Mechanistic Insights into Base-Catalyzed Tautomerization of Alcohols
JUL 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Tautomerization Fundamentals and Research Objectives
Tautomerization is a fundamental process in organic chemistry, involving the rapid interconversion between structural isomers. This phenomenon plays a crucial role in various chemical and biological systems, influencing reactivity, stability, and molecular properties. The base-catalyzed tautomerization of alcohols represents a specific subset of this broader concept, offering unique insights into reaction mechanisms and catalytic processes.
The historical development of tautomerization research dates back to the late 19th century, with significant advancements made throughout the 20th century. Early studies focused on keto-enol tautomerism, gradually expanding to encompass a wider range of functional groups and molecular structures. The advent of advanced spectroscopic techniques and computational methods in recent decades has greatly enhanced our understanding of tautomeric processes, including those involving alcohols.
In the context of base-catalyzed tautomerization of alcohols, the primary objective is to elucidate the mechanistic pathways and energetics involved in the interconversion process. This includes identifying key intermediates, transition states, and the role of the base catalyst in facilitating proton transfer. Understanding these mechanistic details is crucial for predicting and controlling tautomeric equilibria, which has implications for various applications in synthetic chemistry, drug design, and materials science.
The research objectives in this field encompass several key areas. Firstly, there is a focus on developing more accurate models for predicting tautomeric equilibria in different solvent environments and under varying pH conditions. This involves combining experimental data with advanced computational methods to create robust predictive tools. Secondly, researchers aim to explore the influence of structural factors on tautomerization rates and equilibria, including the effects of substituents, ring size, and molecular geometry.
Another important objective is to investigate the role of specific base catalysts in promoting tautomerization, with the goal of designing more efficient and selective catalytic systems. This includes studying both traditional inorganic bases and emerging organic catalysts, such as N-heterocyclic carbenes and organophosphorus compounds. Additionally, there is growing interest in understanding the interplay between tautomerization and other chemical processes, such as isomerization, cyclization, and polymerization reactions.
The ultimate goal of these research efforts is to gain a comprehensive mechanistic understanding of base-catalyzed alcohol tautomerization, enabling the rational design of new chemical transformations and the optimization of existing processes. This knowledge has far-reaching implications, from improving the efficiency of industrial chemical processes to developing novel pharmaceutical compounds with enhanced properties.
The historical development of tautomerization research dates back to the late 19th century, with significant advancements made throughout the 20th century. Early studies focused on keto-enol tautomerism, gradually expanding to encompass a wider range of functional groups and molecular structures. The advent of advanced spectroscopic techniques and computational methods in recent decades has greatly enhanced our understanding of tautomeric processes, including those involving alcohols.
In the context of base-catalyzed tautomerization of alcohols, the primary objective is to elucidate the mechanistic pathways and energetics involved in the interconversion process. This includes identifying key intermediates, transition states, and the role of the base catalyst in facilitating proton transfer. Understanding these mechanistic details is crucial for predicting and controlling tautomeric equilibria, which has implications for various applications in synthetic chemistry, drug design, and materials science.
The research objectives in this field encompass several key areas. Firstly, there is a focus on developing more accurate models for predicting tautomeric equilibria in different solvent environments and under varying pH conditions. This involves combining experimental data with advanced computational methods to create robust predictive tools. Secondly, researchers aim to explore the influence of structural factors on tautomerization rates and equilibria, including the effects of substituents, ring size, and molecular geometry.
Another important objective is to investigate the role of specific base catalysts in promoting tautomerization, with the goal of designing more efficient and selective catalytic systems. This includes studying both traditional inorganic bases and emerging organic catalysts, such as N-heterocyclic carbenes and organophosphorus compounds. Additionally, there is growing interest in understanding the interplay between tautomerization and other chemical processes, such as isomerization, cyclization, and polymerization reactions.
