Zinc coating corrosion resistance under salt spray and humidity conditions
OCT 11, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Zinc Coating Technology Background and Objectives
Zinc coating technology has evolved significantly over the past century as a primary method for protecting steel and iron from corrosion. Initially developed in the early 19th century, hot-dip galvanizing became commercially viable by the 1840s, revolutionizing metal protection. The fundamental principle behind zinc coating's effectiveness lies in its sacrificial protection mechanism, where zinc corrodes preferentially to protect the underlying steel substrate.
The evolution of zinc coating technologies has been driven by increasing demands for durability in aggressive environments. Traditional hot-dip galvanizing has been supplemented by electrogalvanizing, thermal spraying, sherardizing, and mechanical plating processes. Each advancement has aimed to enhance corrosion resistance while optimizing coating thickness, adhesion, and uniformity for specific applications.
Salt spray and humidity conditions represent particularly challenging environments for zinc coatings, as they accelerate the corrosion process through electrochemical reactions. The marine atmosphere, with its high chloride content, and industrial environments with sulfur dioxide emissions pose significant threats to zinc-coated materials. Understanding the performance of zinc coatings under these conditions is crucial for applications in coastal infrastructure, automotive components, and outdoor equipment.
Recent technological developments have focused on improving zinc coating formulations through alloying elements such as aluminum, magnesium, and nickel. These zinc-alloy coatings have demonstrated superior corrosion resistance compared to pure zinc coatings, particularly in salt-rich environments. Additionally, advancements in application techniques have enabled more precise control over coating thickness and composition, resulting in more consistent protection.
The primary objective of current zinc coating research is to develop next-generation protective systems that can withstand increasingly severe environmental conditions while meeting sustainability requirements. This includes extending service life in high-salt and high-humidity environments, reducing coating thickness without compromising protection, and eliminating environmentally harmful substances from the coating process.
Another key goal is to establish standardized testing methodologies that accurately predict real-world performance. Current accelerated testing protocols often fail to correlate precisely with field performance, creating challenges for product development and quality assurance. Developing more representative testing methods would enable more accurate lifetime predictions and performance comparisons between different coating systems.
Furthermore, research aims to integrate zinc coating technology with smart monitoring capabilities, allowing for real-time assessment of coating integrity and early detection of corrosion initiation. This integration of traditional protection methods with digital technologies represents the frontier of corrosion protection systems for the coming decade.
The evolution of zinc coating technologies has been driven by increasing demands for durability in aggressive environments. Traditional hot-dip galvanizing has been supplemented by electrogalvanizing, thermal spraying, sherardizing, and mechanical plating processes. Each advancement has aimed to enhance corrosion resistance while optimizing coating thickness, adhesion, and uniformity for specific applications.
Salt spray and humidity conditions represent particularly challenging environments for zinc coatings, as they accelerate the corrosion process through electrochemical reactions. The marine atmosphere, with its high chloride content, and industrial environments with sulfur dioxide emissions pose significant threats to zinc-coated materials. Understanding the performance of zinc coatings under these conditions is crucial for applications in coastal infrastructure, automotive components, and outdoor equipment.
Recent technological developments have focused on improving zinc coating formulations through alloying elements such as aluminum, magnesium, and nickel. These zinc-alloy coatings have demonstrated superior corrosion resistance compared to pure zinc coatings, particularly in salt-rich environments. Additionally, advancements in application techniques have enabled more precise control over coating thickness and composition, resulting in more consistent protection.
The primary objective of current zinc coating research is to develop next-generation protective systems that can withstand increasingly severe environmental conditions while meeting sustainability requirements. This includes extending service life in high-salt and high-humidity environments, reducing coating thickness without compromising protection, and eliminating environmentally harmful substances from the coating process.
Another key goal is to establish standardized testing methodologies that accurately predict real-world performance. Current accelerated testing protocols often fail to correlate precisely with field performance, creating challenges for product development and quality assurance. Developing more representative testing methods would enable more accurate lifetime predictions and performance comparisons between different coating systems.
