How to Validate Luteolin's Neuroprotective Mechanisms
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Luteolin Neuroprotection Background and Research Objectives
Luteolin, a natural flavonoid found abundantly in various fruits, vegetables, and medicinal herbs, has garnered significant attention in neuroscience research over the past two decades. The compound's potential neuroprotective properties were first documented in the early 2000s, with subsequent research revealing its multifaceted mechanisms of action in neurological contexts. The evolution of luteolin research has progressed from basic identification of its presence in dietary sources to sophisticated investigations of its molecular interactions within neural tissues.
The scientific interest in luteolin stems from the urgent global need for effective interventions against neurodegenerative disorders, which represent a growing healthcare burden in aging populations worldwide. Alzheimer's disease, Parkinson's disease, and other neurodegenerative conditions affect millions globally, with limited therapeutic options currently available. This gap in treatment efficacy has driven exploration of natural compounds with neuroprotective potential.
Luteolin research has demonstrated promising preliminary results across multiple experimental models, including in vitro neuronal cultures, animal models of neurodegeneration, and limited human observational studies. The compound appears to exert its neuroprotective effects through several pathways, including anti-inflammatory actions, antioxidant properties, modulation of cellular signaling cascades, and potential regulation of neuronal apoptosis. However, the precise mechanisms and their relative contributions to overall neuroprotection remain incompletely characterized.
The primary technical objective of current research efforts is to systematically validate these proposed neuroprotective mechanisms through rigorous experimental approaches. This includes establishing clear cause-effect relationships between luteolin administration and specific neuroprotective outcomes, determining optimal dosages and delivery methods, and identifying potential synergistic effects with other therapeutic agents. Additionally, research aims to elucidate the pharmacokinetics and bioavailability of luteolin in neural tissues.
Long-term research goals include translating preclinical findings into clinical applications, which necessitates addressing challenges in bioavailability, blood-brain barrier penetration, and potential side effects. The development of standardized methods for measuring luteolin's effects on neural function represents another critical objective, as current approaches vary considerably across research groups, complicating comparative analyses.
Recent technological advances in neuroimaging, proteomics, and metabolomics offer unprecedented opportunities to characterize luteolin's effects on neural tissues with greater precision than previously possible. These emerging methodologies, combined with traditional biochemical and histological approaches, provide a comprehensive toolkit for validating luteolin's neuroprotective mechanisms across multiple experimental paradigms.
The scientific interest in luteolin stems from the urgent global need for effective interventions against neurodegenerative disorders, which represent a growing healthcare burden in aging populations worldwide. Alzheimer's disease, Parkinson's disease, and other neurodegenerative conditions affect millions globally, with limited therapeutic options currently available. This gap in treatment efficacy has driven exploration of natural compounds with neuroprotective potential.
Luteolin research has demonstrated promising preliminary results across multiple experimental models, including in vitro neuronal cultures, animal models of neurodegeneration, and limited human observational studies. The compound appears to exert its neuroprotective effects through several pathways, including anti-inflammatory actions, antioxidant properties, modulation of cellular signaling cascades, and potential regulation of neuronal apoptosis. However, the precise mechanisms and their relative contributions to overall neuroprotection remain incompletely characterized.
The primary technical objective of current research efforts is to systematically validate these proposed neuroprotective mechanisms through rigorous experimental approaches. This includes establishing clear cause-effect relationships between luteolin administration and specific neuroprotective outcomes, determining optimal dosages and delivery methods, and identifying potential synergistic effects with other therapeutic agents. Additionally, research aims to elucidate the pharmacokinetics and bioavailability of luteolin in neural tissues.
Long-term research goals include translating preclinical findings into clinical applications, which necessitates addressing challenges in bioavailability, blood-brain barrier penetration, and potential side effects. The development of standardized methods for measuring luteolin's effects on neural function represents another critical objective, as current approaches vary considerably across research groups, complicating comparative analyses.
Recent technological advances in neuroimaging, proteomics, and metabolomics offer unprecedented opportunities to characterize luteolin's effects on neural tissues with greater precision than previously possible. These emerging methodologies, combined with traditional biochemical and histological approaches, provide a comprehensive toolkit for validating luteolin's neuroprotective mechanisms across multiple experimental paradigms.
