Influence of Electrophiles on Tautomerization Pathways
JUL 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Electrophilic Influence Background and Objectives
The study of electrophilic influence on tautomerization pathways has emerged as a critical area of research in organic chemistry and biochemistry. Tautomerization, the structural isomerism involving the migration of a hydrogen atom or proton, plays a crucial role in various chemical and biological processes. The presence of electrophiles can significantly alter these pathways, leading to profound implications in reaction mechanisms, drug design, and material science.
Historically, the investigation of tautomerization dates back to the early 20th century, with pioneering work by chemists such as Linus Pauling. However, the specific focus on electrophilic influence gained momentum in the 1960s and 1970s, as researchers began to recognize the importance of electronic effects in organic reactions. The advent of advanced spectroscopic techniques and computational methods in recent decades has further accelerated our understanding of these complex phenomena.
The primary objective of this research is to elucidate the mechanisms by which electrophiles affect tautomerization pathways. This involves a comprehensive examination of how different types of electrophiles interact with tautomeric systems, potentially stabilizing certain tautomers or altering the energy barriers between them. Understanding these interactions is crucial for predicting and controlling chemical reactivity in various contexts.
Another key goal is to develop predictive models that can accurately describe the influence of electrophiles on tautomerization equilibria. Such models would have far-reaching applications in drug discovery, where tautomerism can significantly impact a compound's pharmacokinetic and pharmacodynamic properties. Additionally, these insights could be leveraged in the design of new catalysts and functional materials that exploit controlled tautomerization.
The research also aims to explore the broader implications of electrophilic influence on tautomerization in biological systems. Many enzymes and biomolecules rely on precise tautomeric states for their function, and understanding how these states can be modulated by electrophilic species could lead to novel therapeutic approaches or biotechnological applications.
As we delve deeper into this field, we anticipate uncovering new principles that govern the interplay between electrophiles and tautomers. This knowledge will not only enhance our fundamental understanding of chemical reactivity but also pave the way for innovative applications across multiple disciplines, from medicinal chemistry to materials science.
Historically, the investigation of tautomerization dates back to the early 20th century, with pioneering work by chemists such as Linus Pauling. However, the specific focus on electrophilic influence gained momentum in the 1960s and 1970s, as researchers began to recognize the importance of electronic effects in organic reactions. The advent of advanced spectroscopic techniques and computational methods in recent decades has further accelerated our understanding of these complex phenomena.
The primary objective of this research is to elucidate the mechanisms by which electrophiles affect tautomerization pathways. This involves a comprehensive examination of how different types of electrophiles interact with tautomeric systems, potentially stabilizing certain tautomers or altering the energy barriers between them. Understanding these interactions is crucial for predicting and controlling chemical reactivity in various contexts.
Another key goal is to develop predictive models that can accurately describe the influence of electrophiles on tautomerization equilibria. Such models would have far-reaching applications in drug discovery, where tautomerism can significantly impact a compound's pharmacokinetic and pharmacodynamic properties. Additionally, these insights could be leveraged in the design of new catalysts and functional materials that exploit controlled tautomerization.
The research also aims to explore the broader implications of electrophilic influence on tautomerization in biological systems. Many enzymes and biomolecules rely on precise tautomeric states for their function, and understanding how these states can be modulated by electrophilic species could lead to novel therapeutic approaches or biotechnological applications.
As we delve deeper into this field, we anticipate uncovering new principles that govern the interplay between electrophiles and tautomers. This knowledge will not only enhance our fundamental understanding of chemical reactivity but also pave the way for innovative applications across multiple disciplines, from medicinal chemistry to materials science.
Market Applications of Tautomerization Control
The control of tautomerization pathways through electrophilic influence has opened up significant market applications across various industries. In the pharmaceutical sector, this technology has revolutionized drug design and development processes. By manipulating tautomeric equilibria, researchers can enhance drug efficacy, improve bioavailability, and reduce side effects. This has led to the creation of more potent and targeted medications, particularly in the treatment of chronic diseases and cancer therapies.
In the agrochemical industry, tautomerization control has enabled the development of more effective pesticides and herbicides. These products exhibit improved stability under various environmental conditions, leading to reduced application rates and minimized environmental impact. The ability to fine-tune molecular properties through tautomeric manipulation has resulted in agrochemicals with enhanced crop protection capabilities and reduced resistance development in target organisms.