Furthermore, research aims to integrate zinc coating technology with smart monitoring capabilities, allowing for real-time assessment of coating integrity and early detection of corrosion initiation. This integration of traditional protection methods with digital technologies represents the frontier of corrosion protection systems for the coming decade.
Market Demand Analysis for Corrosion-Resistant Coatings
The global market for corrosion-resistant coatings, particularly zinc-based solutions, has experienced substantial growth driven by increasing industrial applications and infrastructure development. Current market valuations indicate the corrosion-resistant coating sector exceeds $30 billion annually, with zinc coatings representing approximately 25% of this market share. Industry forecasts project a compound annual growth rate of 5.7% through 2028, significantly outpacing general economic growth indicators.
The automotive industry remains the largest consumer of zinc corrosion-resistant coatings, accounting for roughly 38% of total market demand. This sector's requirements are increasingly stringent, with manufacturers seeking coatings that can withstand 1,000+ hours of salt spray testing while maintaining aesthetic qualities. The construction industry follows closely at 27% market share, where demand focuses on coatings that can provide 15-20 year protection in diverse environmental conditions.
Marine and offshore applications represent the fastest-growing segment, expanding at 7.3% annually due to increased offshore wind farm development and maritime infrastructure projects. These applications demand exceptional performance under extreme salt spray conditions, with resistance requirements often exceeding 3,000 hours in accelerated testing environments.
Regional analysis reveals Asia-Pacific as the dominant market, consuming 42% of global zinc coating production, followed by Europe (28%) and North America (21%). Notably, emerging economies in Southeast Asia and Latin America are experiencing demand growth rates exceeding 9% annually, primarily driven by rapid industrialization and infrastructure development projects.
Customer requirements have evolved significantly, with end-users increasingly prioritizing environmentally sustainable solutions. The market shows growing preference for zinc coating systems that eliminate hexavalent chromium and reduce volatile organic compounds while maintaining performance standards. This trend is particularly pronounced in European markets, where regulatory frameworks like REACH have accelerated the transition toward greener alternatives.
Price sensitivity varies considerably across application segments. While automotive and aerospace sectors demonstrate willingness to pay premium prices for high-performance coatings, construction and general industrial applications remain highly cost-conscious, creating distinct market tiers with different value propositions and performance expectations.
The aftermarket segment for zinc coating repair and maintenance has emerged as a significant opportunity, currently valued at approximately $4.2 billion globally with projected growth of 6.5% annually, outpacing the primary coating market and offering higher profit margins for suppliers who can provide effective restoration solutions for existing zinc-coated structures.
The automotive industry remains the largest consumer of zinc corrosion-resistant coatings, accounting for roughly 38% of total market demand. This sector's requirements are increasingly stringent, with manufacturers seeking coatings that can withstand 1,000+ hours of salt spray testing while maintaining aesthetic qualities. The construction industry follows closely at 27% market share, where demand focuses on coatings that can provide 15-20 year protection in diverse environmental conditions.
Marine and offshore applications represent the fastest-growing segment, expanding at 7.3% annually due to increased offshore wind farm development and maritime infrastructure projects. These applications demand exceptional performance under extreme salt spray conditions, with resistance requirements often exceeding 3,000 hours in accelerated testing environments.
Regional analysis reveals Asia-Pacific as the dominant market, consuming 42% of global zinc coating production, followed by Europe (28%) and North America (21%). Notably, emerging economies in Southeast Asia and Latin America are experiencing demand growth rates exceeding 9% annually, primarily driven by rapid industrialization and infrastructure development projects.
Customer requirements have evolved significantly, with end-users increasingly prioritizing environmentally sustainable solutions. The market shows growing preference for zinc coating systems that eliminate hexavalent chromium and reduce volatile organic compounds while maintaining performance standards. This trend is particularly pronounced in European markets, where regulatory frameworks like REACH have accelerated the transition toward greener alternatives.
Price sensitivity varies considerably across application segments. While automotive and aerospace sectors demonstrate willingness to pay premium prices for high-performance coatings, construction and general industrial applications remain highly cost-conscious, creating distinct market tiers with different value propositions and performance expectations.
