Comparing Luteolin Absorption in Human Gut Models
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Luteolin Absorption Background and Research Objectives
Luteolin, a flavonoid compound found abundantly in various fruits, vegetables, and medicinal herbs, has garnered significant attention in the scientific community due to its potential health benefits. The historical trajectory of luteolin research dates back to traditional medicine practices across different cultures, where plants rich in this compound were used for their anti-inflammatory and antioxidant properties. Over the past two decades, scientific interest in luteolin has intensified, with research expanding from basic biochemical characterization to sophisticated absorption and bioavailability studies.
The evolution of luteolin research has been marked by significant technological advancements in analytical methods. Early studies relied on rudimentary extraction and identification techniques, while contemporary research employs high-performance liquid chromatography (HPLC), mass spectrometry, and other advanced analytical tools to precisely quantify luteolin and its metabolites in biological samples. This technological progression has enabled more accurate assessment of luteolin absorption patterns and metabolic fate.
Current scientific understanding indicates that luteolin absorption in the human gut is complex and influenced by multiple factors including food matrix effects, gut microbiota composition, and individual genetic variations. Despite growing knowledge, significant gaps remain in understanding the precise mechanisms of luteolin absorption, particularly regarding the role of intestinal transporters and the impact of different gut environments on bioavailability.
The primary objective of this technical research is to systematically compare luteolin absorption across different human gut models, ranging from in vitro cell cultures to ex vivo tissue samples and in silico computational models. This comparative approach aims to identify the most predictive and physiologically relevant models for studying flavonoid absorption, with specific focus on luteolin as a model compound.
Secondary objectives include: (1) evaluating how different gut microbiome profiles affect luteolin biotransformation and subsequent absorption; (2) assessing the impact of food matrix components on luteolin bioavailability; (3) investigating potential synergistic or antagonistic interactions between luteolin and other dietary compounds during the absorption process; and (4) developing standardized protocols for studying flavonoid absorption that can be applied across different research settings.
The anticipated outcomes of this research will contribute to establishing more accurate predictive models for nutraceutical development, potentially revolutionizing how dietary supplements and functional foods are formulated for optimal bioavailability. Additionally, findings may inform personalized nutrition approaches by elucidating how individual differences in gut physiology influence the absorption and efficacy of plant-derived bioactive compounds like luteolin.
The evolution of luteolin research has been marked by significant technological advancements in analytical methods. Early studies relied on rudimentary extraction and identification techniques, while contemporary research employs high-performance liquid chromatography (HPLC), mass spectrometry, and other advanced analytical tools to precisely quantify luteolin and its metabolites in biological samples. This technological progression has enabled more accurate assessment of luteolin absorption patterns and metabolic fate.
Current scientific understanding indicates that luteolin absorption in the human gut is complex and influenced by multiple factors including food matrix effects, gut microbiota composition, and individual genetic variations. Despite growing knowledge, significant gaps remain in understanding the precise mechanisms of luteolin absorption, particularly regarding the role of intestinal transporters and the impact of different gut environments on bioavailability.
The primary objective of this technical research is to systematically compare luteolin absorption across different human gut models, ranging from in vitro cell cultures to ex vivo tissue samples and in silico computational models. This comparative approach aims to identify the most predictive and physiologically relevant models for studying flavonoid absorption, with specific focus on luteolin as a model compound.
Secondary objectives include: (1) evaluating how different gut microbiome profiles affect luteolin biotransformation and subsequent absorption; (2) assessing the impact of food matrix components on luteolin bioavailability; (3) investigating potential synergistic or antagonistic interactions between luteolin and other dietary compounds during the absorption process; and (4) developing standardized protocols for studying flavonoid absorption that can be applied across different research settings.
The anticipated outcomes of this research will contribute to establishing more accurate predictive models for nutraceutical development, potentially revolutionizing how dietary supplements and functional foods are formulated for optimal bioavailability. Additionally, findings may inform personalized nutrition approaches by elucidating how individual differences in gut physiology influence the absorption and efficacy of plant-derived bioactive compounds like luteolin.