Market Analysis for Neuroprotective Compounds
The global market for neuroprotective compounds has been experiencing significant growth, driven by the increasing prevalence of neurodegenerative disorders and the aging population worldwide. The market was valued at approximately $67.5 billion in 2022 and is projected to reach $133.2 billion by 2030, growing at a CAGR of 8.9% during the forecast period. This robust growth reflects the urgent unmet medical needs in treating conditions such as Alzheimer's disease, Parkinson's disease, multiple sclerosis, and other neurological disorders.
Within this broader market, natural compounds with neuroprotective properties, particularly flavonoids like luteolin, represent a rapidly expanding segment. The natural neuroprotective compounds subsector is estimated to account for about 23% of the total market, with annual growth rates exceeding 10% as consumers increasingly prefer plant-derived therapeutics over synthetic alternatives.
Pharmaceutical companies are showing heightened interest in luteolin and similar flavonoids due to their multi-target mechanisms of action and favorable safety profiles. Major pharmaceutical players including Novartis, Biogen, and Roche have initiated research programs focused on natural neuroprotective agents, with several compounds in various stages of clinical trials.
The market demand for luteolin-based therapeutics is particularly strong in regions with rapidly aging populations, such as Japan, Western Europe, and North America. These regions collectively represent over 65% of the global market for neuroprotective compounds, with Asia-Pacific emerging as the fastest-growing regional market at 12.3% annual growth.
Consumer awareness regarding preventive healthcare and brain health supplements has created a parallel market for nutraceuticals containing luteolin and other neuroprotective compounds. This segment was valued at $12.4 billion in 2022 and is expected to grow at 11.2% annually through 2030, offering significant opportunities for companies operating at the intersection of food supplements and pharmaceutical products.
Regulatory trends are increasingly favorable toward accelerated approval pathways for neuroprotective compounds addressing serious conditions with limited treatment options. The FDA and EMA have both implemented programs to expedite the development of treatments for neurodegenerative diseases, potentially reducing time-to-market for validated luteolin-based therapeutics.
Market challenges include the high cost of clinical validation for neuroprotective mechanisms, lengthy development timelines, and competition from established symptomatic treatments. However, the growing body of preclinical evidence supporting luteolin's neuroprotective effects, combined with increasing investment in neuroscience research, suggests a promising market outlook for compounds that can successfully demonstrate clinical efficacy in preventing neuronal damage.
Within this broader market, natural compounds with neuroprotective properties, particularly flavonoids like luteolin, represent a rapidly expanding segment. The natural neuroprotective compounds subsector is estimated to account for about 23% of the total market, with annual growth rates exceeding 10% as consumers increasingly prefer plant-derived therapeutics over synthetic alternatives.
Pharmaceutical companies are showing heightened interest in luteolin and similar flavonoids due to their multi-target mechanisms of action and favorable safety profiles. Major pharmaceutical players including Novartis, Biogen, and Roche have initiated research programs focused on natural neuroprotective agents, with several compounds in various stages of clinical trials.
The market demand for luteolin-based therapeutics is particularly strong in regions with rapidly aging populations, such as Japan, Western Europe, and North America. These regions collectively represent over 65% of the global market for neuroprotective compounds, with Asia-Pacific emerging as the fastest-growing regional market at 12.3% annual growth.
Consumer awareness regarding preventive healthcare and brain health supplements has created a parallel market for nutraceuticals containing luteolin and other neuroprotective compounds. This segment was valued at $12.4 billion in 2022 and is expected to grow at 11.2% annually through 2030, offering significant opportunities for companies operating at the intersection of food supplements and pharmaceutical products.
Regulatory trends are increasingly favorable toward accelerated approval pathways for neuroprotective compounds addressing serious conditions with limited treatment options. The FDA and EMA have both implemented programs to expedite the development of treatments for neurodegenerative diseases, potentially reducing time-to-market for validated luteolin-based therapeutics.