The aftermarket segment for zinc coating repair and maintenance has emerged as a significant opportunity, currently valued at approximately $4.2 billion globally with projected growth of 6.5% annually, outpacing the primary coating market and offering higher profit margins for suppliers who can provide effective restoration solutions for existing zinc-coated structures.
Current State and Challenges in Zinc Coating Technology
Zinc coating technology has evolved significantly over the past decades, establishing itself as one of the most effective methods for protecting steel against corrosion. Currently, the global market employs several zinc coating techniques including hot-dip galvanizing, electrogalvanizing, zinc spraying, and zinc-rich paints. Each method offers varying degrees of protection depending on coating thickness, composition, and application process. Hot-dip galvanizing remains the most widely used technique due to its cost-effectiveness and durability, providing coating thicknesses ranging from 45 to 200 μm.
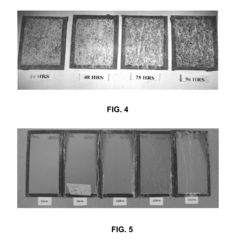
Despite widespread adoption, zinc coatings face significant challenges in aggressive environments, particularly under salt spray and high humidity conditions. Recent studies indicate that traditional zinc coatings begin to deteriorate after 500-1000 hours of salt spray testing (ASTM B117), with red rust formation accelerating thereafter. This performance gap becomes particularly problematic in coastal regions, road infrastructure exposed to de-icing salts, and marine applications where chloride ions rapidly attack the protective layer.
The primary technical challenge lies in the inherent electrochemical properties of zinc. While zinc provides sacrificial protection to steel, its relatively rapid dissolution rate in chloride-rich environments limits long-term effectiveness. Current research indicates that pure zinc coatings dissolve at rates of 5-15 μm per year in moderate marine environments, but this can increase to 20-65 μm annually in severe coastal or industrial settings.
Another significant limitation is the "white rust" phenomenon—the formation of zinc hydroxide and zinc carbonate on the coating surface. These corrosion products, while temporarily protective, can accelerate degradation under cyclic wet-dry conditions typical in real-world applications. Industry testing reveals that humidity cycling between 60-95% relative humidity significantly reduces coating lifespan compared to constant exposure conditions.
Geographically, zinc coating technology development shows distinct patterns. European research focuses heavily on environmentally friendly alternatives to traditional chromate passivation treatments, while North American efforts concentrate on enhancing coating durability for infrastructure applications. Asian markets, particularly China and Japan, lead in developing novel zinc alloy coatings that incorporate aluminum, magnesium, and rare earth elements to enhance performance.
The technical constraints are further complicated by regulatory pressures. Global restrictions on hexavalent chromium—historically used as a passivation treatment—have necessitated the development of alternative surface treatments that often do not match the performance of chromate systems, particularly in harsh environments. This regulatory landscape has created a significant innovation gap that industry and research institutions are actively working to address.
Despite widespread adoption, zinc coatings face significant challenges in aggressive environments, particularly under salt spray and high humidity conditions. Recent studies indicate that traditional zinc coatings begin to deteriorate after 500-1000 hours of salt spray testing (ASTM B117), with red rust formation accelerating thereafter. This performance gap becomes particularly problematic in coastal regions, road infrastructure exposed to de-icing salts, and marine applications where chloride ions rapidly attack the protective layer.
The primary technical challenge lies in the inherent electrochemical properties of zinc. While zinc provides sacrificial protection to steel, its relatively rapid dissolution rate in chloride-rich environments limits long-term effectiveness. Current research indicates that pure zinc coatings dissolve at rates of 5-15 μm per year in moderate marine environments, but this can increase to 20-65 μm annually in severe coastal or industrial settings.
Another significant limitation is the "white rust" phenomenon—the formation of zinc hydroxide and zinc carbonate on the coating surface. These corrosion products, while temporarily protective, can accelerate degradation under cyclic wet-dry conditions typical in real-world applications. Industry testing reveals that humidity cycling between 60-95% relative humidity significantly reduces coating lifespan compared to constant exposure conditions.
