Computational Studies of Tautomerization in Enzyme Catalysis
JUL 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Tautomerization in Enzymes: Background and Objectives
Tautomerization, a fundamental process in organic chemistry, plays a crucial role in enzyme catalysis. This phenomenon involves the rapid interconversion between structural isomers, known as tautomers, which differ in the position of a proton and a π bond. In the context of enzyme catalysis, tautomerization can significantly influence reaction mechanisms, substrate binding, and overall catalytic efficiency.
The study of tautomerization in enzymes has a rich history dating back to the mid-20th century. Early investigations focused on understanding the basic principles of tautomerism in small organic molecules. As biochemical research advanced, scientists began to recognize the importance of tautomerization in biological systems, particularly in enzyme-catalyzed reactions.
Over the past few decades, computational studies have emerged as a powerful tool for investigating tautomerization in enzyme catalysis. These studies have allowed researchers to explore the energetics, kinetics, and structural dynamics of tautomeric transitions at an atomic level. The advent of quantum mechanical methods, molecular dynamics simulations, and hybrid QM/MM approaches has greatly enhanced our ability to model and predict tautomerization events in complex enzymatic environments.
The primary objective of computational studies in this field is to elucidate the mechanistic details of tautomerization-mediated enzyme catalysis. This includes identifying key tautomeric states, characterizing transition states, and quantifying the energetic barriers associated with tautomeric interconversions. Additionally, these studies aim to reveal how enzymes may stabilize specific tautomeric forms of substrates or intermediates to facilitate catalysis.
Another critical goal is to understand the role of the enzyme's microenvironment in modulating tautomerization. This involves investigating how factors such as electrostatic interactions, hydrogen bonding networks, and conformational dynamics of the enzyme influence tautomeric equilibria and transition rates. By gaining insights into these aspects, researchers hope to develop a more comprehensive understanding of enzyme function and potentially guide the design of novel catalysts or therapeutic interventions.
Furthermore, computational studies seek to bridge the gap between experimental observations and theoretical predictions. This involves developing and refining computational methods that can accurately reproduce and explain experimental data related to tautomerization in enzymes. Such efforts are crucial for validating computational approaches and enhancing their predictive power in studying complex biochemical processes.
As we look towards the future, the field of computational studies of tautomerization in enzyme catalysis continues to evolve. Emerging trends include the integration of machine learning techniques, the development of more accurate and efficient computational methods, and the exploration of tautomerization in increasingly complex enzymatic systems. These advancements promise to deepen our understanding of enzyme function and potentially unlock new avenues for enzyme engineering and drug design.
The study of tautomerization in enzymes has a rich history dating back to the mid-20th century. Early investigations focused on understanding the basic principles of tautomerism in small organic molecules. As biochemical research advanced, scientists began to recognize the importance of tautomerization in biological systems, particularly in enzyme-catalyzed reactions.
Over the past few decades, computational studies have emerged as a powerful tool for investigating tautomerization in enzyme catalysis. These studies have allowed researchers to explore the energetics, kinetics, and structural dynamics of tautomeric transitions at an atomic level. The advent of quantum mechanical methods, molecular dynamics simulations, and hybrid QM/MM approaches has greatly enhanced our ability to model and predict tautomerization events in complex enzymatic environments.
The primary objective of computational studies in this field is to elucidate the mechanistic details of tautomerization-mediated enzyme catalysis. This includes identifying key tautomeric states, characterizing transition states, and quantifying the energetic barriers associated with tautomeric interconversions. Additionally, these studies aim to reveal how enzymes may stabilize specific tautomeric forms of substrates or intermediates to facilitate catalysis.
Another critical goal is to understand the role of the enzyme's microenvironment in modulating tautomerization. This involves investigating how factors such as electrostatic interactions, hydrogen bonding networks, and conformational dynamics of the enzyme influence tautomeric equilibria and transition rates. By gaining insights into these aspects, researchers hope to develop a more comprehensive understanding of enzyme function and potentially guide the design of novel catalysts or therapeutic interventions.