The electronics and materials science sectors have also benefited from advancements in tautomerization control. Organic electronic materials, such as those used in OLED displays and photovoltaic cells, have seen significant improvements in performance and longevity. By controlling tautomeric forms, researchers have optimized charge transport properties and increased the stability of these materials, leading to more efficient and durable electronic devices.
In the field of chemical sensing and detection, tautomerization control has enabled the development of highly sensitive and selective sensors. These sensors find applications in environmental monitoring, food safety testing, and medical diagnostics. The ability to design molecules that undergo specific tautomeric shifts in response to target analytes has resulted in more accurate and reliable detection methods across various industries.
The cosmetics and personal care industry has also embraced tautomerization control technologies. This has led to the creation of more stable and effective skincare formulations, hair dyes, and UV protection products. By manipulating tautomeric equilibria, formulators can enhance the longevity and efficacy of active ingredients, resulting in improved product performance and consumer satisfaction.
In the field of catalysis, tautomerization control has opened up new possibilities for designing more efficient and selective catalysts. This has implications for various industrial processes, including petrochemical production, fine chemical synthesis, and biofuel production. The ability to fine-tune catalyst properties through tautomeric manipulation has led to improved reaction yields, reduced energy consumption, and decreased waste generation in chemical manufacturing processes.
In the agrochemical industry, tautomerization control has enabled the development of more effective pesticides and herbicides. These products exhibit improved stability under various environmental conditions, leading to reduced application rates and minimized environmental impact. The ability to fine-tune molecular properties through tautomeric manipulation has resulted in agrochemicals with enhanced crop protection capabilities and reduced resistance development in target organisms.
The electronics and materials science sectors have also benefited from advancements in tautomerization control. Organic electronic materials, such as those used in OLED displays and photovoltaic cells, have seen significant improvements in performance and longevity. By controlling tautomeric forms, researchers have optimized charge transport properties and increased the stability of these materials, leading to more efficient and durable electronic devices.
In the field of chemical sensing and detection, tautomerization control has enabled the development of highly sensitive and selective sensors. These sensors find applications in environmental monitoring, food safety testing, and medical diagnostics. The ability to design molecules that undergo specific tautomeric shifts in response to target analytes has resulted in more accurate and reliable detection methods across various industries.
The cosmetics and personal care industry has also embraced tautomerization control technologies. This has led to the creation of more stable and effective skincare formulations, hair dyes, and UV protection products. By manipulating tautomeric equilibria, formulators can enhance the longevity and efficacy of active ingredients, resulting in improved product performance and consumer satisfaction.
In the field of catalysis, tautomerization control has opened up new possibilities for designing more efficient and selective catalysts. This has implications for various industrial processes, including petrochemical production, fine chemical synthesis, and biofuel production. The ability to fine-tune catalyst properties through tautomeric manipulation has led to improved reaction yields, reduced energy consumption, and decreased waste generation in chemical manufacturing processes.
Current Challenges in Electrophile-Induced Tautomerization
The field of electrophile-induced tautomerization faces several significant challenges that hinder its full understanding and practical application. One of the primary obstacles is the complexity of the reaction mechanisms involved. The interaction between electrophiles and tautomeric systems often leads to multiple competing pathways, making it difficult to predict and control the outcome of these reactions.
The transient nature of tautomeric intermediates poses another major challenge. These short-lived species are often difficult to detect and characterize using conventional analytical techniques. This limitation hampers the detailed study of reaction kinetics and thermodynamics, which are crucial for optimizing reaction conditions and developing predictive models.
Furthermore, the influence of solvent effects on tautomerization pathways remains a significant area of uncertainty. Different solvents can dramatically alter the stability of tautomeric forms and affect the rate of interconversion. This variability complicates the development of general principles for electrophile-induced tautomerization across diverse reaction environments.
The lack of comprehensive computational models also presents a substantial hurdle. While quantum chemical calculations have provided valuable insights, accurately modeling the complex interplay between electrophiles, tautomers, and solvents remains computationally expensive and often requires simplifying assumptions that may not fully capture the intricacies of real systems.
