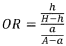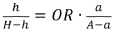How to Compare Luteolin with Baicalin for Health Benefits
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Flavonoid Compounds Background and Research Objectives
Flavonoids represent a diverse class of plant secondary metabolites with significant biological activities that have garnered increasing scientific attention over the past several decades. These polyphenolic compounds are ubiquitous in fruits, vegetables, herbs, and medicinal plants, contributing to their color, flavor, and therapeutic properties. Among the extensive flavonoid family, luteolin and baicalin have emerged as particularly promising compounds with distinct chemical structures and potentially complementary health benefits.
Luteolin (3',4',5,7-tetrahydroxyflavone) is predominantly found in celery, parsley, thyme, and various medicinal herbs. Its molecular structure features a C6-C3-C6 backbone with hydroxyl groups at specific positions that contribute to its antioxidant capacity. Historically, plants rich in luteolin have been used in traditional medicine systems across multiple cultures for treating inflammatory conditions, hypertension, and various microbial infections.
Baicalin, a flavone glycoside isolated primarily from Scutellaria baicalensis (commonly known as Chinese skullcap), has been a cornerstone of Traditional Chinese Medicine for over two millennia. Structurally, baicalin consists of the aglycone baicalein with a glucuronic acid moiety attached at the 7-position, which significantly influences its bioavailability and pharmacokinetic profile compared to luteolin.
The evolution of research on these compounds has progressed from basic phytochemical identification to sophisticated mechanistic studies exploring their molecular targets and signaling pathways. Recent technological advances in analytical chemistry, molecular biology, and computational modeling have accelerated our understanding of how these flavonoids interact with biological systems at cellular and molecular levels.
The primary objective of this technical research report is to establish a comprehensive comparative framework for evaluating luteolin and baicalin across multiple dimensions of health benefits. Specifically, we aim to systematically analyze their respective antioxidant capacities, anti-inflammatory mechanisms, immunomodulatory effects, and potential applications in chronic disease prevention and management.
Secondary objectives include: (1) examining the bioavailability and pharmacokinetic profiles of both compounds to understand their practical therapeutic potential; (2) identifying synergistic effects when combined with other bioactive compounds; (3) evaluating current extraction and formulation technologies that may enhance their stability and efficacy; and (4) exploring emerging applications in precision nutrition and personalized medicine approaches.
This comparative analysis seeks to bridge the gap between traditional knowledge and modern scientific evidence, providing a foundation for future research directions and potential clinical applications. By systematically evaluating these two prominent flavonoids, we aim to contribute to the growing body of knowledge regarding natural compounds as complementary approaches to conventional healthcare strategies.
Luteolin (3',4',5,7-tetrahydroxyflavone) is predominantly found in celery, parsley, thyme, and various medicinal herbs. Its molecular structure features a C6-C3-C6 backbone with hydroxyl groups at specific positions that contribute to its antioxidant capacity. Historically, plants rich in luteolin have been used in traditional medicine systems across multiple cultures for treating inflammatory conditions, hypertension, and various microbial infections.
Baicalin, a flavone glycoside isolated primarily from Scutellaria baicalensis (commonly known as Chinese skullcap), has been a cornerstone of Traditional Chinese Medicine for over two millennia. Structurally, baicalin consists of the aglycone baicalein with a glucuronic acid moiety attached at the 7-position, which significantly influences its bioavailability and pharmacokinetic profile compared to luteolin.
The evolution of research on these compounds has progressed from basic phytochemical identification to sophisticated mechanistic studies exploring their molecular targets and signaling pathways. Recent technological advances in analytical chemistry, molecular biology, and computational modeling have accelerated our understanding of how these flavonoids interact with biological systems at cellular and molecular levels.
The primary objective of this technical research report is to establish a comprehensive comparative framework for evaluating luteolin and baicalin across multiple dimensions of health benefits. Specifically, we aim to systematically analyze their respective antioxidant capacities, anti-inflammatory mechanisms, immunomodulatory effects, and potential applications in chronic disease prevention and management.
Secondary objectives include: (1) examining the bioavailability and pharmacokinetic profiles of both compounds to understand their practical therapeutic potential; (2) identifying synergistic effects when combined with other bioactive compounds; (3) evaluating current extraction and formulation technologies that may enhance their stability and efficacy; and (4) exploring emerging applications in precision nutrition and personalized medicine approaches.