The ultimate goal of these research efforts is to gain a comprehensive mechanistic understanding of base-catalyzed alcohol tautomerization, enabling the rational design of new chemical transformations and the optimization of existing processes. This knowledge has far-reaching implications, from improving the efficiency of industrial chemical processes to developing novel pharmaceutical compounds with enhanced properties.
Industrial Applications of Alcohol Tautomerization
The industrial applications of alcohol tautomerization have gained significant attention in recent years due to their potential to revolutionize various chemical processes. This base-catalyzed reaction, which involves the interconversion of alcohols between different tautomeric forms, has found applications across multiple sectors, including pharmaceuticals, materials science, and energy production.
In the pharmaceutical industry, alcohol tautomerization plays a crucial role in drug discovery and development. The ability to control and manipulate tautomeric equilibria has led to the creation of novel drug candidates with improved efficacy and bioavailability. For instance, researchers have utilized this process to design prodrugs that can be activated through tautomerization in specific physiological conditions, enhancing targeted drug delivery and reducing side effects.
The materials science sector has also benefited from alcohol tautomerization applications. This process has been employed in the development of smart materials with switchable properties. By controlling the tautomeric state of alcohol-containing polymers, scientists have created materials that can change their physical and chemical characteristics in response to external stimuli such as pH, temperature, or light. These adaptive materials have potential uses in sensors, actuators, and self-healing coatings.
In the field of organic synthesis, alcohol tautomerization has emerged as a powerful tool for creating complex molecular structures. Industrial chemists have leveraged this reaction to develop more efficient and selective synthetic routes for high-value chemicals. The ability to control tautomeric equilibria has enabled the production of specific isomers, reducing waste and improving overall process efficiency in large-scale manufacturing.
The energy sector has also explored the potential of alcohol tautomerization in fuel cell technology. Researchers have investigated the use of tautomeric alcohol-based electrolytes to enhance proton conductivity in fuel cells, potentially leading to more efficient and cost-effective energy conversion devices. This application could contribute to the advancement of clean energy technologies and support the transition towards a more sustainable energy landscape.
Furthermore, the food and beverage industry has found applications for alcohol tautomerization in flavor chemistry. By manipulating the tautomeric forms of certain alcohol-containing flavor compounds, manufacturers can fine-tune the sensory properties of their products, creating unique taste profiles and enhancing overall consumer experience.
As industrial applications of alcohol tautomerization continue to expand, there is a growing need for further research and development in this field. Advancements in catalysis, process engineering, and analytical techniques will be crucial in unlocking the full potential of this versatile reaction across various industries. The ongoing exploration of novel applications and the optimization of existing processes promise to drive innovation and create new opportunities for sustainable and efficient chemical manufacturing in the coming years.
In the pharmaceutical industry, alcohol tautomerization plays a crucial role in drug discovery and development. The ability to control and manipulate tautomeric equilibria has led to the creation of novel drug candidates with improved efficacy and bioavailability. For instance, researchers have utilized this process to design prodrugs that can be activated through tautomerization in specific physiological conditions, enhancing targeted drug delivery and reducing side effects.
The materials science sector has also benefited from alcohol tautomerization applications. This process has been employed in the development of smart materials with switchable properties. By controlling the tautomeric state of alcohol-containing polymers, scientists have created materials that can change their physical and chemical characteristics in response to external stimuli such as pH, temperature, or light. These adaptive materials have potential uses in sensors, actuators, and self-healing coatings.
In the field of organic synthesis, alcohol tautomerization has emerged as a powerful tool for creating complex molecular structures. Industrial chemists have leveraged this reaction to develop more efficient and selective synthetic routes for high-value chemicals. The ability to control tautomeric equilibria has enabled the production of specific isomers, reducing waste and improving overall process efficiency in large-scale manufacturing.
The energy sector has also explored the potential of alcohol tautomerization in fuel cell technology. Researchers have investigated the use of tautomeric alcohol-based electrolytes to enhance proton conductivity in fuel cells, potentially leading to more efficient and cost-effective energy conversion devices. This application could contribute to the advancement of clean energy technologies and support the transition towards a more sustainable energy landscape.