Another challenge lies in controlling the regioselectivity of electrophilic attack in systems with multiple tautomerization sites. This is particularly problematic in the synthesis of complex organic molecules, where precise control over the site of tautomerization is crucial for achieving the desired product.
The development of catalysts that can selectively promote specific tautomerization pathways is an ongoing challenge. While some progress has been made in this area, designing catalysts that can effectively discriminate between different tautomeric forms and guide the reaction towards a desired outcome remains a significant obstacle.
Lastly, the application of electrophile-induced tautomerization in industrial processes faces scale-up challenges. Issues such as product isolation, purification, and process efficiency need to be addressed to make these reactions viable for large-scale production. Overcoming these challenges will be crucial for realizing the full potential of electrophile-induced tautomerization in various fields, including pharmaceutical synthesis, materials science, and organic electronics.
The transient nature of tautomeric intermediates poses another major challenge. These short-lived species are often difficult to detect and characterize using conventional analytical techniques. This limitation hampers the detailed study of reaction kinetics and thermodynamics, which are crucial for optimizing reaction conditions and developing predictive models.
Furthermore, the influence of solvent effects on tautomerization pathways remains a significant area of uncertainty. Different solvents can dramatically alter the stability of tautomeric forms and affect the rate of interconversion. This variability complicates the development of general principles for electrophile-induced tautomerization across diverse reaction environments.
The lack of comprehensive computational models also presents a substantial hurdle. While quantum chemical calculations have provided valuable insights, accurately modeling the complex interplay between electrophiles, tautomers, and solvents remains computationally expensive and often requires simplifying assumptions that may not fully capture the intricacies of real systems.
Another challenge lies in controlling the regioselectivity of electrophilic attack in systems with multiple tautomerization sites. This is particularly problematic in the synthesis of complex organic molecules, where precise control over the site of tautomerization is crucial for achieving the desired product.
The development of catalysts that can selectively promote specific tautomerization pathways is an ongoing challenge. While some progress has been made in this area, designing catalysts that can effectively discriminate between different tautomeric forms and guide the reaction towards a desired outcome remains a significant obstacle.
Lastly, the application of electrophile-induced tautomerization in industrial processes faces scale-up challenges. Issues such as product isolation, purification, and process efficiency need to be addressed to make these reactions viable for large-scale production. Overcoming these challenges will be crucial for realizing the full potential of electrophile-induced tautomerization in various fields, including pharmaceutical synthesis, materials science, and organic electronics.
Existing Methodologies for Tautomerization Pathway Analysis
01 Computational methods for predicting tautomerization pathways
Advanced computational techniques are employed to predict and analyze tautomerization pathways. These methods involve quantum mechanical calculations, molecular dynamics simulations, and machine learning algorithms to model the structural changes and energy barriers associated with tautomerization. Such computational approaches help in understanding the influence of tautomerization on molecular properties and reactivity.- Computational methods for predicting tautomerization pathways: Advanced computational techniques are employed to predict and analyze tautomerization pathways. These methods involve quantum mechanical calculations, molecular dynamics simulations, and machine learning algorithms to model the structural changes and energy profiles associated with tautomeric transformations. Such computational approaches help in understanding the influence of tautomerization on molecular properties and reactivity.
- Impact of tautomerization on drug design and development: Tautomerization pathways significantly influence drug design and development processes. The interconversion between tautomeric forms can affect a compound's physicochemical properties, binding affinity to target proteins, and overall pharmacokinetic profile. Understanding these pathways is crucial for optimizing drug candidates and predicting their behavior in biological systems.
- Tautomerization effects on chemical reactivity and catalysis: Tautomerization pathways play a significant role in chemical reactivity and catalysis. The interconversion between tautomers can influence reaction mechanisms, transition states, and product distributions. Understanding these effects is essential for designing efficient catalysts and optimizing chemical processes in various industries, including pharmaceuticals and materials science.
- Analytical techniques for studying tautomerization dynamics: Various analytical techniques are employed to study tautomerization dynamics and their influence on molecular systems. These include spectroscopic methods such as NMR, IR, and UV-Vis spectroscopy, as well as advanced mass spectrometry techniques. These tools allow researchers to observe and quantify tautomeric equilibria, providing insights into the factors that control tautomerization pathways.