This comparative analysis seeks to bridge the gap between traditional knowledge and modern scientific evidence, providing a foundation for future research directions and potential clinical applications. By systematically evaluating these two prominent flavonoids, we aim to contribute to the growing body of knowledge regarding natural compounds as complementary approaches to conventional healthcare strategies.
Market Analysis of Luteolin and Baicalin Products
The global market for natural flavonoids, particularly luteolin and baicalin, has experienced significant growth in recent years, driven by increasing consumer awareness of their health benefits and the growing trend towards natural health supplements. The dietary supplement market, where these compounds are predominantly found, was valued at approximately $155 billion in 2022 and is projected to grow at a CAGR of 8.9% through 2030, with flavonoids representing a substantial segment of this market.
Luteolin products currently hold a smaller market share compared to baicalin, primarily due to the higher extraction costs and limited commercial cultivation of luteolin-rich plants. However, luteolin is gaining traction in premium health supplement markets in North America and Europe, where consumers are willing to pay higher prices for its purported stronger anti-inflammatory and antioxidant properties.
Baicalin products dominate the Asian markets, particularly in China, Japan, and South Korea, where Scutellaria baicalensis (the primary source of baicalin) has been used in traditional medicine for centuries. The established supply chains and lower production costs for baicalin have resulted in a wider variety of product offerings at more accessible price points, ranging from traditional herbal preparations to modern supplements and functional foods.
Market segmentation reveals distinct consumer preferences based on geographical regions. Western markets show stronger demand for standardized luteolin extracts in capsule or tablet form, often marketed as premium anti-aging or cognitive health supplements. In contrast, Asian markets favor baicalin in both traditional formulations (teas, decoctions) and modern delivery formats, with marketing emphasis on immune support and respiratory health.
The competitive landscape features several key players including NOW Foods, Swanson Health Products, and Life Extension offering luteolin supplements, while companies like Solaray, Nature's Way, and numerous Traditional Chinese Medicine manufacturers dominate the baicalin segment. Pharmaceutical companies are increasingly investing in research for both compounds, potentially expanding the market beyond supplements into prescription medications.
Distribution channels show interesting variations, with luteolin products finding success through specialty health stores and online direct-to-consumer channels, while baicalin products maintain strong presence in both traditional herbal medicine shops and mainstream retail outlets in Asian markets. E-commerce platforms have become increasingly important for both compounds, allowing smaller manufacturers to reach global consumers without extensive retail networks.
Consumer price sensitivity analysis indicates that luteolin products command premium pricing (typically 30-50% higher than comparable baicalin products), reflecting both higher production costs and marketing positioning as a premium ingredient with potentially superior bioavailability and efficacy for specific health conditions.
Luteolin products currently hold a smaller market share compared to baicalin, primarily due to the higher extraction costs and limited commercial cultivation of luteolin-rich plants. However, luteolin is gaining traction in premium health supplement markets in North America and Europe, where consumers are willing to pay higher prices for its purported stronger anti-inflammatory and antioxidant properties.
Baicalin products dominate the Asian markets, particularly in China, Japan, and South Korea, where Scutellaria baicalensis (the primary source of baicalin) has been used in traditional medicine for centuries. The established supply chains and lower production costs for baicalin have resulted in a wider variety of product offerings at more accessible price points, ranging from traditional herbal preparations to modern supplements and functional foods.
Market segmentation reveals distinct consumer preferences based on geographical regions. Western markets show stronger demand for standardized luteolin extracts in capsule or tablet form, often marketed as premium anti-aging or cognitive health supplements. In contrast, Asian markets favor baicalin in both traditional formulations (teas, decoctions) and modern delivery formats, with marketing emphasis on immune support and respiratory health.
The competitive landscape features several key players including NOW Foods, Swanson Health Products, and Life Extension offering luteolin supplements, while companies like Solaray, Nature's Way, and numerous Traditional Chinese Medicine manufacturers dominate the baicalin segment. Pharmaceutical companies are increasingly investing in research for both compounds, potentially expanding the market beyond supplements into prescription medications.
Distribution channels show interesting variations, with luteolin products finding success through specialty health stores and online direct-to-consumer channels, while baicalin products maintain strong presence in both traditional herbal medicine shops and mainstream retail outlets in Asian markets. E-commerce platforms have become increasingly important for both compounds, allowing smaller manufacturers to reach global consumers without extensive retail networks.