Furthermore, computational studies seek to bridge the gap between experimental observations and theoretical predictions. This involves developing and refining computational methods that can accurately reproduce and explain experimental data related to tautomerization in enzymes. Such efforts are crucial for validating computational approaches and enhancing their predictive power in studying complex biochemical processes.
As we look towards the future, the field of computational studies of tautomerization in enzyme catalysis continues to evolve. Emerging trends include the integration of machine learning techniques, the development of more accurate and efficient computational methods, and the exploration of tautomerization in increasingly complex enzymatic systems. These advancements promise to deepen our understanding of enzyme function and potentially unlock new avenues for enzyme engineering and drug design.
Market Demand for Enzyme Catalysis Simulations
The market demand for enzyme catalysis simulations has been steadily growing in recent years, driven by the increasing need for efficient and sustainable chemical processes across various industries. Pharmaceutical companies, in particular, have shown a keen interest in computational studies of tautomerization in enzyme catalysis, as these simulations can significantly reduce the time and cost associated with drug discovery and development.
The global enzyme market, which includes both industrial enzymes and specialty enzymes used in research, is projected to reach a substantial value in the coming years. This growth is largely attributed to the rising demand for enzyme-based solutions in various applications, including pharmaceuticals, food and beverages, biofuels, and environmental remediation. Within this broader market, the demand for computational tools and services for enzyme catalysis simulations is experiencing rapid expansion.
Pharmaceutical companies are increasingly relying on in silico methods to streamline their drug discovery processes. Computational studies of tautomerization in enzyme catalysis play a crucial role in predicting drug-target interactions, optimizing lead compounds, and understanding the mechanisms of enzymatic reactions. This approach not only accelerates the drug development pipeline but also reduces the need for extensive laboratory experiments, thereby cutting costs and minimizing the use of resources.
The biotechnology sector is another major driver of demand for enzyme catalysis simulations. As the industry moves towards more sustainable and eco-friendly production methods, there is a growing need for computational tools that can help design and optimize biocatalysts for industrial applications. These simulations enable researchers to explore the potential of novel enzymes and engineer existing ones for improved performance under specific conditions.
Academic institutions and research organizations also contribute significantly to the market demand for enzyme catalysis simulations. The increasing focus on understanding complex biological systems at the molecular level has led to a surge in research projects utilizing computational methods. This trend is further supported by the availability of more powerful computing resources and advanced algorithms, making it possible to simulate larger and more complex enzymatic systems.
The food and beverage industry is emerging as a new frontier for enzyme catalysis simulations. Manufacturers are exploring the use of enzymes to improve food quality, extend shelf life, and develop novel products. Computational studies in this field can help identify and optimize enzymes for specific food processing applications, leading to more efficient and cost-effective production methods.
As the demand for enzyme catalysis simulations continues to grow, there is an increasing need for specialized software tools and expertise in this field. This has created opportunities for software companies and service providers specializing in computational chemistry and molecular modeling. The market is seeing a rise in cloud-based platforms and software-as-a-service (SaaS) solutions that make advanced simulation capabilities more accessible to a wider range of users, from small biotech startups to large pharmaceutical corporations.
The global enzyme market, which includes both industrial enzymes and specialty enzymes used in research, is projected to reach a substantial value in the coming years. This growth is largely attributed to the rising demand for enzyme-based solutions in various applications, including pharmaceuticals, food and beverages, biofuels, and environmental remediation. Within this broader market, the demand for computational tools and services for enzyme catalysis simulations is experiencing rapid expansion.
Pharmaceutical companies are increasingly relying on in silico methods to streamline their drug discovery processes. Computational studies of tautomerization in enzyme catalysis play a crucial role in predicting drug-target interactions, optimizing lead compounds, and understanding the mechanisms of enzymatic reactions. This approach not only accelerates the drug development pipeline but also reduces the need for extensive laboratory experiments, thereby cutting costs and minimizing the use of resources.
The biotechnology sector is another major driver of demand for enzyme catalysis simulations. As the industry moves towards more sustainable and eco-friendly production methods, there is a growing need for computational tools that can help design and optimize biocatalysts for industrial applications. These simulations enable researchers to explore the potential of novel enzymes and engineer existing ones for improved performance under specific conditions.