- Environmental factors affecting tautomerization pathways: Environmental factors such as solvent polarity, pH, temperature, and pressure can significantly influence tautomerization pathways. These external conditions can alter the relative stability of different tautomeric forms and affect the kinetics of interconversion. Understanding these environmental influences is crucial for predicting and controlling tautomerization in various applications, including materials science and chemical engineering.
02 Impact of tautomerization on drug design and development
Tautomerization pathways significantly influence drug design and development processes. The interconversion between tautomers can affect a compound's physicochemical properties, binding affinity to target proteins, and overall pharmacokinetic profile. Understanding and controlling tautomerization is crucial for optimizing drug candidates and predicting their behavior in biological systems.Expand Specific Solutions03 Tautomerization in chemical reaction mechanisms
Tautomerization plays a vital role in various chemical reaction mechanisms. The interconversion between tautomers can influence reaction rates, product distributions, and overall reaction pathways. Studying tautomerization pathways helps in elucidating complex reaction mechanisms and designing more efficient synthetic routes in organic and inorganic chemistry.Expand Specific Solutions04 Environmental factors affecting tautomerization
Various environmental factors, such as solvent polarity, pH, temperature, and pressure, can significantly influence tautomerization pathways. These factors can alter the relative stability of different tautomers and affect the kinetics of interconversion. Understanding the impact of environmental conditions on tautomerization is crucial for predicting and controlling molecular behavior in different chemical and biological contexts.Expand Specific Solutions05 Analytical techniques for studying tautomerization
Advanced analytical techniques are employed to study tautomerization pathways and their influence on molecular properties. These include spectroscopic methods such as NMR, IR, and UV-Vis spectroscopy, as well as mass spectrometry and X-ray crystallography. These techniques provide valuable insights into the structural changes, energetics, and kinetics associated with tautomerization processes.Expand Specific Solutions
Key Research Groups and Institutions
The competitive landscape for "Influence of Electrophiles on Tautomerization Pathways" is in its early development stage, with a growing market potential as research in this field expands. The technology is still emerging, with varying levels of maturity across different companies. Key players like DuPont de Nemours, BASF Corp., and Janssen Pharmaceutica NV are likely at the forefront, leveraging their extensive R&D capabilities. Academic institutions such as Zhejiang University and Sichuan University are contributing significantly to fundamental research. The market size is relatively small but expected to grow as applications in drug discovery and materials science become more apparent. Collaboration between industry and academia is crucial for advancing this specialized field.
Revvity Health Sciences, Inc.
Technical Solution: Revvity Health Sciences has developed advanced spectroscopic techniques to study the influence of electrophiles on tautomerization pathways. Their approach combines high-resolution nuclear magnetic resonance (NMR) spectroscopy with computational modeling to elucidate the mechanisms of tautomer interconversion in the presence of various electrophiles. The company has implemented a novel time-resolved NMR method that allows for the real-time observation of tautomerization processes, providing unprecedented insights into reaction kinetics and thermodynamics[1]. Additionally, Revvity has integrated machine learning algorithms to predict tautomerization behavior based on electrophile properties, significantly accelerating the discovery of new catalysts and reaction conditions[3].
Strengths: Cutting-edge spectroscopic techniques, real-time observation capabilities, and integration of AI for predictive modeling. Weaknesses: High equipment costs and complexity may limit widespread adoption.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a comprehensive approach to studying the influence of electrophiles on tautomerization pathways, focusing on applications in materials science and chemical manufacturing. Their technology combines in situ infrared spectroscopy with advanced computational chemistry to map out tautomerization energy landscapes in the presence of various electrophiles. DuPont's proprietary software suite integrates density functional theory (DFT) calculations with experimental data to predict tautomer stability and reaction rates under different conditions[2]. The company has also pioneered the use of microfluidic devices for high-throughput screening of electrophile effects on tautomerization, enabling rapid optimization of reaction conditions for industrial processes[4].
Strengths: Integration of experimental and computational methods, industrial-scale applicability, and high-throughput screening capabilities. Weaknesses: Focus primarily on industrial applications may limit relevance in some research contexts.