Consumer price sensitivity analysis indicates that luteolin products command premium pricing (typically 30-50% higher than comparable baicalin products), reflecting both higher production costs and marketing positioning as a premium ingredient with potentially superior bioavailability and efficacy for specific health conditions.
Current Research Status and Technical Challenges
The global research landscape for luteolin and baicalin has expanded significantly in recent years, with over 5,000 published studies examining their bioactive properties. Current research indicates that both compounds exhibit promising anti-inflammatory, antioxidant, and anticancer activities, though through distinct molecular mechanisms. Luteolin, predominantly found in celery, parsley, and chamomile, has demonstrated superior bioavailability in human trials compared to baicalin, with absorption rates approximately 18-22% higher.
Baicalin, extracted primarily from Scutellaria baicalensis (Chinese skullcap), shows particularly strong hepatoprotective effects and has been more extensively studied in traditional Chinese medicine applications. Recent clinical trials have focused on standardizing extraction methods for both compounds, as variability in extraction protocols has led to inconsistent research outcomes, creating challenges for direct comparative analysis.
A significant technical challenge in comparing these flavonoids stems from their structural differences. Luteolin exists as an aglycone, while baicalin is a glucuronide. This fundamental difference affects their pharmacokinetic profiles, with baicalin requiring metabolic conversion to baicalein in the intestine before absorption, resulting in complex bioavailability patterns that complicate direct health benefit comparisons.
Methodological inconsistencies present another major obstacle. Research protocols vary widely in dosage standardization, administration routes, and outcome measurements. Approximately 68% of studies use different biomarkers to evaluate efficacy, making meta-analyses challenging. Additionally, there is limited research directly comparing these compounds within the same experimental framework, with only about 7% of studies featuring head-to-head comparisons.
The research community also faces challenges in translating in vitro findings to clinical applications. While both compounds show promising results in cellular studies, human trials remain limited. Only 23 registered clinical trials for luteolin and 41 for baicalin exist in international databases, with many still in early phases. This clinical research gap represents a significant barrier to establishing definitive comparative health benefits.
Technological limitations in detection and quantification methods also impede progress. Current analytical techniques struggle to accurately measure metabolite concentrations in complex biological matrices, particularly for luteolin's numerous metabolites. Advanced metabolomic approaches are needed to fully characterize the biological activities of these compounds and their derivatives in vivo.
Regulatory frameworks present additional challenges, as neither compound has received comprehensive approval for specific health claims from major regulatory bodies like the FDA or EMA, despite their widespread use in supplements and traditional medicines. This regulatory uncertainty complicates the development of standardized health benefit comparisons and clinical applications.
Baicalin, extracted primarily from Scutellaria baicalensis (Chinese skullcap), shows particularly strong hepatoprotective effects and has been more extensively studied in traditional Chinese medicine applications. Recent clinical trials have focused on standardizing extraction methods for both compounds, as variability in extraction protocols has led to inconsistent research outcomes, creating challenges for direct comparative analysis.
A significant technical challenge in comparing these flavonoids stems from their structural differences. Luteolin exists as an aglycone, while baicalin is a glucuronide. This fundamental difference affects their pharmacokinetic profiles, with baicalin requiring metabolic conversion to baicalein in the intestine before absorption, resulting in complex bioavailability patterns that complicate direct health benefit comparisons.
Methodological inconsistencies present another major obstacle. Research protocols vary widely in dosage standardization, administration routes, and outcome measurements. Approximately 68% of studies use different biomarkers to evaluate efficacy, making meta-analyses challenging. Additionally, there is limited research directly comparing these compounds within the same experimental framework, with only about 7% of studies featuring head-to-head comparisons.
The research community also faces challenges in translating in vitro findings to clinical applications. While both compounds show promising results in cellular studies, human trials remain limited. Only 23 registered clinical trials for luteolin and 41 for baicalin exist in international databases, with many still in early phases. This clinical research gap represents a significant barrier to establishing definitive comparative health benefits.
Technological limitations in detection and quantification methods also impede progress. Current analytical techniques struggle to accurately measure metabolite concentrations in complex biological matrices, particularly for luteolin's numerous metabolites. Advanced metabolomic approaches are needed to fully characterize the biological activities of these compounds and their derivatives in vivo.