Geographically, zinc coating technology development shows distinct patterns. European research focuses heavily on environmentally friendly alternatives to traditional chromate passivation treatments, while North American efforts concentrate on enhancing coating durability for infrastructure applications. Asian markets, particularly China and Japan, lead in developing novel zinc alloy coatings that incorporate aluminum, magnesium, and rare earth elements to enhance performance.
The technical constraints are further complicated by regulatory pressures. Global restrictions on hexavalent chromium—historically used as a passivation treatment—have necessitated the development of alternative surface treatments that often do not match the performance of chromate systems, particularly in harsh environments. This regulatory landscape has created a significant innovation gap that industry and research institutions are actively working to address.
Existing Zinc Coating Solutions for Harsh Environments
01 Zinc coating composition for enhanced corrosion resistance
Specific compositions of zinc coatings can significantly enhance corrosion resistance properties. These compositions may include zinc alloys with elements such as aluminum, magnesium, or other metals that create a more effective barrier against corrosive environments. The precise formulation of these coatings affects their protective capabilities, durability, and performance in various environmental conditions.- Zinc coating composition for enhanced corrosion resistance: Specific compositions of zinc coatings can significantly enhance corrosion resistance properties. These compositions may include zinc alloys with elements such as aluminum, magnesium, or other metals that create a more effective barrier against corrosive environments. The precise formulation of these coatings affects their durability, adhesion, and protective qualities, making them suitable for various industrial applications where metal components are exposed to harsh conditions.
- Surface treatment methods for zinc coatings: Various surface treatment methods can be applied to zinc coatings to improve their corrosion resistance. These treatments include passivation processes, chromate treatments, phosphating, and other chemical or electrochemical processes that modify the surface properties of the zinc coating. These treatments create protective layers that enhance the barrier properties of the coating and provide additional protection against corrosive elements.
- Multi-layer zinc coating systems: Multi-layer coating systems that incorporate zinc layers provide superior corrosion protection compared to single-layer applications. These systems typically consist of a zinc base layer followed by one or more additional protective layers, which may include other metals, polymers, or conversion coatings. The combination of different materials creates a synergistic effect that enhances overall corrosion resistance while maintaining other desirable properties such as appearance and wear resistance.
- Zinc coating application techniques: Various application techniques for zinc coatings significantly impact their corrosion resistance properties. These techniques include hot-dip galvanizing, electroplating, thermal spraying, and mechanical plating. Each method produces coatings with different microstructures, thicknesses, and adhesion characteristics, which directly affect their protective capabilities. The selection of the appropriate application technique depends on the specific requirements of the end-use application and the desired level of corrosion protection.
- Additives and inhibitors for zinc coatings: Incorporating specific additives and corrosion inhibitors into zinc coatings can significantly enhance their protective properties. These additives may include organic compounds, nanoparticles, or inorganic substances that provide additional barrier protection or actively inhibit corrosion processes. Some additives also improve the coating's adhesion, flexibility, or self-healing capabilities, extending the service life of the protected substrate even in aggressive environments.