Furthermore, the food and beverage industry has found applications for alcohol tautomerization in flavor chemistry. By manipulating the tautomeric forms of certain alcohol-containing flavor compounds, manufacturers can fine-tune the sensory properties of their products, creating unique taste profiles and enhancing overall consumer experience.
As industrial applications of alcohol tautomerization continue to expand, there is a growing need for further research and development in this field. Advancements in catalysis, process engineering, and analytical techniques will be crucial in unlocking the full potential of this versatile reaction across various industries. The ongoing exploration of novel applications and the optimization of existing processes promise to drive innovation and create new opportunities for sustainable and efficient chemical manufacturing in the coming years.
Current Challenges in Base-Catalyzed Tautomerization
Base-catalyzed tautomerization of alcohols presents several significant challenges that hinder its widespread application and understanding. One of the primary obstacles is the complexity of the reaction mechanism, which involves multiple steps and intermediates. This complexity makes it difficult to predict and control the reaction outcomes, especially in complex molecular systems.
The reversibility of the tautomerization process poses another challenge. The equilibrium between different tautomeric forms can be highly sensitive to reaction conditions, making it challenging to selectively produce and isolate specific tautomers. This sensitivity often results in mixtures of products, complicating purification and characterization processes.
Another significant hurdle is the lack of general and efficient catalysts for base-catalyzed tautomerization. While some bases have shown promise, their effectiveness is often limited to specific substrates or reaction conditions. The development of versatile catalysts that can promote tautomerization across a wide range of alcohols remains an ongoing challenge in the field.
The control of regioselectivity in base-catalyzed tautomerization is also a persistent issue. For molecules with multiple potential tautomerization sites, directing the reaction to a specific position can be challenging. This lack of regiocontrol can lead to the formation of undesired isomers, reducing reaction efficiency and complicating product isolation.
Furthermore, the energy barriers associated with base-catalyzed tautomerization can be substantial, particularly for certain classes of alcohols. Overcoming these energetic hurdles often requires harsh reaction conditions, which can lead to unwanted side reactions or decomposition of sensitive substrates. Developing milder reaction conditions that maintain efficiency is a key challenge in this area.
The influence of solvent effects on base-catalyzed tautomerization adds another layer of complexity. Solvents can significantly impact the stability of different tautomeric forms and affect the reaction kinetics. Understanding and predicting these solvent effects remains a challenge, particularly when scaling up reactions or transferring them to different solvent systems.
Lastly, the application of base-catalyzed tautomerization in asymmetric synthesis presents unique challenges. Controlling the stereochemistry of the products formed during tautomerization, especially in prochiral systems, is a complex task that requires careful catalyst design and reaction optimization.
The reversibility of the tautomerization process poses another challenge. The equilibrium between different tautomeric forms can be highly sensitive to reaction conditions, making it challenging to selectively produce and isolate specific tautomers. This sensitivity often results in mixtures of products, complicating purification and characterization processes.
Another significant hurdle is the lack of general and efficient catalysts for base-catalyzed tautomerization. While some bases have shown promise, their effectiveness is often limited to specific substrates or reaction conditions. The development of versatile catalysts that can promote tautomerization across a wide range of alcohols remains an ongoing challenge in the field.
The control of regioselectivity in base-catalyzed tautomerization is also a persistent issue. For molecules with multiple potential tautomerization sites, directing the reaction to a specific position can be challenging. This lack of regiocontrol can lead to the formation of undesired isomers, reducing reaction efficiency and complicating product isolation.
Furthermore, the energy barriers associated with base-catalyzed tautomerization can be substantial, particularly for certain classes of alcohols. Overcoming these energetic hurdles often requires harsh reaction conditions, which can lead to unwanted side reactions or decomposition of sensitive substrates. Developing milder reaction conditions that maintain efficiency is a key challenge in this area.
The influence of solvent effects on base-catalyzed tautomerization adds another layer of complexity. Solvents can significantly impact the stability of different tautomeric forms and affect the reaction kinetics. Understanding and predicting these solvent effects remains a challenge, particularly when scaling up reactions or transferring them to different solvent systems.