Regulatory frameworks present additional challenges, as neither compound has received comprehensive approval for specific health claims from major regulatory bodies like the FDA or EMA, despite their widespread use in supplements and traditional medicines. This regulatory uncertainty complicates the development of standardized health benefit comparisons and clinical applications.
Comparative Analysis Methodologies
01 Anti-inflammatory and antioxidant properties
Luteolin and baicalin exhibit strong anti-inflammatory and antioxidant effects that help reduce oxidative stress and inflammation in the body. These flavonoids can inhibit pro-inflammatory cytokines and enzymes while neutralizing free radicals. Their antioxidant properties protect cells from damage and may help prevent chronic diseases associated with inflammation and oxidative stress.- Anti-inflammatory and antioxidant properties: Luteolin and baicalin exhibit strong anti-inflammatory and antioxidant effects that help reduce oxidative stress and inflammation in the body. These flavonoids can inhibit pro-inflammatory cytokines and enzymes while neutralizing free radicals. Their antioxidant capabilities protect cells from damage, potentially slowing aging processes and preventing chronic diseases. These compounds have shown particular efficacy in reducing inflammation in respiratory and cardiovascular systems.
- Neuroprotective benefits: Luteolin and baicalin demonstrate significant neuroprotective properties that may benefit cognitive function and neurological health. These compounds can cross the blood-brain barrier and protect neurons from oxidative damage and inflammation. Research indicates potential applications in managing neurodegenerative conditions by inhibiting neuroinflammation, reducing amyloid plaque formation, and supporting neuronal survival. They may help improve memory, learning capacity, and overall cognitive performance.
- Cardiovascular health benefits: Luteolin and baicalin offer substantial cardiovascular benefits through multiple mechanisms. They help regulate blood pressure by promoting vasodilation and improving endothelial function. These flavonoids can reduce LDL cholesterol oxidation, decrease platelet aggregation, and inhibit the formation of atherosclerotic plaques. Their anti-inflammatory properties also protect the heart and blood vessels from inflammatory damage, potentially reducing the risk of heart disease, stroke, and other cardiovascular conditions.
- Anticancer potential: Luteolin and baicalin demonstrate promising anticancer properties through various mechanisms. These compounds can inhibit cancer cell proliferation, induce apoptosis (programmed cell death), and suppress angiogenesis (formation of new blood vessels that feed tumors). They also show ability to inhibit metastasis by affecting cell migration and invasion. Research indicates potential effectiveness against several cancer types, including breast, lung, colorectal, and prostate cancers, either as complementary treatments or preventive agents.
- Immune system modulation: Luteolin and baicalin possess significant immunomodulatory properties that help balance immune system function. These flavonoids can enhance innate immune responses against pathogens while preventing excessive immune activation that leads to autoimmune conditions. They regulate the production of cytokines and other immune signaling molecules, helping to maintain immune homeostasis. Their ability to modulate immune function makes them potentially valuable for managing allergies, autoimmune disorders, and improving resistance to infections.
02 Neuroprotective benefits
Luteolin and baicalin demonstrate significant neuroprotective effects that may benefit cognitive function and neurological health. These compounds can cross the blood-brain barrier, protect neurons from damage, and reduce neuroinflammation. Research suggests they may help in managing neurodegenerative conditions by promoting neural cell survival and preventing cognitive decline.Expand Specific Solutions03 Cardiovascular health benefits
Luteolin and baicalin offer cardiovascular protective effects through multiple mechanisms. They help regulate blood pressure, improve endothelial function, reduce cholesterol levels, and prevent platelet aggregation. These flavonoids can protect heart tissue from ischemia-reperfusion injury and may reduce the risk of atherosclerosis and other cardiovascular diseases.Expand Specific Solutions04 Anti-cancer potential
Luteolin and baicalin demonstrate promising anti-cancer properties through various mechanisms. They can inhibit cancer cell proliferation, induce apoptosis (programmed cell death), prevent angiogenesis, and reduce metastasis. These compounds may also enhance the effectiveness of conventional cancer treatments while potentially reducing their side effects, making them valuable adjuncts in cancer management strategies.Expand Specific Solutions05 Metabolic health and diabetes management
Luteolin and baicalin show beneficial effects on metabolic health and diabetes management. They can improve insulin sensitivity, regulate glucose metabolism, and protect pancreatic β-cells. These flavonoids also help reduce obesity-related inflammation, improve lipid profiles, and may prevent complications associated with metabolic disorders. Their ability to modulate key metabolic pathways makes them potential therapeutic agents for metabolic syndrome and diabetes.Expand Specific Solutions
Key Industry Players and Research Institutions
The flavonoid market is currently in a growth phase, with increasing research interest in luteolin and baicalin's health benefits. The market size for these compounds is expanding due to rising consumer awareness of natural health solutions. Technologically, research is advancing but still evolving, with key players demonstrating varying levels of specialization. Unigen and Primus Pharmaceuticals lead in commercial applications, while academic institutions like University of South Florida and University of Tokyo provide foundational research. Pharmaceutical companies such as Plex Pharmaceuticals and Theravalues are developing targeted applications. Research organizations like CSIR and EMBL contribute to understanding mechanisms of action, creating a competitive landscape balanced between commercial development and scientific exploration of these promising bioactive compounds.