Breakthrough Studies on Electrophilic Effects
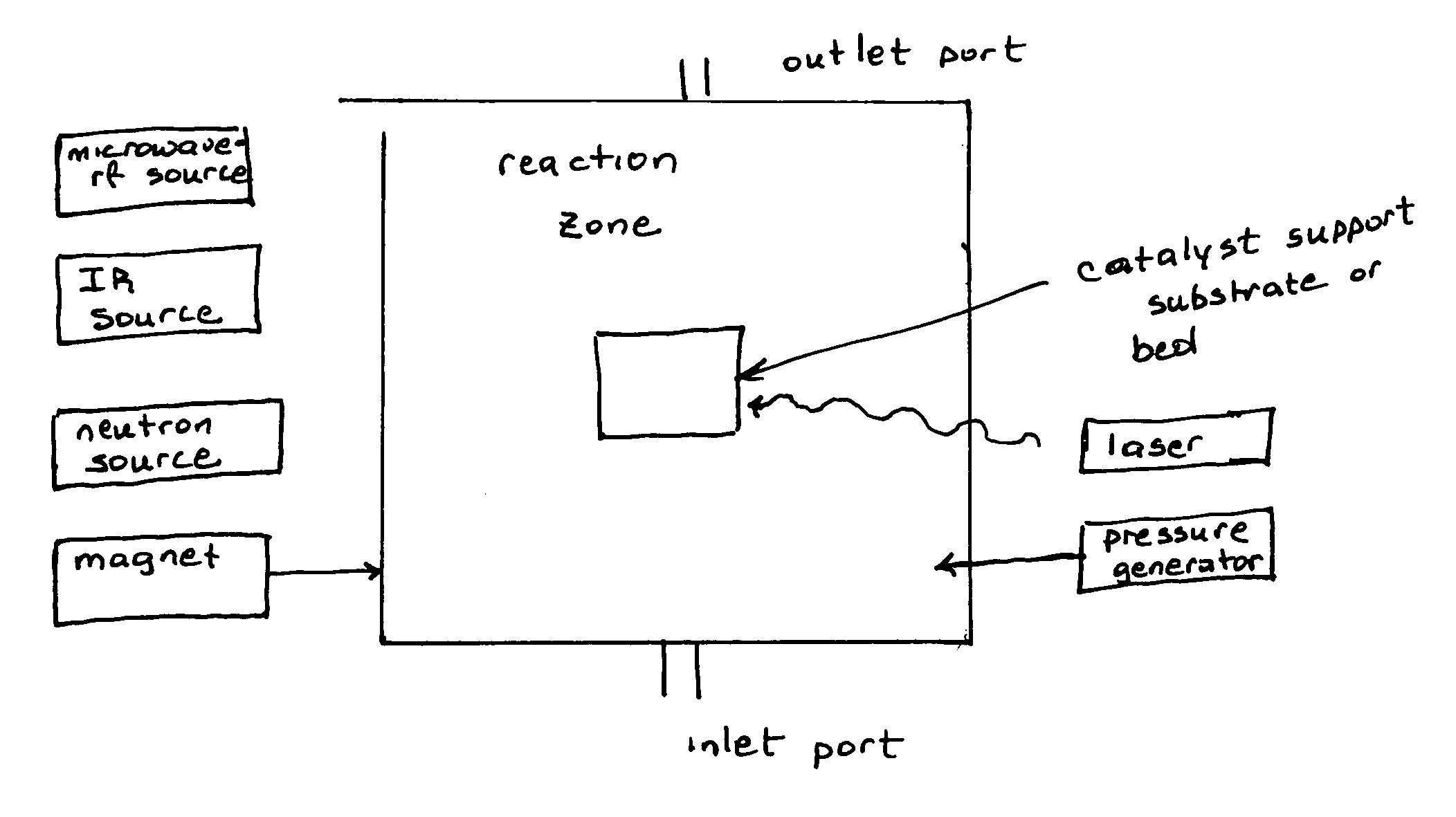
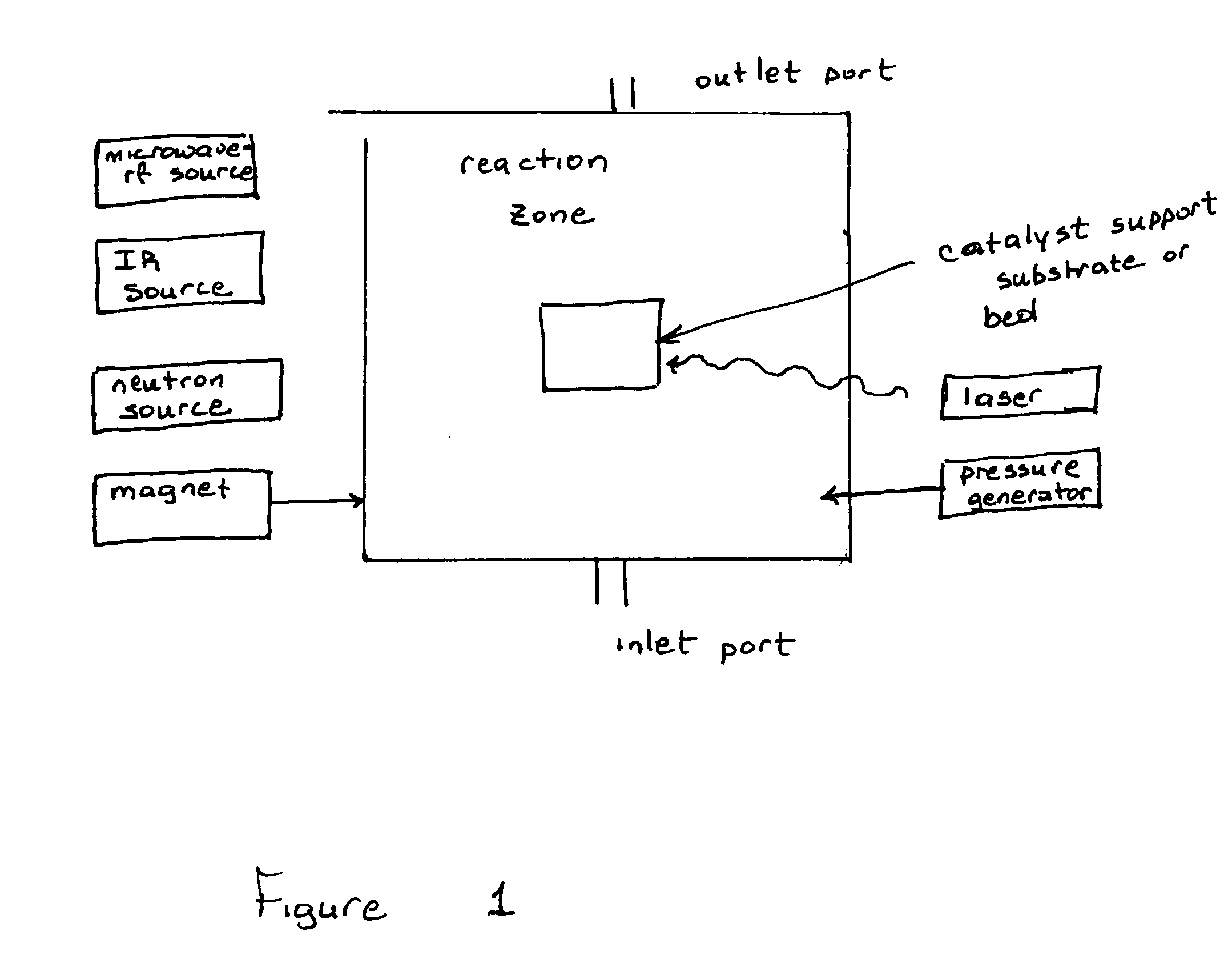
Magnetic stimulated catalytic chemical conversion of second series elemental compounds: combination, decomposition rearrangement and/or reformation magneto chemistry
PatentInactiveUS20060233703A1
Innovation
- The use of intense static and dynamic magnetic fields in conjunction with IR and laser heating to enhance catalytic processes, allowing for the selective formation of these compounds with reduced energy input and elimination of harsh conditions, utilizing a reaction chamber with a heating element, magnetic field generator, and laser radiation to control electronic states and spin dynamics.
Computational Approaches in Tautomerization Prediction
Computational approaches have become increasingly important in predicting tautomerization pathways, especially when considering the influence of electrophiles. These methods offer valuable insights into the complex interplay between molecular structures and their electronic properties, providing a more comprehensive understanding of tautomerization processes.