Academic institutions and research organizations also contribute significantly to the market demand for enzyme catalysis simulations. The increasing focus on understanding complex biological systems at the molecular level has led to a surge in research projects utilizing computational methods. This trend is further supported by the availability of more powerful computing resources and advanced algorithms, making it possible to simulate larger and more complex enzymatic systems.
The food and beverage industry is emerging as a new frontier for enzyme catalysis simulations. Manufacturers are exploring the use of enzymes to improve food quality, extend shelf life, and develop novel products. Computational studies in this field can help identify and optimize enzymes for specific food processing applications, leading to more efficient and cost-effective production methods.
As the demand for enzyme catalysis simulations continues to grow, there is an increasing need for specialized software tools and expertise in this field. This has created opportunities for software companies and service providers specializing in computational chemistry and molecular modeling. The market is seeing a rise in cloud-based platforms and software-as-a-service (SaaS) solutions that make advanced simulation capabilities more accessible to a wider range of users, from small biotech startups to large pharmaceutical corporations.
Current Challenges in Computational Tautomerization Studies
Computational studies of tautomerization in enzyme catalysis face several significant challenges that hinder our complete understanding of these complex processes. One of the primary difficulties lies in accurately modeling the dynamic nature of tautomerization events within the enzyme active site. The constant fluctuations and rearrangements of atoms and bonds make it challenging to capture the precise energetics and kinetics of these transformations.
Another major hurdle is the accurate representation of the enzyme environment. Enzymes are large, complex molecules with intricate three-dimensional structures that play a crucial role in catalyzing tautomerization reactions. Simulating the entire enzyme structure while maintaining computational efficiency is a delicate balance that researchers must strike. Simplified models may miss critical interactions, while full-atom simulations can be prohibitively expensive in terms of computational resources.
The choice of appropriate computational methods and levels of theory presents another challenge. Quantum mechanical calculations are often necessary to describe the electronic rearrangements involved in tautomerization accurately. However, these methods are computationally intensive and typically limited to small systems. Hybrid quantum mechanics/molecular mechanics (QM/MM) approaches offer a compromise but introduce their own set of challenges in terms of boundary treatments and coupling between the QM and MM regions.
Accounting for solvent effects and long-range interactions adds another layer of complexity to computational studies. Water molecules and ions in the enzyme's environment can significantly influence tautomerization processes, but including these effects in simulations without dramatically increasing computational cost remains a challenge. Advanced solvation models and implicit solvent techniques have been developed, but their accuracy in reproducing the specific environment of enzyme active sites is still a subject of ongoing research.
The multiscale nature of enzyme catalysis poses yet another challenge. Tautomerization events occur on femtosecond to picosecond timescales, while overall enzyme dynamics can span milliseconds or longer. Bridging these vastly different timescales in a single computational framework is a formidable task that requires innovative approaches and algorithms.
Lastly, validating computational results against experimental data remains a critical challenge. While experimental techniques have advanced significantly, directly observing tautomerization events in enzyme active sites is still extremely difficult. This lack of detailed experimental benchmarks makes it challenging to assess the accuracy of computational predictions and refine theoretical models.
Another major hurdle is the accurate representation of the enzyme environment. Enzymes are large, complex molecules with intricate three-dimensional structures that play a crucial role in catalyzing tautomerization reactions. Simulating the entire enzyme structure while maintaining computational efficiency is a delicate balance that researchers must strike. Simplified models may miss critical interactions, while full-atom simulations can be prohibitively expensive in terms of computational resources.
The choice of appropriate computational methods and levels of theory presents another challenge. Quantum mechanical calculations are often necessary to describe the electronic rearrangements involved in tautomerization accurately. However, these methods are computationally intensive and typically limited to small systems. Hybrid quantum mechanics/molecular mechanics (QM/MM) approaches offer a compromise but introduce their own set of challenges in terms of boundary treatments and coupling between the QM and MM regions.