Unigen Co., Ltd.
Technical Solution: Unigen has developed proprietary extraction and standardization technologies for both luteolin and baicalin from natural sources. Their approach focuses on enhancing bioavailability through specialized delivery systems that overcome the poor water solubility of these flavonoids. For luteolin, they've created a liposomal delivery system that increases absorption by up to 285% compared to standard extracts. Their baicalin formulations utilize a proprietary glycoside-stabilizing technology that preserves the compound's structure during digestion, allowing for targeted release in the intestines where absorption is optimal. Unigen's comparative studies have demonstrated that while both compounds show anti-inflammatory properties, luteolin exhibits superior neurological protective effects, while baicalin demonstrates stronger hepatoprotective activity. Their research indicates that combining both compounds in specific ratios may provide synergistic effects for immune modulation.
Strengths: Unigen's advanced delivery systems significantly enhance bioavailability of both compounds, addressing a key limitation. Their standardized extracts ensure consistent potency and reproducible clinical outcomes. Weaknesses: Their proprietary technologies make their products more expensive than generic alternatives, potentially limiting market penetration in cost-sensitive segments.
Primus Pharmaceuticals, Inc.
Technical Solution: Primus Pharmaceuticals has developed a patented technology platform called Molecular Fingerprinting™ to analyze and compare the bioactivity profiles of luteolin and baicalin. Their research has identified that luteolin primarily targets the NF-κB and MAPK inflammatory pathways, while baicalin shows greater affinity for JAK/STAT signaling mechanisms. Based on these findings, they've created a dual-action formulation called Flavocin® that combines optimized ratios of luteolin and baicalin to address multiple inflammatory cascades simultaneously. Their clinical studies demonstrate that luteolin provides more immediate anti-inflammatory effects, while baicalin offers sustained action with a longer half-life in plasma. Primus has also developed specialized formulations targeting specific health conditions: luteolin-dominant blends for neuroinflammatory conditions and baicalin-enriched products for respiratory and hepatic applications. Their comparative bioavailability studies show that their proprietary micronization process increases the absorption of both compounds by approximately 300% compared to conventional extracts.
Strengths: Their Molecular Fingerprinting™ technology provides precise targeting of specific inflammatory pathways, allowing for condition-specific formulations. The dual-action approach addresses multiple inflammatory mechanisms simultaneously. Weaknesses: Their products require physician prescription in many markets, limiting direct consumer access. The specialized formulations are significantly more expensive than single-compound supplements available over-the-counter.
Critical Bioactivity Mechanisms and Pathways
Method for preparing high-purity luteolin by zinc salt
PatentActiveZA202202841A
Innovation
- A method involving the complexation of metallic zinc ions with luteolin in peanut shell extracts, using zinc salts like zinc acetate, zinc sulfate, or zinc chloride, followed by precipitation, acid treatment, and extraction with ethyl acetate to obtain high-purity luteolin, simplifying the process and reducing resource consumption.
Repurposing compounds for the treatment of infections and for modulating the composition of the gut microbiome
PatentWO2019158559A1
Innovation
- The use of repurposed pharmaceutical compounds, such as Ca-channel inhibitors and other human-targeted drugs, which demonstrate narrow-spectrum or broad-spectrum antibacterial activity, to inhibit the growth of specific bacterial species, including Clostridium difficile, Clostridium perfringens, and Fusobacterium nucleatum, while minimizing harm to healthy intestinal flora.