One of the primary computational techniques employed in this field is density functional theory (DFT). DFT calculations allow researchers to explore the potential energy surfaces of tautomeric systems, identifying stable conformations and transition states. By incorporating the effects of electrophiles into these calculations, scientists can elucidate how these species influence the energetics and kinetics of tautomerization reactions.
Molecular dynamics simulations represent another powerful tool for investigating tautomerization pathways. These simulations can model the time-dependent behavior of molecular systems, capturing the dynamic nature of tautomerization processes. When combined with advanced sampling techniques, such as metadynamics or umbrella sampling, researchers can overcome energy barriers and explore rare events that may be crucial in understanding the influence of electrophiles on tautomerization.
Quantum mechanical/molecular mechanical (QM/MM) methods have also proven valuable in studying tautomerization in complex environments. These hybrid approaches allow for the accurate treatment of the tautomerizing region using quantum mechanics while considering the surrounding environment with classical molecular mechanics. This is particularly useful when investigating the effects of electrophiles in biological systems or solution-phase reactions.
Machine learning algorithms are increasingly being applied to predict tautomerization pathways and the influence of electrophiles. By training on large datasets of known tautomeric systems, these models can rapidly predict tautomer distributions and reaction rates for novel compounds. Graph neural networks, in particular, have shown promise in capturing the structural features that govern tautomerization processes.
Ab initio molecular dynamics (AIMD) simulations offer a unique perspective on tautomerization by combining electronic structure calculations with classical molecular dynamics. This approach allows for the real-time observation of bond breaking and formation events, providing detailed mechanistic insights into how electrophiles modulate tautomerization pathways.
As computational power continues to increase, more sophisticated methods are being developed to address the challenges of predicting tautomerization in the presence of electrophiles. These include the use of multiscale modeling techniques, advanced electronic structure methods, and the integration of experimental data with computational predictions to improve accuracy and reliability.
One of the primary computational techniques employed in this field is density functional theory (DFT). DFT calculations allow researchers to explore the potential energy surfaces of tautomeric systems, identifying stable conformations and transition states. By incorporating the effects of electrophiles into these calculations, scientists can elucidate how these species influence the energetics and kinetics of tautomerization reactions.
Molecular dynamics simulations represent another powerful tool for investigating tautomerization pathways. These simulations can model the time-dependent behavior of molecular systems, capturing the dynamic nature of tautomerization processes. When combined with advanced sampling techniques, such as metadynamics or umbrella sampling, researchers can overcome energy barriers and explore rare events that may be crucial in understanding the influence of electrophiles on tautomerization.
Quantum mechanical/molecular mechanical (QM/MM) methods have also proven valuable in studying tautomerization in complex environments. These hybrid approaches allow for the accurate treatment of the tautomerizing region using quantum mechanics while considering the surrounding environment with classical molecular mechanics. This is particularly useful when investigating the effects of electrophiles in biological systems or solution-phase reactions.
Machine learning algorithms are increasingly being applied to predict tautomerization pathways and the influence of electrophiles. By training on large datasets of known tautomeric systems, these models can rapidly predict tautomer distributions and reaction rates for novel compounds. Graph neural networks, in particular, have shown promise in capturing the structural features that govern tautomerization processes.
Ab initio molecular dynamics (AIMD) simulations offer a unique perspective on tautomerization by combining electronic structure calculations with classical molecular dynamics. This approach allows for the real-time observation of bond breaking and formation events, providing detailed mechanistic insights into how electrophiles modulate tautomerization pathways.
As computational power continues to increase, more sophisticated methods are being developed to address the challenges of predicting tautomerization in the presence of electrophiles. These include the use of multiscale modeling techniques, advanced electronic structure methods, and the integration of experimental data with computational predictions to improve accuracy and reliability.
Environmental Factors Affecting Tautomeric Equilibria
Environmental factors play a crucial role in influencing tautomeric equilibria, particularly in the context of electrophilic effects on tautomerization pathways. The interplay between environmental conditions and tautomeric systems can significantly impact the distribution of tautomers and their reactivity.
Temperature is a key environmental factor affecting tautomeric equilibria. Higher temperatures generally increase the rate of tautomerization and can shift the equilibrium towards less stable tautomers. This effect is particularly pronounced in systems where electrophiles are involved, as elevated temperatures can enhance their reactivity and influence the energy barriers between tautomeric forms.