Lastly, the application of base-catalyzed tautomerization in asymmetric synthesis presents unique challenges. Controlling the stereochemistry of the products formed during tautomerization, especially in prochiral systems, is a complex task that requires careful catalyst design and reaction optimization.
Existing Mechanistic Models for Base-Catalyzed Tautomerization
01 Tautomerization of alcohols in organic synthesis
Alcohol tautomerization plays a crucial role in organic synthesis, particularly in the interconversion between enol and carbonyl forms. This process is important in various chemical reactions and can be influenced by factors such as pH, temperature, and catalysts. Understanding and controlling alcohol tautomerization is essential for developing efficient synthetic routes and improving reaction yields.- Tautomerization of alcohols in organic synthesis: Alcohol tautomerization plays a crucial role in organic synthesis, particularly in the interconversion between enol and carbonyl forms. This process is essential for various chemical reactions and can be influenced by factors such as pH, temperature, and catalysts. Understanding and controlling alcohol tautomerization is vital for the development of new synthetic methodologies and the optimization of existing processes.
- Catalysts for alcohol tautomerization: Various catalysts can be employed to facilitate alcohol tautomerization. These may include metal-based catalysts, enzymes, or organic compounds. The choice of catalyst can significantly affect the rate and selectivity of the tautomerization process. Researchers continue to develop novel catalytic systems to improve the efficiency and sustainability of alcohol tautomerization reactions in industrial applications.
- Applications of alcohol tautomerization in the pharmaceutical industry: Alcohol tautomerization is widely utilized in the pharmaceutical industry for the synthesis of drug precursors and active pharmaceutical ingredients. This process allows for the modification of molecular structures, potentially enhancing drug efficacy or altering pharmacokinetic properties. Understanding and controlling tautomerization is crucial for drug design and the development of new therapeutic compounds.
- Analytical methods for studying alcohol tautomerization: Various analytical techniques are employed to study alcohol tautomerization, including spectroscopic methods such as NMR, IR, and UV-Vis spectroscopy. These techniques allow researchers to observe and quantify the tautomeric equilibrium, providing valuable insights into reaction mechanisms and kinetics. Advanced computational methods are also used to predict and model tautomerization processes, aiding in the design of new chemical reactions and materials.
- Industrial applications of alcohol tautomerization: Alcohol tautomerization finds applications in various industrial processes, including the production of polymers, fine chemicals, and materials. This process can be utilized to modify the properties of materials, such as improving thermal stability or enhancing reactivity. Understanding and controlling tautomerization is essential for optimizing industrial processes and developing new products with desired characteristics.
02 Catalysts for alcohol tautomerization
Various catalysts can be employed to facilitate alcohol tautomerization. These may include metal-based catalysts, enzymes, or acid-base catalysts. The choice of catalyst can significantly affect the rate and selectivity of the tautomerization process, allowing for better control over the desired products in chemical reactions involving alcohols.Expand Specific Solutions03 Industrial applications of alcohol tautomerization
Alcohol tautomerization has numerous industrial applications, including the production of pharmaceuticals, fragrances, and fine chemicals. The process can be utilized in the synthesis of complex organic molecules, the modification of natural products, and the development of new materials. Understanding and harnessing alcohol tautomerization can lead to more efficient and cost-effective industrial processes.Expand Specific Solutions04 Analytical methods for studying alcohol tautomerization
Various analytical techniques can be employed to study alcohol tautomerization, including spectroscopic methods such as NMR, IR, and UV-Vis spectroscopy. These techniques allow researchers to observe and quantify the tautomeric equilibrium, determine reaction kinetics, and identify intermediates. Advanced computational methods can also be used to model and predict tautomerization behavior in different chemical environments.Expand Specific Solutions05 Environmental factors affecting alcohol tautomerization
Environmental factors such as solvent polarity, pH, temperature, and pressure can significantly influence alcohol tautomerization. Understanding these effects is crucial for optimizing reaction conditions and controlling the tautomeric equilibrium. Researchers can manipulate these factors to favor specific tautomeric forms or to enhance the rate of tautomerization in various chemical processes.Expand Specific Solutions
Key Players in Tautomerization Research
The field of base-catalyzed tautomerization of alcohols is in a mature stage of development, with significant research contributions from both academic institutions and industrial players. The market for this technology is substantial, driven by its applications in organic synthesis and pharmaceutical manufacturing. Companies like BASF Corp., DuPont de Nemours, Inc., and ExxonMobil Chemical Patents, Inc. are leading industrial players, leveraging their extensive R&D capabilities to advance this technology. Academic institutions such as the University of Bologna and Hong Kong Polytechnic University are also making notable contributions, particularly in mechanistic studies. The technology's maturity is evident in the diverse range of applications and the depth of understanding of the underlying mechanisms, with ongoing research focused on optimizing catalysts and improving reaction efficiency.