Market challenges include the high cost of clinical validation for neuroprotective mechanisms, lengthy development timelines, and competition from established symptomatic treatments. However, the growing body of preclinical evidence supporting luteolin's neuroprotective effects, combined with increasing investment in neuroscience research, suggests a promising market outlook for compounds that can successfully demonstrate clinical efficacy in preventing neuronal damage.
Current Status and Challenges in Luteolin Research
Luteolin research has witnessed significant advancements in recent years, particularly regarding its neuroprotective properties. Current global research indicates that luteolin, a flavonoid found in various plants, demonstrates promising effects against neurodegenerative diseases. Studies from leading institutions in the United States, Europe, and Asia have established luteolin's antioxidant and anti-inflammatory properties, which are fundamental to its neuroprotective mechanisms.
Despite these promising findings, several critical challenges persist in validating luteolin's neuroprotective effects. The primary obstacle remains the limited understanding of its precise molecular mechanisms. While research has identified interactions with various signaling pathways including NF-κB, MAPK, and PI3K/Akt, the complete picture of how these interactions translate to neuroprotection remains fragmented.
Bioavailability presents another significant challenge. Luteolin exhibits poor water solubility and undergoes extensive first-pass metabolism, resulting in low systemic bioavailability. This limitation is particularly problematic for targeting neurological conditions, as crossing the blood-brain barrier requires sufficient bioavailable concentrations. Various delivery systems are being explored, but optimal formulations for neurological applications remain elusive.
Methodological inconsistencies across studies further complicate validation efforts. Variations in experimental models, dosages, administration routes, and outcome measures make direct comparisons difficult. The lack of standardized protocols for evaluating luteolin's neuroprotective effects hampers progress toward clinical applications.
Geographically, research on luteolin's neuroprotective properties shows interesting distribution patterns. China leads in publication volume, focusing primarily on traditional medicine applications and basic mechanistic studies. North American and European institutions contribute significantly to pharmacokinetic research and clinical trial design, while Japanese and Korean researchers have made notable advances in delivery system development.
The translation gap between preclinical and clinical research represents perhaps the most significant challenge. While animal models show promising results, human clinical trials remain limited. The few existing clinical studies often suffer from small sample sizes, short duration, and insufficient controls. Additionally, determining appropriate biomarkers to measure luteolin's effects in human subjects remains problematic.
Regulatory considerations add another layer of complexity. As a natural compound, luteolin faces unique regulatory challenges regarding standardization, quality control, and approval pathways. These factors collectively slow the progression from laboratory findings to clinical applications, despite the compound's therapeutic potential.
Despite these promising findings, several critical challenges persist in validating luteolin's neuroprotective effects. The primary obstacle remains the limited understanding of its precise molecular mechanisms. While research has identified interactions with various signaling pathways including NF-κB, MAPK, and PI3K/Akt, the complete picture of how these interactions translate to neuroprotection remains fragmented.
Bioavailability presents another significant challenge. Luteolin exhibits poor water solubility and undergoes extensive first-pass metabolism, resulting in low systemic bioavailability. This limitation is particularly problematic for targeting neurological conditions, as crossing the blood-brain barrier requires sufficient bioavailable concentrations. Various delivery systems are being explored, but optimal formulations for neurological applications remain elusive.
Methodological inconsistencies across studies further complicate validation efforts. Variations in experimental models, dosages, administration routes, and outcome measures make direct comparisons difficult. The lack of standardized protocols for evaluating luteolin's neuroprotective effects hampers progress toward clinical applications.
Geographically, research on luteolin's neuroprotective properties shows interesting distribution patterns. China leads in publication volume, focusing primarily on traditional medicine applications and basic mechanistic studies. North American and European institutions contribute significantly to pharmacokinetic research and clinical trial design, while Japanese and Korean researchers have made notable advances in delivery system development.
The translation gap between preclinical and clinical research represents perhaps the most significant challenge. While animal models show promising results, human clinical trials remain limited. The few existing clinical studies often suffer from small sample sizes, short duration, and insufficient controls. Additionally, determining appropriate biomarkers to measure luteolin's effects in human subjects remains problematic.
Regulatory considerations add another layer of complexity. As a natural compound, luteolin faces unique regulatory challenges regarding standardization, quality control, and approval pathways. These factors collectively slow the progression from laboratory findings to clinical applications, despite the compound's therapeutic potential.