Accounting for solvent effects and long-range interactions adds another layer of complexity to computational studies. Water molecules and ions in the enzyme's environment can significantly influence tautomerization processes, but including these effects in simulations without dramatically increasing computational cost remains a challenge. Advanced solvation models and implicit solvent techniques have been developed, but their accuracy in reproducing the specific environment of enzyme active sites is still a subject of ongoing research.
The multiscale nature of enzyme catalysis poses yet another challenge. Tautomerization events occur on femtosecond to picosecond timescales, while overall enzyme dynamics can span milliseconds or longer. Bridging these vastly different timescales in a single computational framework is a formidable task that requires innovative approaches and algorithms.
Lastly, validating computational results against experimental data remains a critical challenge. While experimental techniques have advanced significantly, directly observing tautomerization events in enzyme active sites is still extremely difficult. This lack of detailed experimental benchmarks makes it challenging to assess the accuracy of computational predictions and refine theoretical models.
Existing Computational Approaches for Tautomerization
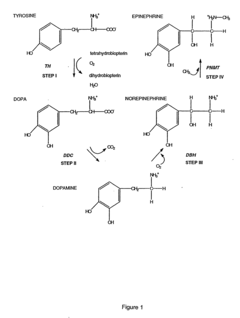
01 Role of tautomerization in enzyme catalysis
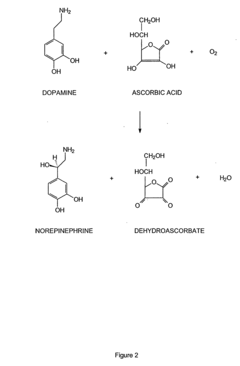
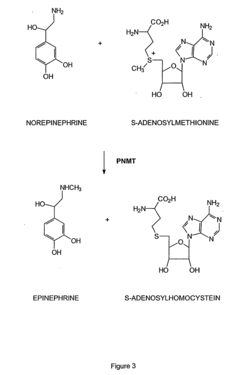
Tautomerization plays a crucial role in enzyme catalysis by facilitating the interconversion between different structural isomers of substrates or intermediates. This process can alter the reactivity of molecules, enabling enzymes to catalyze specific reactions more efficiently. Understanding tautomerization mechanisms in enzyme active sites is essential for elucidating catalytic mechanisms and designing novel biocatalysts.- Tautomerization mechanisms in enzyme catalysis: Enzymes can catalyze tautomerization reactions, which involve the interconversion of structural isomers. This process plays a crucial role in various biochemical pathways and can affect substrate binding, reactivity, and product formation. Understanding these mechanisms is essential for elucidating enzyme function and developing new catalytic strategies.
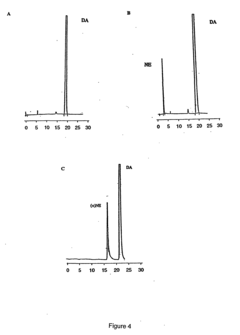
- Detection and analysis of tautomeric forms in enzymatic reactions: Advanced analytical techniques are employed to detect and characterize tautomeric forms during enzyme-catalyzed reactions. These methods may include spectroscopic techniques, chromatography, and computational modeling. Such analyses provide insights into reaction intermediates and help elucidate the role of tautomerization in enzyme function.
- Influence of tautomerization on enzyme substrate specificity: Tautomerization can significantly impact enzyme substrate specificity by altering the structural and electronic properties of substrates. This phenomenon can affect binding affinity, reactivity, and overall catalytic efficiency. Understanding these effects is crucial for enzyme engineering and the development of novel biocatalysts.
- Tautomerization in enzyme-mediated drug metabolism: Tautomerization plays a significant role in enzyme-mediated drug metabolism, affecting drug bioavailability, efficacy, and toxicity. Understanding these processes is crucial for drug design and development, as well as for predicting potential drug-drug interactions and metabolic pathways.
- Computational modeling of tautomerization in enzyme catalysis: Advanced computational methods are used to model and predict tautomerization processes in enzyme catalysis. These approaches include quantum mechanical calculations, molecular dynamics simulations, and machine learning algorithms. Such models help in understanding reaction mechanisms, designing new enzymes, and optimizing catalytic processes.