BASF Corp.
Technical Solution: BASF has developed advanced catalytic systems for base-catalyzed tautomerization of alcohols. Their approach involves using novel heterogeneous catalysts with tailored surface properties to enhance selectivity and yield. The company has implemented a multi-step process that includes alcohol dehydrogenation, followed by controlled tautomerization and subsequent hydrogenation[1]. This method allows for precise control over the reaction equilibrium, minimizing unwanted side products. BASF has also incorporated in-situ spectroscopic monitoring techniques to gain real-time mechanistic insights into the tautomerization process, enabling optimization of reaction conditions for various alcohol substrates[3].
Strengths: Extensive expertise in catalysis, large-scale production capabilities, and advanced analytical tools. Weaknesses: Potential high costs associated with specialized catalyst development and complex process control systems.
DuPont de Nemours, Inc.
Technical Solution: DuPont has focused on developing environmentally friendly approaches to base-catalyzed tautomerization of alcohols. Their research has led to the creation of bio-based catalysts derived from renewable resources, which exhibit high activity and selectivity for alcohol tautomerization[2]. The company has also explored the use of ionic liquids as reaction media, which has shown promise in enhancing reaction rates and improving product separation[4]. DuPont's mechanistic studies have revealed the importance of hydrogen bonding networks in stabilizing transition states during the tautomerization process, leading to the design of catalysts with optimized hydrogen bond donor and acceptor sites[5].
Strengths: Strong focus on sustainable chemistry and innovative catalyst design. Weaknesses: Potential scalability issues with bio-based catalysts and higher costs associated with ionic liquid technologies.
Critical Insights from Recent Tautomerization Studies
Ring opening polymerisation of cyclic carbonates with organic catalyst system
PatentInactiveIN1775DELNP2012A
Innovation
- The use of small amounts of metal-free organocatalysts, such as amine, guanidine, or phosphazene precursors in combination with excess alcohol as both a co-initiator and transfer agent for immortal ring-opening polymerization of cyclic carbonates, allowing for controlled polymerization and functionalization of polycarbonates without the need for metallic impurities.
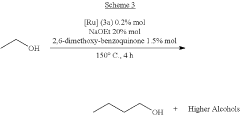
Process for the transformation of primary aliphatic alcohols into higher aliphatic alcohols
PatentActiveUS11932592B2
Innovation
- A homogeneous condensation process using a catalyst mixture comprising a transition metal complex, a base, and specific additives like pyridines N-oxide, isoquinolines N-oxide, or benzoquinones, which enhances the conversion and yield of higher aliphatic alcohols by accelerating the reaction rate and maintaining high selectivity.
Computational Approaches in Tautomerization Research
Computational approaches have become indispensable tools in tautomerization research, offering valuable insights into the mechanistic details of base-catalyzed alcohol tautomerization. These methods provide a powerful complement to experimental techniques, allowing researchers to explore reaction pathways, transition states, and energetics at the molecular level.