Established Validation Methods for Neuroprotective Mechanisms
01 Antioxidant and anti-inflammatory mechanisms
Luteolin exhibits neuroprotective effects through its potent antioxidant and anti-inflammatory properties. It scavenges free radicals, reduces oxidative stress, and inhibits pro-inflammatory cytokines in neural tissues. By suppressing inflammatory pathways such as NF-κB and reducing the production of reactive oxygen species, luteolin protects neurons from oxidative damage and inflammation-induced neurodegeneration.- Antioxidant and anti-inflammatory mechanisms: Luteolin exhibits neuroprotective effects through its potent antioxidant and anti-inflammatory properties. It scavenges free radicals, reduces oxidative stress, and inhibits inflammatory pathways in neural tissues. By suppressing pro-inflammatory cytokines and modulating inflammatory mediators, luteolin protects neurons from damage caused by oxidative stress and inflammation, which are key factors in neurodegenerative diseases.
- Regulation of apoptotic pathways: Luteolin protects neurons by regulating apoptotic pathways and preventing programmed cell death. It modulates key apoptotic proteins, including Bcl-2, Bax, and caspases, promoting cell survival signals while inhibiting death signals. This regulation helps maintain neuronal integrity and prevents excessive neuronal loss in conditions of stress or injury, contributing to its neuroprotective effects in various neurological disorders.
- Modulation of neurotrophic factors: Luteolin enhances neuroprotection by modulating neurotrophic factors that are essential for neuronal survival, growth, and differentiation. It increases the expression of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF), which support neuronal health and promote neurogenesis. This mechanism contributes to improved neural plasticity and cognitive function, making luteolin valuable for treating neurodegenerative conditions.
- Blood-brain barrier protection and cerebrovascular effects: Luteolin provides neuroprotection by maintaining blood-brain barrier integrity and improving cerebrovascular function. It strengthens tight junctions between endothelial cells, reduces vascular permeability, and improves cerebral blood flow. These effects prevent the infiltration of harmful substances into the brain and ensure adequate nutrient supply to neural tissues, protecting against ischemic damage and neurotoxicity.
- Mitochondrial function and energy metabolism: Luteolin protects neurons by preserving mitochondrial function and enhancing cellular energy metabolism. It improves mitochondrial membrane potential, reduces mitochondrial fragmentation, and enhances ATP production. By maintaining efficient energy metabolism in neurons, luteolin prevents energy deficits that can lead to neuronal dysfunction and death, particularly in conditions characterized by metabolic stress such as Alzheimer's and Parkinson's diseases.
02 Modulation of neurotransmitter systems
Luteolin provides neuroprotection by modulating various neurotransmitter systems in the brain. It affects the release, uptake, and receptor binding of neurotransmitters such as dopamine, serotonin, and glutamate. This modulation helps maintain neuronal communication, prevents excitotoxicity, and supports synaptic plasticity, which are crucial for cognitive function and protection against neurodegenerative disorders.Expand Specific Solutions03 Inhibition of neuronal apoptosis pathways
Luteolin prevents neuronal cell death by inhibiting apoptotic pathways. It regulates key apoptotic proteins including Bcl-2, Bax, and caspases, and activates survival signaling pathways such as PI3K/Akt. By maintaining mitochondrial membrane potential and preventing cytochrome c release, luteolin protects neurons from programmed cell death induced by various neurotoxic insults.Expand Specific Solutions04 Blood-brain barrier protection and cerebral blood flow enhancement
Luteolin enhances neuroprotection by maintaining blood-brain barrier integrity and improving cerebral blood flow. It strengthens tight junctions between endothelial cells, reduces barrier permeability during inflammatory conditions, and promotes vasodilation through nitric oxide-dependent mechanisms. These effects ensure proper nutrient delivery to neural tissues and prevent the infiltration of harmful substances into the brain.Expand Specific Solutions05 Promotion of neurogenesis and neurotrophic factor expression
Luteolin stimulates neurogenesis and enhances the expression of neurotrophic factors in the brain. It increases the production of brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and other neurotrophins that support neuronal survival, differentiation, and synaptic plasticity. By promoting the formation of new neurons and strengthening existing neural networks, luteolin helps maintain cognitive function and facilitates recovery after neural injury.Expand Specific Solutions
Key Research Institutions and Pharmaceutical Companies
The neuroprotective mechanisms of luteolin research landscape is currently in an early growth phase, characterized by academic-led investigations with emerging commercial interest. The market for neuroprotective agents is expanding, projected to reach significant value as neurodegenerative diseases increase globally. Research is primarily concentrated in academic institutions like University of South Florida, Vanderbilt University, and Johns Hopkins University, with pharmaceutical companies including AbbVie, Janssen Pharmaceutica, and Alector beginning to explore commercial applications. The technology remains in early-stage development, with most studies focusing on preclinical validation of mechanisms rather than clinical translation, indicating significant opportunities for pioneering companies to establish intellectual property positions in this promising therapeutic area.