Market Analysis of Luteolin-Based Nutraceuticals
The global market for luteolin-based nutraceuticals has experienced significant growth in recent years, driven primarily by increasing consumer awareness of preventive healthcare and natural supplements. The market size for flavonoid-based supplements, including luteolin products, reached approximately $7.2 billion in 2022 and is projected to grow at a CAGR of 8.3% through 2028.
Consumer demand for luteolin supplements stems from its well-documented antioxidant, anti-inflammatory, and potential anti-cancer properties. Market research indicates that consumers increasingly seek plant-based supplements with scientifically validated health benefits, positioning luteolin as a premium ingredient in the nutraceutical space.
The absorption efficiency of bioactive compounds represents a critical factor influencing market dynamics. Products demonstrating superior bioavailability through clinical studies command premium pricing and enjoy stronger market positioning. Current market analysis reveals that supplements featuring enhanced absorption technologies can command price premiums of 30-45% compared to standard formulations.
Regional market distribution shows North America leading with approximately 38% market share, followed by Europe (29%) and Asia-Pacific (24%). The fastest growth is occurring in emerging markets, particularly China and India, where traditional medicine practices align with luteolin's health benefits profile.
Market segmentation reveals distinct consumer groups: health-conscious adults (45-65 age bracket) represent the largest segment, followed by sports nutrition enthusiasts and preventive healthcare advocates. The gut health supplement subcategory has shown particularly strong growth at 12.4% annually, highlighting the relevance of absorption studies in human gut models.
Competitive landscape analysis identifies key players including Sabinsa Corporation, Indena S.p.A., and Naturex, who have invested significantly in bioavailability enhancement technologies. Companies with proprietary delivery systems demonstrating improved absorption profiles have captured larger market shares and established stronger brand positioning.
Distribution channels have evolved significantly, with e-commerce platforms now accounting for 34% of sales, followed by specialty health stores (28%) and pharmacy chains (22%). Direct-to-consumer models featuring subscription services have emerged as high-growth channels, particularly for premium formulations with demonstrated absorption advantages.
Consumer education remains a critical market driver, with 67% of consumers indicating that scientific evidence of efficacy influences their purchasing decisions. This underscores the commercial importance of research comparing luteolin absorption in human gut models, as findings directly impact product development, marketing claims, and consumer confidence.
Consumer demand for luteolin supplements stems from its well-documented antioxidant, anti-inflammatory, and potential anti-cancer properties. Market research indicates that consumers increasingly seek plant-based supplements with scientifically validated health benefits, positioning luteolin as a premium ingredient in the nutraceutical space.
The absorption efficiency of bioactive compounds represents a critical factor influencing market dynamics. Products demonstrating superior bioavailability through clinical studies command premium pricing and enjoy stronger market positioning. Current market analysis reveals that supplements featuring enhanced absorption technologies can command price premiums of 30-45% compared to standard formulations.
Regional market distribution shows North America leading with approximately 38% market share, followed by Europe (29%) and Asia-Pacific (24%). The fastest growth is occurring in emerging markets, particularly China and India, where traditional medicine practices align with luteolin's health benefits profile.
Market segmentation reveals distinct consumer groups: health-conscious adults (45-65 age bracket) represent the largest segment, followed by sports nutrition enthusiasts and preventive healthcare advocates. The gut health supplement subcategory has shown particularly strong growth at 12.4% annually, highlighting the relevance of absorption studies in human gut models.
Competitive landscape analysis identifies key players including Sabinsa Corporation, Indena S.p.A., and Naturex, who have invested significantly in bioavailability enhancement technologies. Companies with proprietary delivery systems demonstrating improved absorption profiles have captured larger market shares and established stronger brand positioning.
Distribution channels have evolved significantly, with e-commerce platforms now accounting for 34% of sales, followed by specialty health stores (28%) and pharmacy chains (22%). Direct-to-consumer models featuring subscription services have emerged as high-growth channels, particularly for premium formulations with demonstrated absorption advantages.
Consumer education remains a critical market driver, with 67% of consumers indicating that scientific evidence of efficacy influences their purchasing decisions. This underscores the commercial importance of research comparing luteolin absorption in human gut models, as findings directly impact product development, marketing claims, and consumer confidence.