02 Detection and analysis of tautomeric forms in enzymatic reactions
Advanced analytical techniques are employed to detect and characterize tautomeric forms involved in enzyme-catalyzed reactions. These methods may include spectroscopic techniques, computational modeling, and high-resolution structural analysis. Such approaches help in identifying transient tautomeric species and understanding their role in the overall catalytic process.Expand Specific Solutions03 Tautomerization in enzyme engineering and directed evolution
Enzyme engineering and directed evolution strategies can be applied to modulate tautomerization processes in enzyme catalysis. By altering the enzyme's active site or introducing specific mutations, researchers can influence tautomeric equilibria and potentially enhance catalytic efficiency or alter substrate specificity. This approach has implications for the development of improved biocatalysts for industrial applications.Expand Specific Solutions04 Computational modeling of tautomerization in enzyme catalysis
Computational methods, including quantum mechanical calculations and molecular dynamics simulations, are used to model tautomerization processes in enzyme active sites. These in silico approaches provide insights into the energetics and kinetics of tautomeric transitions, helping to predict and rationalize experimental observations. Such computational studies contribute to a deeper understanding of enzyme mechanisms and aid in the design of novel catalysts.Expand Specific Solutions05 Tautomerization in specific enzyme classes and reaction types
Different enzyme classes exhibit unique tautomerization patterns relevant to their catalytic mechanisms. For instance, isomerases often rely on tautomerization to catalyze their reactions, while some oxidoreductases and transferases may involve tautomeric intermediates. Understanding these class-specific tautomerization processes is crucial for elucidating reaction mechanisms and developing targeted enzyme engineering strategies.Expand Specific Solutions
Key Players in Enzyme Simulation Software
The field of computational studies of tautomerization in enzyme catalysis is in a mature stage of development, with significant research contributions from academic institutions and pharmaceutical companies. The market size for this specialized area is relatively niche but growing, driven by its importance in drug discovery and design. Key players like Carnegie Mellon University, Harvard College, and Roche are at the forefront, leveraging advanced computational methods and experimental techniques. The technology's maturity is evident in the diverse applications across pharmaceutical research, with companies like Bayer Pharma AG and Novozymes A/S applying these studies to enhance enzyme engineering and drug development processes. The competitive landscape is characterized by collaboration between academia and industry, fostering innovation and practical applications in enzyme catalysis research.
President & Fellows of Harvard College
Technical Solution: Harvard researchers have developed a multiscale modeling approach to study tautomerization in enzyme catalysis. Their method combines ab initio quantum chemistry calculations with molecular dynamics simulations to explore the free energy landscapes of enzymatic tautomerization reactions[4]. They have implemented enhanced sampling techniques, such as metadynamics, to overcome the limitations of traditional molecular dynamics in capturing rare events like tautomerization[5]. Additionally, they have developed a novel reaction coordinate analysis tool that can identify and characterize the key molecular motions involved in enzyme-catalyzed tautomerization processes[6].
Strengths: Comprehensive multiscale modeling approach; Advanced sampling techniques for rare events. Weaknesses: Computationally intensive; May be challenging to apply to very large enzyme systems.
The Regents of the University of California
Technical Solution: UC researchers have pioneered the use of hybrid quantum mechanics/molecular mechanics (QM/MM) methods for studying enzyme-catalyzed tautomerization reactions. Their approach combines high-level quantum mechanical calculations for the active site with classical molecular mechanics for the rest of the enzyme and solvent[7]. They have developed specialized force fields that accurately capture the electronic effects involved in tautomerization processes[8]. Additionally, they have implemented advanced free energy calculation methods, such as thermodynamic integration and umbrella sampling, to compute the energetics of tautomerization reactions in enzyme environments[9].
Strengths: Highly accurate QM/MM methods; Specialized force fields for tautomerization. Weaknesses: Computationally expensive for large systems; Requires expertise in both quantum chemistry and molecular mechanics.