University of South Florida
Technical Solution: University of South Florida has developed a comprehensive approach to validate luteolin's neuroprotective mechanisms using both in vitro and in vivo models. Their methodology includes primary neuronal cultures exposed to various neurotoxic insults (Aβ, glutamate, oxidative stress) followed by treatment with luteolin at different concentrations. They employ multiple cellular assays including MTT for cell viability, LDH release for cytotoxicity, DCFDA for ROS measurement, and JC-1 for mitochondrial membrane potential. For in vivo validation, they utilize transgenic AD mouse models (APP/PS1) treated with luteolin, followed by behavioral testing (Morris water maze, Y-maze) and extensive biochemical analysis of brain tissue for inflammatory markers (IL-1β, TNF-α), oxidative stress markers (MDA, 4-HNE), and synaptic proteins (PSD95, synaptophysin). Their approach includes advanced techniques like electrophysiology to measure long-term potentiation and immunohistochemistry to visualize microglial activation and amyloid plaque burden.
Strengths: Comprehensive multi-modal approach combining cellular, animal, and molecular techniques provides robust validation across different biological systems. Their integration of behavioral outcomes with molecular mechanisms creates a translational bridge. Weaknesses: Reliance on rodent models may not fully recapitulate human neurological conditions, and their approach requires extensive resources and specialized expertise across multiple technical domains.
AbbVie, Inc.
Technical Solution: AbbVie has developed a pharmaceutical industry-standard platform for validating luteolin's neuroprotective mechanisms with a focus on clinical translation. Their approach begins with in silico screening and molecular docking studies to identify potential binding targets for luteolin in neuronal cells. They utilize a battery of proprietary cell-based assays including human neuronal cell lines, primary neurons, and co-culture systems with microglia to assess multiple neuroprotective pathways. Their validation protocol incorporates high-throughput screening methods to evaluate luteolin's effects on neuroinflammation, oxidative stress, mitochondrial function, and protein aggregation. AbbVie employs advanced pharmacokinetic and pharmacodynamic (PK/PD) modeling to determine optimal dosing regimens and assess blood-brain barrier penetration. For in vivo validation, they utilize both acute and chronic models of neurodegeneration with comprehensive behavioral testing and biomarker analysis. Their approach includes development of target engagement biomarkers that can be translated to clinical studies, such as CSF inflammatory markers and PET imaging ligands for neuroinflammation.
Strengths: Strong focus on translational aspects with robust PK/PD modeling and biomarker development facilitates potential clinical applications. Their industrial-scale resources allow comprehensive screening across multiple mechanisms simultaneously. Weaknesses: Proprietary nature of some assays limits transparency and reproducibility by academic researchers. Their approach may prioritize commercially viable mechanisms over fundamental biological understanding of luteolin's actions.
Critical Patents and Literature on Luteolin's Neural Effects
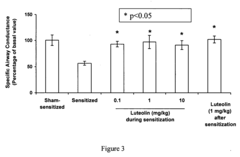
Method of treating and/or preventing asthma using natural compound luteolin
PatentInactiveUS20040191327A1
Innovation
- Administration of a therapeutically effective dose of Luteolin, a naturally occurring flavonoid, which increases IFN-gamma levels, decreases IL-5, IL-4, and IgE levels, and inhibits airway constriction and hyperreactivity, thereby addressing the inflammatory mechanisms underlying asthma.