02 Surface treatment methods for zinc coatings
Various surface treatment methods can be applied to zinc coatings to improve their corrosion resistance. These treatments may include chemical passivation, chromate conversion coatings, or post-treatment sealers. Such processes create additional protective layers or modify the surface properties of the zinc coating to enhance its ability to withstand corrosive elements and extend the service life of the coated material.Expand Specific Solutions03 Multi-layer zinc coating systems
Multi-layer coating systems that incorporate zinc layers provide superior corrosion protection compared to single-layer applications. These systems typically consist of a zinc base layer followed by one or more additional protective layers, which may include organic coatings, sealers, or other metallic layers. The combination of different materials creates a synergistic effect that enhances overall corrosion resistance and extends the durability of the protected substrate.Expand Specific Solutions04 Zinc coating application techniques
The method of applying zinc coatings significantly impacts their corrosion resistance properties. Various application techniques include hot-dip galvanizing, electroplating, thermal spraying, and mechanical plating. Each technique produces coatings with different microstructures, thicknesses, and adhesion characteristics, which in turn affect their corrosion resistance performance. The selection of the appropriate application method depends on the specific requirements of the end-use application.Expand Specific Solutions05 Additives and inhibitors for zinc coatings
Incorporating specific additives and corrosion inhibitors into zinc coatings can substantially improve their protective capabilities. These additives may include organic compounds, nanoparticles, or specialized inhibitors that enhance the coating's barrier properties or provide active corrosion protection. Such formulations can offer targeted protection against specific corrosive environments or extend the effective lifespan of the zinc coating under challenging conditions.Expand Specific Solutions
Major Industry Players in Zinc Coating Solutions
The zinc coating corrosion resistance market is currently in a growth phase, driven by increasing demand for durable protective coatings across automotive, construction, and industrial sectors. The global market size is estimated to exceed $5 billion, with projected annual growth of 4-6% through 2028. Technologically, the field shows varying maturity levels, with established players like Tata Steel and NIPPON STEEL offering traditional hot-dip galvanizing solutions, while innovation leaders such as BASF Coatings, PPG Industries, and Global Graphene Group are developing advanced nano-coatings and environmentally friendly alternatives. Companies like NOF Metal Coatings and Atotech Deutschland are pioneering zinc flake technologies that demonstrate superior performance under extreme salt spray and humidity conditions, positioning themselves at the forefront of next-generation corrosion protection solutions.
BASF Coatings GmbH
Technical Solution: BASF Coatings has developed advanced zinc-rich primer systems that combine organic and inorganic zinc components to enhance corrosion protection in harsh environments. Their ZeroKote technology incorporates nano-zinc particles dispersed in specialized polymer matrices, creating a more uniform protective layer that provides superior barrier protection and cathodic protection simultaneously. The system includes a proprietary sealing technology that prevents zinc leaching during salt spray exposure while maintaining electrical conductivity for continued cathodic protection. BASF's multi-layer approach combines zinc-rich primers with specialized topcoats containing corrosion inhibitors that activate under specific environmental triggers, providing dynamic protection that responds to changing conditions. Their coatings demonstrate excellent performance in ASTM B117 salt spray tests, maintaining integrity for over 3,000 hours.
Strengths: Superior barrier and cathodic protection through nano-zinc technology; responsive corrosion inhibitor systems; excellent adhesion to various substrates. Weaknesses: Higher initial cost compared to conventional zinc coatings; requires precise application parameters; may have limited effectiveness in extremely acidic environments.
Bekaert SA
Technical Solution: Bekaert has pioneered advanced zinc coating technologies for steel wire and related products, focusing on enhanced corrosion resistance in demanding environments. Their Bezinal® coating technology incorporates a precisely controlled zinc-aluminum alloy that forms a more stable and corrosion-resistant surface compared to pure zinc coatings. The aluminum content creates a thin, transparent aluminum oxide layer that provides additional barrier protection while maintaining the sacrificial properties of zinc. Bekaert's process includes proprietary drawing techniques that maintain coating integrity even during severe deformation, ensuring continued protection in formed products. Their research has demonstrated that Bezinal® coatings provide 3-4 times longer protection than standard galvanized coatings in salt spray tests and significantly improved performance in cyclic humidity testing. For specialized applications, Bekaert has developed Bezinal®+ with additional magnesium content, further enhancing corrosion resistance in chloride-rich environments.
Strengths: Superior protection in marine and industrial environments; excellent coating adhesion during wire drawing and forming; reduced zinc consumption. Weaknesses: Higher production complexity; more sensitive to process parameters; potentially higher initial cost compared to standard galvanizing.
Key Technical Innovations in Zinc Coating Formulations
Dry-in-place corrosion-resistant coating for zinc or zinc-alloy coated substrates
PatentInactiveUS20150176135A1
Innovation
- A corrosion-resistant coating is developed by combining zinc phosphate, chromium, and silicate compounds with water to form a mixed aqueous solution, which is applied to zinc or zinc-alloy surfaces, forming a covalent bond and providing enhanced chemical resistance exceeding 150 hours in ASTM B117 standards.