Core Algorithms for Tautomer Prediction in Enzymes
Modular approach to on-line synthesis, drug discovery and biochemical transformations using immobilized enzyme reactors
PatentInactiveUS20040053355A1
Innovation
- Development of a liquid chromatographic system using coupled on-line immobilized enzyme reactors (IMERs) that immobilize dopamine beta-hydroxylase and phenylethanolamine N-methyltransferase, allowing for on-line chromatographic purification and structural identification of products, and enabling the exploration of enzyme interrelationships and inhibitor screening.
Inhibitors of RNA guided nucleases and uses thereof
PatentActiveUS20190263807A1
Innovation
- Development of specific compounds and methods to inhibit RNA-guided endonuclease activity, including small molecules that can rapidly and reversibly control the activity of Cas9 and Cpf1, using high-throughput biochemical and cellular assays to detect and screen for inhibitory agents.
Interdisciplinary Applications of Tautomerization Studies
The interdisciplinary applications of tautomerization studies in enzyme catalysis extend far beyond the realm of biochemistry, offering valuable insights and methodologies to various scientific fields. In materials science, understanding tautomerization processes has led to the development of novel smart materials with switchable properties. These materials can respond to environmental stimuli by altering their molecular structure, enabling applications in sensors, drug delivery systems, and adaptive coatings.
In the pharmaceutical industry, tautomerization studies have revolutionized drug design and development. By predicting and controlling tautomeric equilibria, researchers can optimize drug-target interactions, improve bioavailability, and reduce side effects. This has resulted in more effective and safer medications for a wide range of diseases, from cancer to neurological disorders.
The field of organic electronics has also benefited significantly from tautomerization research. Organic semiconductors that exhibit tautomerism can be used to create tunable electronic devices, such as organic light-emitting diodes (OLEDs) and organic field-effect transistors (OFETs) with enhanced performance and efficiency.
In environmental science, tautomerization studies have contributed to the development of advanced water purification technologies. Tautomeric compounds can be used as efficient adsorbents for removing pollutants from water, offering a sustainable solution to water treatment challenges.
The food industry has leveraged tautomerization knowledge to improve food quality and safety. By understanding the tautomeric behavior of food additives and preservatives, scientists can optimize their stability and functionality, leading to better-preserved and healthier food products.
In the field of nanotechnology, tautomerization-based molecular switches have emerged as promising components for nanoscale devices. These switches can be controlled by external stimuli, paving the way for molecular-scale computing and information storage.
The energy sector has also benefited from tautomerization studies, particularly in the development of advanced battery technologies. Tautomeric compounds can serve as efficient electrolytes or electrode materials, potentially improving the performance and longevity of rechargeable batteries.
In the pharmaceutical industry, tautomerization studies have revolutionized drug design and development. By predicting and controlling tautomeric equilibria, researchers can optimize drug-target interactions, improve bioavailability, and reduce side effects. This has resulted in more effective and safer medications for a wide range of diseases, from cancer to neurological disorders.
The field of organic electronics has also benefited significantly from tautomerization research. Organic semiconductors that exhibit tautomerism can be used to create tunable electronic devices, such as organic light-emitting diodes (OLEDs) and organic field-effect transistors (OFETs) with enhanced performance and efficiency.
In environmental science, tautomerization studies have contributed to the development of advanced water purification technologies. Tautomeric compounds can be used as efficient adsorbents for removing pollutants from water, offering a sustainable solution to water treatment challenges.
The food industry has leveraged tautomerization knowledge to improve food quality and safety. By understanding the tautomeric behavior of food additives and preservatives, scientists can optimize their stability and functionality, leading to better-preserved and healthier food products.
In the field of nanotechnology, tautomerization-based molecular switches have emerged as promising components for nanoscale devices. These switches can be controlled by external stimuli, paving the way for molecular-scale computing and information storage.
The energy sector has also benefited from tautomerization studies, particularly in the development of advanced battery technologies. Tautomeric compounds can serve as efficient electrolytes or electrode materials, potentially improving the performance and longevity of rechargeable batteries.