Density Functional Theory (DFT) calculations have emerged as a particularly effective approach for studying tautomerization processes. DFT methods offer a favorable balance between computational cost and accuracy, enabling the investigation of relatively large molecular systems. Researchers often employ hybrid functionals such as B3LYP or M06-2X, coupled with appropriate basis sets, to optimize geometries and calculate energies of reactants, products, and transition states.
Ab initio methods, including MP2 and coupled cluster theory, provide higher levels of accuracy for smaller systems or benchmark calculations. These approaches are particularly useful for validating DFT results and exploring subtle electronic effects that may influence tautomerization mechanisms.
Molecular dynamics simulations offer a complementary perspective, allowing researchers to study the dynamic behavior of tautomerization reactions in solution. These simulations can reveal the role of solvent molecules in stabilizing intermediates and facilitating proton transfer processes. Advanced sampling techniques, such as metadynamics or umbrella sampling, can be employed to overcome energy barriers and explore rare events in tautomerization reactions.
Quantum mechanical/molecular mechanical (QM/MM) methods have gained prominence in studying base-catalyzed tautomerization in complex environments, such as enzyme active sites. These hybrid approaches allow for the accurate treatment of the reactive center using quantum mechanics while incorporating the effects of the surrounding environment through classical molecular mechanics.
Machine learning techniques are increasingly being applied to tautomerization research, enabling the rapid prediction of tautomeric equilibria and reaction rates. These methods can be trained on large datasets of experimental and computational results, providing a valuable tool for high-throughput screening of potential tautomerization reactions.
Computational approaches also play a crucial role in interpreting experimental data. For instance, calculated vibrational frequencies and NMR chemical shifts can be compared with experimental spectra to identify and characterize tautomeric species. Time-dependent DFT calculations can provide insights into electronic transitions, aiding in the interpretation of UV-Vis spectroscopy data for tautomeric systems.
As computational power continues to increase, these methods are becoming more accessible and applicable to larger and more complex systems. The integration of multiple computational techniques, along with experimental data, is paving the way for a more comprehensive understanding of base-catalyzed tautomerization mechanisms in alcohols and related compounds.
Density Functional Theory (DFT) calculations have emerged as a particularly effective approach for studying tautomerization processes. DFT methods offer a favorable balance between computational cost and accuracy, enabling the investigation of relatively large molecular systems. Researchers often employ hybrid functionals such as B3LYP or M06-2X, coupled with appropriate basis sets, to optimize geometries and calculate energies of reactants, products, and transition states.
Ab initio methods, including MP2 and coupled cluster theory, provide higher levels of accuracy for smaller systems or benchmark calculations. These approaches are particularly useful for validating DFT results and exploring subtle electronic effects that may influence tautomerization mechanisms.
Molecular dynamics simulations offer a complementary perspective, allowing researchers to study the dynamic behavior of tautomerization reactions in solution. These simulations can reveal the role of solvent molecules in stabilizing intermediates and facilitating proton transfer processes. Advanced sampling techniques, such as metadynamics or umbrella sampling, can be employed to overcome energy barriers and explore rare events in tautomerization reactions.
Quantum mechanical/molecular mechanical (QM/MM) methods have gained prominence in studying base-catalyzed tautomerization in complex environments, such as enzyme active sites. These hybrid approaches allow for the accurate treatment of the reactive center using quantum mechanics while incorporating the effects of the surrounding environment through classical molecular mechanics.
Machine learning techniques are increasingly being applied to tautomerization research, enabling the rapid prediction of tautomeric equilibria and reaction rates. These methods can be trained on large datasets of experimental and computational results, providing a valuable tool for high-throughput screening of potential tautomerization reactions.
Computational approaches also play a crucial role in interpreting experimental data. For instance, calculated vibrational frequencies and NMR chemical shifts can be compared with experimental spectra to identify and characterize tautomeric species. Time-dependent DFT calculations can provide insights into electronic transitions, aiding in the interpretation of UV-Vis spectroscopy data for tautomeric systems.