Environmental Impact of Zinc Coating Technologies
The environmental impact of zinc coating technologies extends beyond their primary function of corrosion protection. Traditional zinc coating processes, particularly hot-dip galvanizing, have historically been associated with significant environmental concerns including high energy consumption, greenhouse gas emissions, and the generation of hazardous waste. The zinc smelting process alone accounts for approximately 0.4% of global CO2 emissions, highlighting the carbon footprint of these protective technologies.
Water pollution represents another critical environmental challenge, as zinc coating operations can release heavy metals and acidic compounds into waterways if effluent is not properly treated. Studies indicate that zinc concentrations exceeding 120 μg/L in aquatic environments can adversely affect fish and other aquatic organisms, potentially disrupting entire ecosystems. This is particularly relevant when considering the lifecycle environmental impact of zinc-coated products exposed to salt spray and humidity conditions.
Recent advancements in zinc coating technologies have focused on reducing these environmental impacts. Water-based zinc-rich coatings have emerged as alternatives to traditional solvent-based systems, reducing volatile organic compound (VOC) emissions by up to 80%. Similarly, thermal spray zinc coating processes have been optimized to reduce energy consumption by approximately 30% compared to conventional hot-dip galvanizing methods.
The end-of-life management of zinc-coated products presents both challenges and opportunities. While zinc is theoretically 100% recyclable without loss of physical or chemical properties, the recovery rate from coated products averages only 60% globally. This represents a significant loss of resources and potential environmental contamination, particularly when zinc coatings degrade under harsh salt spray and humidity conditions.
Regulatory frameworks worldwide are increasingly addressing the environmental aspects of zinc coating technologies. The European Union's REACH regulation has placed restrictions on certain zinc compounds used in coating processes, while the EPA in the United States has established effluent guidelines specifically for metal finishing operations including zinc coating. These regulations have driven innovation in more environmentally friendly zinc coating technologies that maintain corrosion resistance performance.
Life cycle assessment (LCA) studies comparing various zinc coating technologies have demonstrated that despite their environmental impacts, the extended service life they provide to steel structures results in net environmental benefits. A properly applied zinc coating can extend the service life of steel by 50-100 years, significantly reducing the need for replacement and the associated environmental impacts of steel production.
Water pollution represents another critical environmental challenge, as zinc coating operations can release heavy metals and acidic compounds into waterways if effluent is not properly treated. Studies indicate that zinc concentrations exceeding 120 μg/L in aquatic environments can adversely affect fish and other aquatic organisms, potentially disrupting entire ecosystems. This is particularly relevant when considering the lifecycle environmental impact of zinc-coated products exposed to salt spray and humidity conditions.
Recent advancements in zinc coating technologies have focused on reducing these environmental impacts. Water-based zinc-rich coatings have emerged as alternatives to traditional solvent-based systems, reducing volatile organic compound (VOC) emissions by up to 80%. Similarly, thermal spray zinc coating processes have been optimized to reduce energy consumption by approximately 30% compared to conventional hot-dip galvanizing methods.
The end-of-life management of zinc-coated products presents both challenges and opportunities. While zinc is theoretically 100% recyclable without loss of physical or chemical properties, the recovery rate from coated products averages only 60% globally. This represents a significant loss of resources and potential environmental contamination, particularly when zinc coatings degrade under harsh salt spray and humidity conditions.
Regulatory frameworks worldwide are increasingly addressing the environmental aspects of zinc coating technologies. The European Union's REACH regulation has placed restrictions on certain zinc compounds used in coating processes, while the EPA in the United States has established effluent guidelines specifically for metal finishing operations including zinc coating. These regulations have driven innovation in more environmentally friendly zinc coating technologies that maintain corrosion resistance performance.