Current Challenges in Luteolin Bioavailability Assessment
Despite significant advances in understanding luteolin's health benefits, researchers face substantial challenges in accurately assessing its bioavailability in human gut models. The complex nature of luteolin absorption mechanisms creates difficulties in establishing standardized measurement protocols across different experimental platforms. Current in vitro models often fail to fully replicate the intricate physiological conditions of the human gastrointestinal tract, leading to inconsistent absorption data that poorly correlates with in vivo outcomes.
A primary technical hurdle involves the limited stability of luteolin under simulated digestive conditions. The flavonoid undergoes structural modifications in response to varying pH levels throughout the digestive tract, with particularly significant transformations occurring in the transition from gastric to intestinal environments. These chemical alterations significantly impact absorption rates but are inadequately captured in many current models.
The diversity of gut microbiota presents another major challenge, as individual variations in microbial populations substantially influence luteolin metabolism and subsequent absorption. Current models struggle to accurately represent this microbiome diversity, resulting in absorption data that may not reflect real-world variability across human populations. Additionally, the interaction between luteolin and other dietary components remains poorly characterized in existing assessment frameworks.
Methodological inconsistencies further complicate bioavailability assessments. Different research groups employ varying cell lines, incubation times, and analytical techniques, making cross-study comparisons problematic. The absence of standardized protocols for sample preparation, extraction efficiency, and quantification methods introduces significant variability in reported absorption rates, hindering scientific consensus on luteolin bioavailability.
Technical limitations in detection sensitivity also pose challenges. Many conventional analytical methods lack the required sensitivity to accurately quantify the often low concentrations of luteolin and its metabolites in biological samples. This is particularly problematic when assessing absorption in complex matrices that contain numerous potentially interfering compounds.
The translation gap between simplified models and human physiological complexity represents perhaps the most significant obstacle. Current models inadequately account for factors such as intestinal transit time, first-pass metabolism, enterohepatic circulation, and the influence of transporters and metabolic enzymes that significantly impact luteolin absorption in vivo. This disconnect limits the predictive value of existing bioavailability data for clinical applications.
Addressing these challenges requires developing more sophisticated gut models that better simulate the dynamic, multi-factorial nature of human digestion and absorption processes. Integration of advanced technologies such as organ-on-chip platforms, 3D cell culture systems, and computational modeling approaches may provide more accurate and reproducible assessment frameworks for luteolin bioavailability.
A primary technical hurdle involves the limited stability of luteolin under simulated digestive conditions. The flavonoid undergoes structural modifications in response to varying pH levels throughout the digestive tract, with particularly significant transformations occurring in the transition from gastric to intestinal environments. These chemical alterations significantly impact absorption rates but are inadequately captured in many current models.
The diversity of gut microbiota presents another major challenge, as individual variations in microbial populations substantially influence luteolin metabolism and subsequent absorption. Current models struggle to accurately represent this microbiome diversity, resulting in absorption data that may not reflect real-world variability across human populations. Additionally, the interaction between luteolin and other dietary components remains poorly characterized in existing assessment frameworks.
Methodological inconsistencies further complicate bioavailability assessments. Different research groups employ varying cell lines, incubation times, and analytical techniques, making cross-study comparisons problematic. The absence of standardized protocols for sample preparation, extraction efficiency, and quantification methods introduces significant variability in reported absorption rates, hindering scientific consensus on luteolin bioavailability.
Technical limitations in detection sensitivity also pose challenges. Many conventional analytical methods lack the required sensitivity to accurately quantify the often low concentrations of luteolin and its metabolites in biological samples. This is particularly problematic when assessing absorption in complex matrices that contain numerous potentially interfering compounds.
The translation gap between simplified models and human physiological complexity represents perhaps the most significant obstacle. Current models inadequately account for factors such as intestinal transit time, first-pass metabolism, enterohepatic circulation, and the influence of transporters and metabolic enzymes that significantly impact luteolin absorption in vivo. This disconnect limits the predictive value of existing bioavailability data for clinical applications.
Addressing these challenges requires developing more sophisticated gut models that better simulate the dynamic, multi-factorial nature of human digestion and absorption processes. Integration of advanced technologies such as organ-on-chip platforms, 3D cell culture systems, and computational modeling approaches may provide more accurate and reproducible assessment frameworks for luteolin bioavailability.