Solvent polarity is another critical environmental factor that can dramatically alter tautomeric equilibria. Polar solvents tend to stabilize more polar tautomers, while non-polar solvents favor less polar forms. In the presence of electrophiles, solvent effects become even more pronounced, as they can modulate the electrophile's reactivity and its interaction with different tautomeric species.
pH is a fundamental environmental factor that directly impacts tautomeric equilibria, especially in protic systems. Acidic or basic conditions can significantly shift the equilibrium by protonating or deprotonating specific sites in the tautomeric molecules. Electrophiles are particularly sensitive to pH changes, as their reactivity and selectivity can be altered, leading to different tautomerization pathways.
Pressure, although less commonly considered, can also influence tautomeric equilibria. High-pressure conditions may favor tautomers with smaller molecular volumes, potentially affecting the interaction between electrophiles and tautomeric species.
The presence of specific ions or metal cations in the environment can have a profound effect on tautomeric equilibria. These species can coordinate with tautomeric molecules, stabilizing certain forms and altering the energy landscape of the tautomerization process. Electrophiles may compete with or be influenced by these ionic species, leading to complex equilibrium shifts.
Light exposure is another environmental factor that can induce tautomerization, particularly in photosensitive systems. Certain wavelengths of light can provide the necessary energy for tautomeric interconversion, potentially altering the reactivity of electrophiles towards different tautomeric forms.
Lastly, the concentration of reactants and products in the system can significantly impact tautomeric equilibria. Higher concentrations may favor aggregation or self-association of tautomers, potentially altering their reactivity towards electrophiles and influencing the overall tautomerization pathways.
Understanding these environmental factors and their interplay with electrophilic influences is crucial for predicting and controlling tautomeric equilibria in various chemical and biological systems. This knowledge can be leveraged to optimize reaction conditions, design more effective drugs, and develop novel materials with tunable properties based on tautomeric behavior.
Temperature is a key environmental factor affecting tautomeric equilibria. Higher temperatures generally increase the rate of tautomerization and can shift the equilibrium towards less stable tautomers. This effect is particularly pronounced in systems where electrophiles are involved, as elevated temperatures can enhance their reactivity and influence the energy barriers between tautomeric forms.
Solvent polarity is another critical environmental factor that can dramatically alter tautomeric equilibria. Polar solvents tend to stabilize more polar tautomers, while non-polar solvents favor less polar forms. In the presence of electrophiles, solvent effects become even more pronounced, as they can modulate the electrophile's reactivity and its interaction with different tautomeric species.
pH is a fundamental environmental factor that directly impacts tautomeric equilibria, especially in protic systems. Acidic or basic conditions can significantly shift the equilibrium by protonating or deprotonating specific sites in the tautomeric molecules. Electrophiles are particularly sensitive to pH changes, as their reactivity and selectivity can be altered, leading to different tautomerization pathways.
Pressure, although less commonly considered, can also influence tautomeric equilibria. High-pressure conditions may favor tautomers with smaller molecular volumes, potentially affecting the interaction between electrophiles and tautomeric species.
The presence of specific ions or metal cations in the environment can have a profound effect on tautomeric equilibria. These species can coordinate with tautomeric molecules, stabilizing certain forms and altering the energy landscape of the tautomerization process. Electrophiles may compete with or be influenced by these ionic species, leading to complex equilibrium shifts.
Light exposure is another environmental factor that can induce tautomerization, particularly in photosensitive systems. Certain wavelengths of light can provide the necessary energy for tautomeric interconversion, potentially altering the reactivity of electrophiles towards different tautomeric forms.
Lastly, the concentration of reactants and products in the system can significantly impact tautomeric equilibria. Higher concentrations may favor aggregation or self-association of tautomers, potentially altering their reactivity towards electrophiles and influencing the overall tautomerization pathways.
Understanding these environmental factors and their interplay with electrophilic influences is crucial for predicting and controlling tautomeric equilibria in various chemical and biological systems. This knowledge can be leveraged to optimize reaction conditions, design more effective drugs, and develop novel materials with tunable properties based on tautomeric behavior.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!