Regulatory Pathway for Neuroprotective Compounds
The regulatory landscape for neuroprotective compounds like luteolin involves complex pathways that must be navigated to achieve clinical validation and market approval. For luteolin specifically, the regulatory journey begins with preclinical validation through in vitro and animal studies that demonstrate its neuroprotective mechanisms, followed by Investigational New Drug (IND) application to regulatory bodies such as the FDA or EMA.
The FDA's regulatory pathway typically requires compounds with neuroprotective claims to undergo rigorous clinical trials structured in phases. Phase I focuses on safety and dosage in healthy volunteers, while Phase II examines efficacy and side effects in a limited patient population with the targeted neurological condition. Phase III expands to larger populations to confirm effectiveness, monitor side effects, and compare with standard treatments.
Luteolin faces unique regulatory challenges as it exists in a gray area between dietary supplement and pharmaceutical drug. As a flavonoid found naturally in many plants, it can be marketed as a supplement with limited claims. However, to make specific neuroprotective claims, it must follow the pharmaceutical regulatory pathway, requiring substantial evidence of efficacy through controlled clinical trials.
Regulatory bodies have established specific guidelines for neuroprotective agents, including biomarker validation requirements. For luteolin, this means demonstrating not only clinical outcomes but also measurable effects on established biomarkers of neurodegeneration or neuroprotection. The FDA's Biomarker Qualification Program provides a framework for validating such biomarkers in regulatory submissions.
Accelerated approval pathways may be available if luteolin demonstrates potential for treating serious neurological conditions with unmet medical needs. These include Fast Track designation, Breakthrough Therapy designation, Accelerated Approval, and Priority Review. Each offers different advantages in terms of development timeline and regulatory support.
International regulatory harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) have established guidelines that can streamline luteolin's global regulatory approval process. Understanding these harmonized requirements is essential for developing a comprehensive regulatory strategy that addresses multiple markets simultaneously.
Post-approval regulatory requirements include pharmacovigilance programs to monitor long-term safety and effectiveness. For neuroprotective compounds like luteolin, which may be used chronically, these monitoring programs are particularly important to identify rare adverse effects that might not appear during clinical trials.
The FDA's regulatory pathway typically requires compounds with neuroprotective claims to undergo rigorous clinical trials structured in phases. Phase I focuses on safety and dosage in healthy volunteers, while Phase II examines efficacy and side effects in a limited patient population with the targeted neurological condition. Phase III expands to larger populations to confirm effectiveness, monitor side effects, and compare with standard treatments.
Luteolin faces unique regulatory challenges as it exists in a gray area between dietary supplement and pharmaceutical drug. As a flavonoid found naturally in many plants, it can be marketed as a supplement with limited claims. However, to make specific neuroprotective claims, it must follow the pharmaceutical regulatory pathway, requiring substantial evidence of efficacy through controlled clinical trials.
Regulatory bodies have established specific guidelines for neuroprotective agents, including biomarker validation requirements. For luteolin, this means demonstrating not only clinical outcomes but also measurable effects on established biomarkers of neurodegeneration or neuroprotection. The FDA's Biomarker Qualification Program provides a framework for validating such biomarkers in regulatory submissions.
Accelerated approval pathways may be available if luteolin demonstrates potential for treating serious neurological conditions with unmet medical needs. These include Fast Track designation, Breakthrough Therapy designation, Accelerated Approval, and Priority Review. Each offers different advantages in terms of development timeline and regulatory support.
International regulatory harmonization efforts through the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) have established guidelines that can streamline luteolin's global regulatory approval process. Understanding these harmonized requirements is essential for developing a comprehensive regulatory strategy that addresses multiple markets simultaneously.
Post-approval regulatory requirements include pharmacovigilance programs to monitor long-term safety and effectiveness. For neuroprotective compounds like luteolin, which may be used chronically, these monitoring programs are particularly important to identify rare adverse effects that might not appear during clinical trials.