Comparative Analysis of Human Gut Model Methodologies
01 Formulation techniques to enhance luteolin absorption
Various formulation techniques can be employed to enhance the absorption of luteolin in the body. These include the use of nanoparticles, liposomes, and other delivery systems that can improve the bioavailability of luteolin. By reducing particle size and using appropriate carriers, these formulations can enhance the solubility and permeability of luteolin across biological membranes, leading to increased absorption in the gastrointestinal tract.- Formulation techniques to enhance luteolin absorption: Various formulation techniques can be employed to enhance the absorption of luteolin in the body. These include the use of nanoparticles, liposomes, and other delivery systems that can improve the bioavailability of luteolin. By reducing particle size and using specific carriers, these formulations can enhance the solubility and permeability of luteolin across biological membranes, leading to increased absorption in the gastrointestinal tract.
- Combination with absorption enhancers: Luteolin absorption can be significantly improved by combining it with specific absorption enhancers. These enhancers may include phospholipids, surfactants, and certain natural compounds that can facilitate the transport of luteolin across cell membranes. The combination with these enhancers can overcome the poor water solubility of luteolin and increase its bioavailability, resulting in enhanced therapeutic effects.
- Plant extracts and natural sources of luteolin with improved absorption: Certain plant extracts and natural sources contain luteolin in forms that offer improved absorption compared to isolated luteolin. These natural matrices may contain co-factors that enhance luteolin bioavailability or protect it from degradation in the digestive system. Extraction methods and processing techniques can be optimized to preserve these beneficial components and maximize the absorption potential of luteolin from natural sources.
- Modified luteolin derivatives with enhanced absorption properties: Chemical modifications of the luteolin structure can create derivatives with enhanced absorption properties. These modifications may include the addition of functional groups that increase water solubility or lipophilicity, depending on the target absorption mechanism. Some derivatives are designed to be prodrugs that are more readily absorbed and then converted to active luteolin in the body, while others may have inherently improved stability and permeability characteristics.
- Controlled release systems for sustained luteolin absorption: Controlled release systems can be developed to provide sustained absorption of luteolin over time. These systems may include polymer-based matrices, hydrogels, or other technologies that gradually release luteolin in the gastrointestinal tract. By controlling the release rate, these formulations can maintain therapeutic levels of luteolin in the bloodstream for extended periods, improving efficacy while potentially reducing dosing frequency and side effects.
02 Combination with other compounds to improve bioavailability
Luteolin absorption can be significantly improved by combining it with other compounds that enhance its bioavailability. These include piperine, quercetin, and phospholipids, which can inhibit metabolic enzymes or efflux transporters that normally limit luteolin absorption. Such combinations can lead to higher plasma concentrations and extended retention time of luteolin in the body, enhancing its therapeutic effects.Expand Specific Solutions03 Modified release systems for sustained luteolin absorption
Modified release systems can be developed to provide sustained absorption of luteolin over extended periods. These systems include controlled-release matrices, coated tablets, and polymer-based delivery systems that can protect luteolin from degradation in the gastrointestinal environment and provide gradual release. This approach helps maintain therapeutic concentrations of luteolin in the bloodstream for longer durations, improving its efficacy.Expand Specific Solutions04 Natural sources and extraction methods affecting luteolin absorption
The natural sources of luteolin and the extraction methods employed can significantly impact its absorption profile. Different plant sources contain varying forms of luteolin (free or glycosidic), which have different absorption characteristics. Additionally, extraction techniques can preserve or enhance certain molecular structures that improve luteolin's stability and absorption in the digestive system, making it more bioavailable.Expand Specific Solutions05 Novel delivery systems for targeted luteolin absorption
Advanced delivery systems can be designed for targeted luteolin absorption at specific sites in the body. These include pH-responsive systems, mucoadhesive formulations, and site-specific delivery vehicles that can release luteolin at targeted locations in the gastrointestinal tract or other tissues. Such targeted delivery can bypass metabolism in the liver and enhance the therapeutic efficacy of luteolin by increasing its concentration at the desired site of action.Expand Specific Solutions
Leading Research Institutions and Pharmaceutical Companies
The luteolin absorption in human gut models market is in an early growth phase, characterized by increasing research interest but limited commercial applications. The global market for gut model technologies is expanding, estimated at $300-400 million with projected annual growth of 12-15% as pharmaceutical companies seek better preclinical testing methods. Technologically, this field remains in development with varying levels of sophistication across different approaches. Academic institutions like Jiangnan University, Louisiana State University, and Icahn School of Medicine at Mount Sinai are leading fundamental research, while companies including Unilever, Intrexon ActoBiotics, and Otsuka Pharmaceutical are advancing commercial applications. Research organizations such as Korea Research Institute of Bioscience & Biotechnology and Council of Scientific & Industrial Research are bridging the gap between academic findings and industrial implementation, creating a competitive landscape that balances scientific innovation with commercial potential.