Life cycle assessment (LCA) studies comparing various zinc coating technologies have demonstrated that despite their environmental impacts, the extended service life they provide to steel structures results in net environmental benefits. A properly applied zinc coating can extend the service life of steel by 50-100 years, significantly reducing the need for replacement and the associated environmental impacts of steel production.
Testing Standards and Quality Control Methodologies
The evaluation of zinc coating corrosion resistance requires adherence to rigorous testing standards and quality control methodologies to ensure consistent and reliable performance assessment. The most widely recognized standard for salt spray testing is ASTM B117, which establishes uniform conditions for creating and maintaining a salt spray (fog) testing environment. This standard specifies precise parameters including temperature (35°C), salt concentration (5% NaCl), pH range (6.5-7.2), and continuous spray exposure protocols.
For humidity testing, ASTM D1735 provides standardized procedures for water fog testing, while ISO 6270-2 establishes methods for determining resistance to humidity under condensation conditions. These standards ensure that zinc coatings are evaluated under controlled environmental conditions that simulate real-world exposure scenarios.
Quality control methodologies for zinc coating corrosion testing incorporate multiple inspection points throughout the testing process. Pre-test quality control includes verification of sample preparation consistency, coating thickness measurement using methods outlined in ASTM B499 or ISO 2178, and surface cleanliness assessment. During testing, regular calibration of equipment, monitoring of environmental parameters, and documentation of chamber conditions are essential for maintaining test validity.
Post-test evaluation follows standardized rating systems such as ASTM D610 for rust evaluation and ASTM D714 for blistering assessment. The ISO 8993 and ISO 8994 standards provide visual methods for assessing the degree of corrosion, while ASTM D1654 establishes procedures for evaluating painted or coated specimens subjected to corrosive environments.
Advanced quality control approaches incorporate statistical process control (SPC) techniques to monitor test variability and ensure reproducibility across multiple test cycles. Six Sigma methodologies are increasingly applied to reduce variation in testing procedures and improve the reliability of corrosion resistance data.
Digital imaging analysis has emerged as a valuable tool for objective assessment of corrosion progression, allowing for quantitative measurement of corroded areas and reducing subjective interpretation bias. These systems typically employ specialized software that can detect color changes, surface irregularities, and corrosion product formation with greater precision than visual inspection alone.
Interlaboratory testing programs, such as those coordinated by ASTM International and ISO technical committees, provide external validation of testing methodologies and help establish confidence intervals for performance expectations across different testing facilities.
For humidity testing, ASTM D1735 provides standardized procedures for water fog testing, while ISO 6270-2 establishes methods for determining resistance to humidity under condensation conditions. These standards ensure that zinc coatings are evaluated under controlled environmental conditions that simulate real-world exposure scenarios.
Quality control methodologies for zinc coating corrosion testing incorporate multiple inspection points throughout the testing process. Pre-test quality control includes verification of sample preparation consistency, coating thickness measurement using methods outlined in ASTM B499 or ISO 2178, and surface cleanliness assessment. During testing, regular calibration of equipment, monitoring of environmental parameters, and documentation of chamber conditions are essential for maintaining test validity.
Post-test evaluation follows standardized rating systems such as ASTM D610 for rust evaluation and ASTM D714 for blistering assessment. The ISO 8993 and ISO 8994 standards provide visual methods for assessing the degree of corrosion, while ASTM D1654 establishes procedures for evaluating painted or coated specimens subjected to corrosive environments.
Advanced quality control approaches incorporate statistical process control (SPC) techniques to monitor test variability and ensure reproducibility across multiple test cycles. Six Sigma methodologies are increasingly applied to reduce variation in testing procedures and improve the reliability of corrosion resistance data.
Digital imaging analysis has emerged as a valuable tool for objective assessment of corrosion progression, allowing for quantitative measurement of corroded areas and reducing subjective interpretation bias. These systems typically employ specialized software that can detect color changes, surface irregularities, and corrosion product formation with greater precision than visual inspection alone.
Interlaboratory testing programs, such as those coordinated by ASTM International and ISO technical committees, provide external validation of testing methodologies and help establish confidence intervals for performance expectations across different testing facilities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!