Validation Methods for Computational Tautomer Predictions
Validation methods for computational tautomer predictions play a crucial role in ensuring the accuracy and reliability of computational studies on tautomerization in enzyme catalysis. These methods involve a systematic approach to evaluate the performance of computational models and algorithms used to predict tautomeric equilibria and transitions.
One of the primary validation techniques is the comparison of computational predictions with experimental data. This involves using high-resolution spectroscopic methods, such as NMR and X-ray crystallography, to determine the tautomeric states of molecules in solution and solid-state. The experimental results serve as a benchmark against which computational predictions can be assessed. Statistical metrics, such as root mean square error (RMSE) and correlation coefficients, are often employed to quantify the agreement between predicted and observed tautomeric ratios.
Cross-validation techniques are also commonly used to evaluate the robustness and generalizability of computational models. This involves partitioning the available data into training and test sets, allowing for the assessment of how well the model performs on unseen data. K-fold cross-validation, in particular, is widely used to provide a more comprehensive evaluation of model performance across different subsets of the data.
Another important validation approach is the use of benchmark datasets specifically designed for tautomer prediction. These datasets typically include a diverse range of molecules with known tautomeric behavior, allowing for a standardized evaluation of different computational methods. The performance of new algorithms or models can be directly compared to established methods using these benchmark sets.
Sensitivity analysis is often employed to assess the impact of various computational parameters on tautomer predictions. This involves systematically varying input parameters, such as basis sets, level of theory, and solvation models, to determine their influence on the predicted tautomeric equilibria. Such analysis helps identify the most critical factors affecting prediction accuracy and guides the optimization of computational protocols.
Furthermore, validation methods often include the assessment of computational efficiency and scalability. This is particularly important for enzyme catalysis studies, where large biomolecular systems are involved. Benchmarking the computational time and resource requirements for different methods helps in selecting appropriate approaches for specific research questions.
In recent years, machine learning approaches have gained prominence in tautomer prediction. Validation methods for these models often involve techniques from the field of artificial intelligence, such as confusion matrices, receiver operating characteristic (ROC) curves, and precision-recall analysis. These methods provide insights into the model's ability to correctly classify tautomeric forms and balance between different types of prediction errors.
One of the primary validation techniques is the comparison of computational predictions with experimental data. This involves using high-resolution spectroscopic methods, such as NMR and X-ray crystallography, to determine the tautomeric states of molecules in solution and solid-state. The experimental results serve as a benchmark against which computational predictions can be assessed. Statistical metrics, such as root mean square error (RMSE) and correlation coefficients, are often employed to quantify the agreement between predicted and observed tautomeric ratios.
Cross-validation techniques are also commonly used to evaluate the robustness and generalizability of computational models. This involves partitioning the available data into training and test sets, allowing for the assessment of how well the model performs on unseen data. K-fold cross-validation, in particular, is widely used to provide a more comprehensive evaluation of model performance across different subsets of the data.
Another important validation approach is the use of benchmark datasets specifically designed for tautomer prediction. These datasets typically include a diverse range of molecules with known tautomeric behavior, allowing for a standardized evaluation of different computational methods. The performance of new algorithms or models can be directly compared to established methods using these benchmark sets.
Sensitivity analysis is often employed to assess the impact of various computational parameters on tautomer predictions. This involves systematically varying input parameters, such as basis sets, level of theory, and solvation models, to determine their influence on the predicted tautomeric equilibria. Such analysis helps identify the most critical factors affecting prediction accuracy and guides the optimization of computational protocols.
Furthermore, validation methods often include the assessment of computational efficiency and scalability. This is particularly important for enzyme catalysis studies, where large biomolecular systems are involved. Benchmarking the computational time and resource requirements for different methods helps in selecting appropriate approaches for specific research questions.
In recent years, machine learning approaches have gained prominence in tautomer prediction. Validation methods for these models often involve techniques from the field of artificial intelligence, such as confusion matrices, receiver operating characteristic (ROC) curves, and precision-recall analysis. These methods provide insights into the model's ability to correctly classify tautomeric forms and balance between different types of prediction errors.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!