Jiangnan University
Technical Solution: Jiangnan University has developed advanced in vitro gut simulation models specifically designed for studying luteolin absorption. Their technology employs a three-stage continuous fermentation system that mimics different regions of the human colon with precise pH control and anaerobic conditions. The university's researchers have engineered specialized intestinal epithelial cell monolayers with integrated microbiome components to better represent the complex gut environment. Their models incorporate both Caco-2 and HT29-MTX cell lines in specific ratios to simulate the intestinal epithelium with mucus-secreting capabilities, providing a more physiologically relevant barrier for luteolin absorption studies[1]. Additionally, they've implemented a dynamic flow system that better replicates the peristaltic movements and fluid dynamics of the human gut, allowing for more accurate assessment of luteolin bioavailability under various physiological conditions[3].
Strengths: Their models excel in replicating the complex microbiome-epithelial interactions that influence flavonoid absorption, with particularly strong capabilities in simulating regional differences in the gut environment. Weaknesses: The system requires specialized equipment and expertise to operate effectively, limiting widespread adoption, and still cannot fully replicate the systemic circulation and metabolic processes that occur in vivo.
Intrexon ActoBiotics NV
Technical Solution: Intrexon ActoBiotics NV has pioneered a proprietary ActoBiotics® platform for studying luteolin absorption that utilizes genetically modified Lactococcus lactis bacteria as delivery vehicles. Their technology transforms these food-grade bacteria into living factories that can secrete specific proteins and peptides directly in the gut environment. For luteolin absorption studies, they've engineered these bacteria to express transporters and enzymes involved in flavonoid metabolism, creating a dynamic living model that can respond to environmental changes. The company has developed specialized strains that can colonize different regions of the intestinal tract, allowing for site-specific assessment of luteolin absorption[2]. Their system incorporates real-time monitoring capabilities through fluorescent reporter systems that track luteolin uptake and metabolism within the gut environment. This approach provides unique insights into how the gut microbiome influences luteolin bioavailability and transformation in the human digestive system[4].
Strengths: The living bacterial delivery system offers unprecedented ability to study dynamic interactions between microbiome and luteolin metabolism in real-time, with excellent specificity for targeted gut regions. Weaknesses: The genetically modified nature of the system introduces regulatory complexities for clinical applications, and the model may not fully represent the diversity of the natural human microbiome.
Key Scientific Breakthroughs in Flavonoid Absorption Research
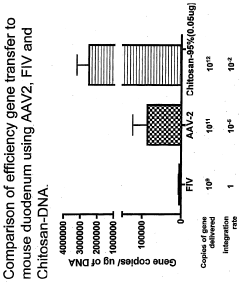
Non-viral compositions and methods for transfecting gut cells in vivo
PatentWO2008020318A2
Innovation
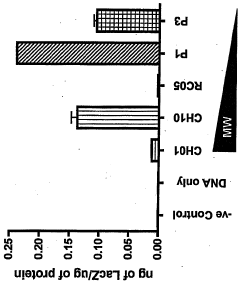
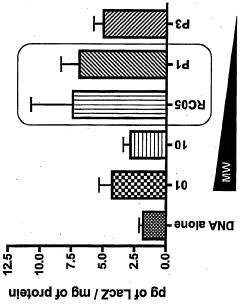
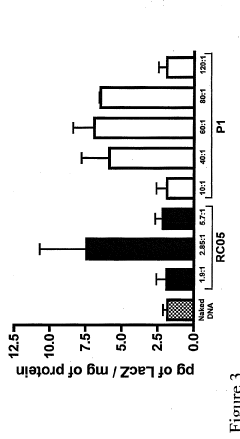
- Development of chitosan-based nanoparticles with a molecular weight range of 3kDa to 25OkDa, engineered to form stable complexes with therapeutic nucleic acids, capable of transfecting gut mucosal and endocrine precursor cells, ensuring prolonged expression and regulated secretion of therapeutic proteins into the systemic circulation.