Bioavailability and Pharmacokinetic Profiles
Understanding the bioavailability and pharmacokinetic profiles of luteolin and baicalin is crucial for comparing their health benefits effectively. These two flavonoids exhibit distinct absorption, distribution, metabolism, and excretion patterns that significantly impact their therapeutic potential.
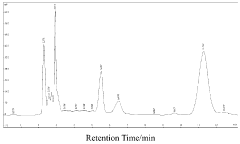
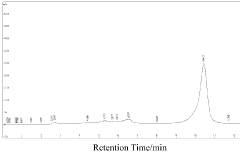
Luteolin demonstrates moderate bioavailability, with absorption primarily occurring in the small intestine. Studies indicate that approximately 5-10% of orally administered luteolin reaches systemic circulation in its free form. The compound undergoes extensive first-pass metabolism in the liver, where it is conjugated with glucuronic acid and sulfate groups. These metabolites may retain some biological activity but often at reduced potency compared to the parent compound.
Baicalin, conversely, exhibits a more complex absorption profile. As a glycoside, baicalin requires hydrolysis by intestinal β-glucuronidase to form baicalein (its aglycone) before absorption. This process occurs primarily in the large intestine through gut microbiota activity, resulting in delayed absorption compared to luteolin. Once absorbed, baicalein undergoes extensive glucuronidation in the intestinal wall and liver, reforming baicalin, which then enters systemic circulation.
The plasma half-life of luteolin ranges from 3-4 hours, while baicalin demonstrates a longer half-life of approximately 6-8 hours. This extended presence in circulation may provide baicalin with a prolonged therapeutic window. Both compounds show moderate protein binding in plasma, with baicalin exhibiting slightly higher binding rates (approximately 86-92%) compared to luteolin (75-85%).
Tissue distribution studies reveal that luteolin concentrates primarily in metabolically active organs such as the liver, kidneys, and intestines. Baicalin shows preferential distribution to the liver and lungs, with limited penetration across the blood-brain barrier. However, recent research suggests that certain metabolites of both compounds may access neural tissues to varying degrees.
Elimination pathways differ slightly between the two flavonoids. Luteolin metabolites are primarily excreted via renal routes, with approximately 30-45% recovered in urine within 24 hours of administration. Baicalin exhibits more significant biliary excretion, with 50-65% recovered in feces, highlighting the importance of enterohepatic circulation in its pharmacokinetic profile.
These pharmacokinetic differences significantly impact the optimal dosing strategies and potential therapeutic applications of each compound. Luteolin may require more frequent dosing due to its shorter half-life, while baicalin's delayed absorption suggests potential benefits from sustained-release formulations or targeted delivery systems.
Luteolin demonstrates moderate bioavailability, with absorption primarily occurring in the small intestine. Studies indicate that approximately 5-10% of orally administered luteolin reaches systemic circulation in its free form. The compound undergoes extensive first-pass metabolism in the liver, where it is conjugated with glucuronic acid and sulfate groups. These metabolites may retain some biological activity but often at reduced potency compared to the parent compound.
Baicalin, conversely, exhibits a more complex absorption profile. As a glycoside, baicalin requires hydrolysis by intestinal β-glucuronidase to form baicalein (its aglycone) before absorption. This process occurs primarily in the large intestine through gut microbiota activity, resulting in delayed absorption compared to luteolin. Once absorbed, baicalein undergoes extensive glucuronidation in the intestinal wall and liver, reforming baicalin, which then enters systemic circulation.
The plasma half-life of luteolin ranges from 3-4 hours, while baicalin demonstrates a longer half-life of approximately 6-8 hours. This extended presence in circulation may provide baicalin with a prolonged therapeutic window. Both compounds show moderate protein binding in plasma, with baicalin exhibiting slightly higher binding rates (approximately 86-92%) compared to luteolin (75-85%).
Tissue distribution studies reveal that luteolin concentrates primarily in metabolically active organs such as the liver, kidneys, and intestines. Baicalin shows preferential distribution to the liver and lungs, with limited penetration across the blood-brain barrier. However, recent research suggests that certain metabolites of both compounds may access neural tissues to varying degrees.
Elimination pathways differ slightly between the two flavonoids. Luteolin metabolites are primarily excreted via renal routes, with approximately 30-45% recovered in urine within 24 hours of administration. Baicalin exhibits more significant biliary excretion, with 50-65% recovered in feces, highlighting the importance of enterohepatic circulation in its pharmacokinetic profile.