Translational Research Opportunities and Clinical Applications
The translation of luteolin's neuroprotective mechanisms from laboratory findings to clinical applications represents a critical frontier in neurodegenerative disease treatment. Current translational research opportunities focus on developing luteolin-based interventions for conditions such as Alzheimer's disease, Parkinson's disease, and stroke, where oxidative stress and neuroinflammation play significant pathological roles.
Bioavailability enhancement strategies present immediate translational opportunities. Novel drug delivery systems including nanoparticle encapsulation, liposomal formulations, and blood-brain barrier penetration technologies can significantly improve luteolin's therapeutic potential. These approaches address the compound's inherent limitations in solubility and metabolic stability, potentially increasing its concentration in neural tissues.
Clinical applications may initially manifest as adjunctive therapies alongside established treatments. Early-phase clinical trials could target specific neurological conditions where biomarkers of oxidative stress and inflammation can be readily measured, providing objective endpoints for efficacy assessment. The development of standardized luteolin formulations with consistent bioactive content represents a prerequisite for meaningful clinical investigation.
Precision medicine approaches offer promising avenues for translational success. Identifying patient subpopulations most likely to benefit from luteolin's mechanisms—particularly those with genetic profiles indicating susceptibility to oxidative stress or neuroinflammation—could enhance clinical trial outcomes and therapeutic efficacy. Biomarker development for patient stratification and treatment response monitoring will be essential components of this strategy.
Preventive applications in high-risk populations constitute another translational pathway. Long-term administration of luteolin formulations in individuals with genetic predispositions to neurodegenerative conditions could potentially delay disease onset or slow progression. Such preventive approaches would require careful safety profiling and extended observation periods to establish efficacy.
Combination therapies incorporating luteolin with other neuroprotective agents may yield synergistic effects. Strategic pairing with compounds targeting complementary pathways could enhance overall neuroprotection while potentially reducing required dosages of individual components. This approach may mitigate side effect profiles while maximizing therapeutic benefits across multiple neuropathological mechanisms.
Regulatory considerations will significantly influence translational timelines. Engaging with regulatory bodies early in development processes can streamline approval pathways, particularly through special designations for neurodegenerative conditions with limited treatment options. Public-private partnerships between academic institutions, biotechnology companies, and pharmaceutical industry could accelerate clinical translation through shared resources and expertise.
Bioavailability enhancement strategies present immediate translational opportunities. Novel drug delivery systems including nanoparticle encapsulation, liposomal formulations, and blood-brain barrier penetration technologies can significantly improve luteolin's therapeutic potential. These approaches address the compound's inherent limitations in solubility and metabolic stability, potentially increasing its concentration in neural tissues.
Clinical applications may initially manifest as adjunctive therapies alongside established treatments. Early-phase clinical trials could target specific neurological conditions where biomarkers of oxidative stress and inflammation can be readily measured, providing objective endpoints for efficacy assessment. The development of standardized luteolin formulations with consistent bioactive content represents a prerequisite for meaningful clinical investigation.
Precision medicine approaches offer promising avenues for translational success. Identifying patient subpopulations most likely to benefit from luteolin's mechanisms—particularly those with genetic profiles indicating susceptibility to oxidative stress or neuroinflammation—could enhance clinical trial outcomes and therapeutic efficacy. Biomarker development for patient stratification and treatment response monitoring will be essential components of this strategy.
Preventive applications in high-risk populations constitute another translational pathway. Long-term administration of luteolin formulations in individuals with genetic predispositions to neurodegenerative conditions could potentially delay disease onset or slow progression. Such preventive approaches would require careful safety profiling and extended observation periods to establish efficacy.
Combination therapies incorporating luteolin with other neuroprotective agents may yield synergistic effects. Strategic pairing with compounds targeting complementary pathways could enhance overall neuroprotection while potentially reducing required dosages of individual components. This approach may mitigate side effect profiles while maximizing therapeutic benefits across multiple neuropathological mechanisms.
Regulatory considerations will significantly influence translational timelines. Engaging with regulatory bodies early in development processes can streamline approval pathways, particularly through special designations for neurodegenerative conditions with limited treatment options. Public-private partnerships between academic institutions, biotechnology companies, and pharmaceutical industry could accelerate clinical translation through shared resources and expertise.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!