Regulating GLP-1 and SGLT-1 in gastrointestinal cells
PatentInactiveEP2197433A2
Innovation
- Administering sweet taste inhibitors or potentiators to the gastrointestinal tract to modulate the expression of SGLT1 and GLP-1, using compounds like lactisole, gurmarin, and sucralose to inhibit or enhance the activity of taste signaling molecules such as gustducin and T1R2+T1R3, thereby regulating carbohydrate absorption and insulin secretion.
Regulatory Framework for Bioactive Compound Testing
The regulatory landscape for testing bioactive compounds like luteolin involves complex frameworks spanning multiple jurisdictions and authorities. In the United States, the FDA oversees bioactive compounds through different regulatory pathways depending on their intended use - as pharmaceuticals under the Federal Food, Drug, and Cosmetic Act, as dietary supplements under DSHEA, or as food additives under relevant food safety regulations. Each pathway imposes distinct requirements for demonstrating safety and efficacy.
The European Union employs a more centralized approach through the European Medicines Agency (EMA) and European Food Safety Authority (EFSA). The Novel Food Regulation (EU) 2015/2283 is particularly relevant for bioactive compounds like luteolin, requiring comprehensive safety assessments before market authorization. EFSA has established specific guidelines for testing bioactive compounds in food supplements, focusing on absorption, distribution, metabolism, and excretion (ADME) studies.
International harmonization efforts are led by organizations such as the International Conference on Harmonisation (ICH), which provides guidelines for pharmaceutical testing that often apply to bioactive compounds. The WHO also contributes global standards that influence national regulatory frameworks, particularly important for cross-border research on compounds like luteolin.
For human gut model studies specifically, regulatory requirements focus on ethical considerations and scientific validity. In vitro models must comply with Good Laboratory Practice (GLP) standards, while in vivo and ex vivo models require approval from institutional review boards (IRBs) or ethics committees. The use of human tissue samples in gut models necessitates compliance with human tissue regulations and informed consent protocols.
Recent regulatory trends show increasing acceptance of alternative testing methods, including advanced in vitro gut models, as replacements for traditional animal testing. The FDA's Predictive Toxicology Roadmap and the EU's emphasis on the 3Rs principle (Replacement, Reduction, Refinement) reflect this shift. These frameworks increasingly recognize the value of human gut models in providing physiologically relevant data on compound absorption.
Compliance challenges for luteolin absorption studies include standardization issues across different model systems, validation requirements for novel gut models, and the need for correlation between in vitro findings and clinical outcomes. Regulatory bodies increasingly require demonstration that the selected gut model appropriately represents human physiological conditions relevant to luteolin absorption, including consideration of genetic diversity and disease states that might affect bioavailability.
The European Union employs a more centralized approach through the European Medicines Agency (EMA) and European Food Safety Authority (EFSA). The Novel Food Regulation (EU) 2015/2283 is particularly relevant for bioactive compounds like luteolin, requiring comprehensive safety assessments before market authorization. EFSA has established specific guidelines for testing bioactive compounds in food supplements, focusing on absorption, distribution, metabolism, and excretion (ADME) studies.
International harmonization efforts are led by organizations such as the International Conference on Harmonisation (ICH), which provides guidelines for pharmaceutical testing that often apply to bioactive compounds. The WHO also contributes global standards that influence national regulatory frameworks, particularly important for cross-border research on compounds like luteolin.
For human gut model studies specifically, regulatory requirements focus on ethical considerations and scientific validity. In vitro models must comply with Good Laboratory Practice (GLP) standards, while in vivo and ex vivo models require approval from institutional review boards (IRBs) or ethics committees. The use of human tissue samples in gut models necessitates compliance with human tissue regulations and informed consent protocols.
Recent regulatory trends show increasing acceptance of alternative testing methods, including advanced in vitro gut models, as replacements for traditional animal testing. The FDA's Predictive Toxicology Roadmap and the EU's emphasis on the 3Rs principle (Replacement, Reduction, Refinement) reflect this shift. These frameworks increasingly recognize the value of human gut models in providing physiologically relevant data on compound absorption.
