision-makers. Electroplating and hot-dip galvanizing represent two distinct approaches with significant economic implications across their lifecycle.
Initial investment costs differ substantially between these methods. Electroplating facilities typically require lower capital expenditure for equipment setup, making them more accessible for smaller operations. Conversely, hot-dip galvanizing demands higher upfront investment in larger facilities, specialized equipment, and more robust safety systems to manage molten zinc baths operating at approximately 450°C.
Operational expenses reveal further distinctions. Electroplating demonstrates higher energy efficiency during application but requires more precise process control and specialized chemicals. Hot-dip galvanizing consumes more energy to maintain zinc baths at operating temperature but offers faster processing times for large batches, potentially reducing labor costs per unit.
Material consumption patterns also impact overall economics. Electroplating achieves thinner coatings (typically 5-25 μm) with more precise thickness control, resulting in lower zinc consumption per item. Hot-dip galvanizing produces thicker coatings (50-200 μm), consuming more zinc but potentially extending service life proportionally.
Durability considerations significantly influence lifetime value calculations. Hot-dip coatings generally demonstrate superior longevity in harsh environments, with service life often exceeding 50 years in non-extreme conditions. This extended protection period can offset higher initial costs through reduced maintenance and replacement expenses. Electroplated coatings, while adequate for many applications, typically offer shorter protection periods, necessitating more frequent reapplication.
Environmental compliance costs increasingly affect total ownership calculations. Both processes face regulatory scrutiny, but electroplating often encounters stricter waste management requirements due to chemical byproducts. Hot-dip operations must address zinc ash and dross disposal, though these materials are more readily recyclable.
Application-specific factors ultimately determine optimal cost-benefit outcomes. For small, precision components produced in high volumes, electroplating often proves more economical despite shorter coating life. For structural steel and large components exposed to harsh environments, hot-dip galvanizing's higher upfront cost is frequently justified through extended maintenance-free periods and reduced lifecycle expenses.
Initial investment costs differ substantially between these methods. Electroplating facilities typically require lower capital expenditure for equipment setup, making them more accessible for smaller operations. Conversely, hot-dip galvanizing demands higher upfront investment in larger facilities, specialized equipment, and more robust safety systems to manage molten zinc baths operating at approximately 450°C.
Operational expenses reveal further distinctions. Electroplating demonstrates higher energy efficiency during application but requires more precise process control and specialized chemicals. Hot-dip galvanizing consumes more energy to maintain zinc baths at operating temperature but offers faster processing times for large batches, potentially reducing labor costs per unit.
Material consumption patterns also impact overall economics. Electroplating achieves thinner coatings (typically 5-25 μm) with more precise thickness control, resulting in lower zinc consumption per item. Hot-dip galvanizing produces thicker coatings (50-200 μm), consuming more zinc but potentially extending service life proportionally.
Durability considerations significantly influence lifetime value calculations. Hot-dip coatings generally demonstrate superior longevity in harsh environments, with service life often exceeding 50 years in non-extreme conditions. This extended protection period can offset higher initial costs through reduced maintenance and replacement expenses. Electroplated coatings, while adequate for many applications, typically offer shorter protection periods, necessitating more frequent reapplication.
Environmental compliance costs increasingly affect total ownership calculations. Both processes face regulatory scrutiny, but electroplating often encounters stricter waste management requirements due to chemical byproducts. Hot-dip operations must address zinc ash and dross disposal, though these materials are more readily recyclable.
Application-specific factors ultimately determine optimal cost-benefit outcomes. For small, precision components produced in high volumes, electroplating often proves more economical despite shorter coating life. For structural steel and large components exposed to harsh environments, hot-dip galvanizing's higher upfront cost is frequently justified through extended maintenance-free periods and reduced lifecycle expenses.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!