As computational power continues to increase, these methods are becoming more accessible and applicable to larger and more complex systems. The integration of multiple computational techniques, along with experimental data, is paving the way for a more comprehensive understanding of base-catalyzed tautomerization mechanisms in alcohols and related compounds.
Environmental Impact of Tautomerization Processes
The environmental impact of tautomerization processes, particularly in the context of base-catalyzed tautomerization of alcohols, is a crucial aspect to consider in both industrial and research settings. These processes, while essential for various chemical transformations, can have significant implications for the environment if not properly managed.
Tautomerization reactions often involve the use of strong bases as catalysts, which can pose environmental risks if released into ecosystems. These bases, such as sodium hydroxide or potassium tert-butoxide, may alter the pH of soil and water systems, potentially disrupting local flora and fauna. Furthermore, the disposal of waste products from these reactions requires careful consideration to prevent contamination of water sources and soil.
The solvents used in tautomerization processes also contribute to the environmental footprint. Many of these reactions are carried out in organic solvents, which can be volatile and potentially harmful if released into the atmosphere. The production, use, and disposal of these solvents must be carefully managed to minimize air and water pollution.
Energy consumption is another environmental concern associated with tautomerization processes. These reactions often require heating or cooling, which contributes to greenhouse gas emissions if the energy source is not renewable. Implementing energy-efficient technologies and exploring alternative energy sources can help mitigate this impact.
On a positive note, tautomerization processes can play a role in green chemistry initiatives. By enabling more efficient synthetic routes, these reactions can potentially reduce the overall environmental impact of chemical production. For instance, base-catalyzed tautomerization of alcohols can lead to the formation of valuable intermediates with fewer steps and less waste compared to traditional methods.
The development of recyclable catalysts and more environmentally friendly solvents is an active area of research that could significantly reduce the environmental impact of tautomerization processes. Ionic liquids and supercritical fluids are being explored as greener alternatives to conventional organic solvents, potentially offering reduced emissions and improved recyclability.
In conclusion, while tautomerization processes, including base-catalyzed tautomerization of alcohols, present certain environmental challenges, ongoing research and technological advancements are paving the way for more sustainable practices. Balancing the benefits of these reactions with their potential environmental impacts remains a key consideration for researchers and industry professionals alike.
Tautomerization reactions often involve the use of strong bases as catalysts, which can pose environmental risks if released into ecosystems. These bases, such as sodium hydroxide or potassium tert-butoxide, may alter the pH of soil and water systems, potentially disrupting local flora and fauna. Furthermore, the disposal of waste products from these reactions requires careful consideration to prevent contamination of water sources and soil.
The solvents used in tautomerization processes also contribute to the environmental footprint. Many of these reactions are carried out in organic solvents, which can be volatile and potentially harmful if released into the atmosphere. The production, use, and disposal of these solvents must be carefully managed to minimize air and water pollution.
Energy consumption is another environmental concern associated with tautomerization processes. These reactions often require heating or cooling, which contributes to greenhouse gas emissions if the energy source is not renewable. Implementing energy-efficient technologies and exploring alternative energy sources can help mitigate this impact.
On a positive note, tautomerization processes can play a role in green chemistry initiatives. By enabling more efficient synthetic routes, these reactions can potentially reduce the overall environmental impact of chemical production. For instance, base-catalyzed tautomerization of alcohols can lead to the formation of valuable intermediates with fewer steps and less waste compared to traditional methods.
The development of recyclable catalysts and more environmentally friendly solvents is an active area of research that could significantly reduce the environmental impact of tautomerization processes. Ionic liquids and supercritical fluids are being explored as greener alternatives to conventional organic solvents, potentially offering reduced emissions and improved recyclability.
In conclusion, while tautomerization processes, including base-catalyzed tautomerization of alcohols, present certain environmental challenges, ongoing research and technological advancements are paving the way for more sustainable practices. Balancing the benefits of these reactions with their potential environmental impacts remains a key consideration for researchers and industry professionals alike.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!