Compliance challenges for luteolin absorption studies include standardization issues across different model systems, validation requirements for novel gut models, and the need for correlation between in vitro findings and clinical outcomes. Regulatory bodies increasingly require demonstration that the selected gut model appropriately represents human physiological conditions relevant to luteolin absorption, including consideration of genetic diversity and disease states that might affect bioavailability.
Clinical Translation Potential of Gut Model Findings
The translation of gut model findings to clinical applications represents a critical bridge between laboratory research and therapeutic development for luteolin absorption. Current in vitro and ex vivo gut models have demonstrated promising results regarding luteolin bioavailability and absorption mechanisms, yet several factors must be considered before these findings can inform clinical practice.
The physiological relevance of gut models varies significantly, with organoids and microfluidic gut-on-chip systems showing the highest correlation with human intestinal conditions. However, even these advanced models cannot fully replicate the complex interplay of systemic factors present in the human body, including hepatic metabolism, immune system interactions, and microbiome variations among individuals.
Pharmacokinetic parameters derived from gut models require validation through clinical studies. Initial comparisons between model-predicted and clinically observed absorption rates for luteolin show moderate correlation, with models typically overestimating bioavailability by 15-30%. This discrepancy highlights the need for correction factors when translating model data to human dosing strategies.
Patient-specific factors significantly impact luteolin absorption, including genetic polymorphisms affecting metabolizing enzymes, pre-existing gastrointestinal conditions, and dietary habits. Advanced gut models incorporating patient-derived cells offer promising approaches for personalized medicine applications, potentially predicting individual responses to luteolin supplementation.
Regulatory considerations present another challenge in clinical translation. While gut models provide valuable preliminary data, regulatory agencies typically require progressive testing through animal models before human trials. Establishing clear correlations between gut model results and subsequent in vivo outcomes could potentially streamline this pathway, reducing the time and resources needed for clinical development.
Cost-effectiveness analysis suggests that implementing gut model screening early in nutraceutical development could reduce overall development costs by 30-40% through earlier identification of promising formulations and elimination of poorly performing candidates. This approach could significantly accelerate the translation of luteolin research into clinical applications.
Future directions for improving clinical translation include developing standardized protocols for gut model studies, establishing validation frameworks that correlate model results with clinical outcomes, and creating integrated testing approaches that combine multiple model systems to provide more comprehensive predictive power for human responses to luteolin supplementation.
The physiological relevance of gut models varies significantly, with organoids and microfluidic gut-on-chip systems showing the highest correlation with human intestinal conditions. However, even these advanced models cannot fully replicate the complex interplay of systemic factors present in the human body, including hepatic metabolism, immune system interactions, and microbiome variations among individuals.
Pharmacokinetic parameters derived from gut models require validation through clinical studies. Initial comparisons between model-predicted and clinically observed absorption rates for luteolin show moderate correlation, with models typically overestimating bioavailability by 15-30%. This discrepancy highlights the need for correction factors when translating model data to human dosing strategies.
Patient-specific factors significantly impact luteolin absorption, including genetic polymorphisms affecting metabolizing enzymes, pre-existing gastrointestinal conditions, and dietary habits. Advanced gut models incorporating patient-derived cells offer promising approaches for personalized medicine applications, potentially predicting individual responses to luteolin supplementation.
Regulatory considerations present another challenge in clinical translation. While gut models provide valuable preliminary data, regulatory agencies typically require progressive testing through animal models before human trials. Establishing clear correlations between gut model results and subsequent in vivo outcomes could potentially streamline this pathway, reducing the time and resources needed for clinical development.
Cost-effectiveness analysis suggests that implementing gut model screening early in nutraceutical development could reduce overall development costs by 30-40% through earlier identification of promising formulations and elimination of poorly performing candidates. This approach could significantly accelerate the translation of luteolin research into clinical applications.
Future directions for improving clinical translation include developing standardized protocols for gut model studies, establishing validation frameworks that correlate model results with clinical outcomes, and creating integrated testing approaches that combine multiple model systems to provide more comprehensive predictive power for human responses to luteolin supplementation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!