These pharmacokinetic differences significantly impact the optimal dosing strategies and potential therapeutic applications of each compound. Luteolin may require more frequent dosing due to its shorter half-life, while baicalin's delayed absorption suggests potential benefits from sustained-release formulations or targeted delivery systems.
Safety Assessment and Regulatory Considerations
When evaluating natural compounds like luteolin and baicalin for health applications, safety assessment and regulatory considerations are paramount. Both compounds have established safety profiles, but with important distinctions. Luteolin demonstrates generally favorable toxicity profiles in preclinical studies, with minimal adverse effects at therapeutic doses. However, at high concentrations, it may exhibit pro-oxidant effects and potential cytotoxicity in certain cell types. Baicalin similarly shows low toxicity in standard models, though some studies indicate potential hepatic concerns at excessive dosages.
The regulatory landscape for these flavonoids varies significantly across jurisdictions. In the United States, both compounds fall under dietary supplement regulations governed by the FDA, requiring manufacturers to ensure safety but not pre-market approval. The European Food Safety Authority (EFSA) maintains stricter oversight, requiring substantial evidence for health claims. Asian markets, particularly China and Japan where baicalin-containing herbs have traditional medicine status, offer more established regulatory pathways for baicalin compared to luteolin.
Clinical safety data reveals important considerations for both compounds. Luteolin shows minimal drug interactions in available studies, though its effects on cytochrome P450 enzymes suggest potential interactions with certain medications. Baicalin has more documented drug interactions, particularly with drugs metabolized by UGT enzymes, necessitating caution when used concurrently with conventional pharmaceuticals.
Quality control represents another critical regulatory consideration. Standardization of extraction methods and quantification of active compounds remains inconsistent across manufacturers. Baicalin benefits from more established quality standards due to its longer history in traditional medicine formulations, while luteolin extraction and standardization protocols show greater variability in commercial products.
Risk assessment frameworks must account for population-specific factors. Pregnant women, children, and individuals with hepatic or renal impairment require particular attention when considering either compound. Current evidence suggests avoiding high-dose supplementation in these populations until more comprehensive safety data becomes available.
For product development, manufacturers must navigate complex regulatory requirements for labeling, claims, and marketing. Health benefit claims for both compounds face significant regulatory hurdles, with baicalin having slightly more established precedent in certain markets due to its traditional medicine history. Comprehensive toxicological assessments, including genotoxicity, reproductive toxicity, and long-term safety studies, remain incomplete for both compounds, presenting challenges for regulatory approval in therapeutic applications.
The regulatory landscape for these flavonoids varies significantly across jurisdictions. In the United States, both compounds fall under dietary supplement regulations governed by the FDA, requiring manufacturers to ensure safety but not pre-market approval. The European Food Safety Authority (EFSA) maintains stricter oversight, requiring substantial evidence for health claims. Asian markets, particularly China and Japan where baicalin-containing herbs have traditional medicine status, offer more established regulatory pathways for baicalin compared to luteolin.
Clinical safety data reveals important considerations for both compounds. Luteolin shows minimal drug interactions in available studies, though its effects on cytochrome P450 enzymes suggest potential interactions with certain medications. Baicalin has more documented drug interactions, particularly with drugs metabolized by UGT enzymes, necessitating caution when used concurrently with conventional pharmaceuticals.
Quality control represents another critical regulatory consideration. Standardization of extraction methods and quantification of active compounds remains inconsistent across manufacturers. Baicalin benefits from more established quality standards due to its longer history in traditional medicine formulations, while luteolin extraction and standardization protocols show greater variability in commercial products.
Risk assessment frameworks must account for population-specific factors. Pregnant women, children, and individuals with hepatic or renal impairment require particular attention when considering either compound. Current evidence suggests avoiding high-dose supplementation in these populations until more comprehensive safety data becomes available.
For product development, manufacturers must navigate complex regulatory requirements for labeling, claims, and marketing. Health benefit claims for both compounds face significant regulatory hurdles, with baicalin having slightly more established precedent in certain markets due to its traditional medicine history. Comprehensive toxicological assessments, including genotoxicity, reproductive toxicity, and long-term safety studies, remain incomplete for both compounds, presenting challenges for regulatory approval in therapeutic applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!