Kinetics of Tautomerization in Unstable Molecular Systems
JUL 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Tautomerization Kinetics Background and Objectives
Tautomerization, a fundamental process in organic chemistry, has been a subject of intense study for decades. This phenomenon, involving the rapid interconversion between structural isomers, plays a crucial role in various chemical and biological systems. The kinetics of tautomerization in unstable molecular systems presents a particularly intriguing area of research, as it combines the challenges of studying fast reactions with the complexities of dealing with transient species.
The historical development of tautomerization research can be traced back to the late 19th century, with early observations of keto-enol equilibria. However, it was not until the mid-20th century that significant progress was made in understanding the kinetics of these processes. The advent of advanced spectroscopic techniques, such as ultrafast laser spectroscopy and NMR, has revolutionized our ability to probe these rapid transformations.
In recent years, the focus has shifted towards understanding tautomerization in increasingly complex and unstable molecular systems. This shift is driven by the recognition of tautomerization's importance in areas such as drug design, materials science, and astrochemistry. The behavior of unstable tautomers in these contexts can significantly influence molecular properties and reactivity, making their study both challenging and essential.
The primary objective of current research in this field is to elucidate the mechanisms and factors governing tautomerization kinetics in unstable systems. This includes investigating the influence of molecular structure, environmental conditions, and external stimuli on tautomerization rates and equilibria. Additionally, there is a growing interest in developing predictive models that can accurately describe tautomerization behavior in diverse chemical environments.
Another key goal is to bridge the gap between theoretical predictions and experimental observations. Computational chemistry has made significant strides in modeling tautomerization processes, but validating these models against experimental data for unstable systems remains a considerable challenge. Overcoming this hurdle is crucial for advancing our understanding and predictive capabilities in this field.
Furthermore, researchers aim to exploit the unique properties of unstable tautomers for practical applications. This includes designing molecular switches, developing new catalytic systems, and creating responsive materials. The potential for harnessing tautomerization in unstable systems for these applications underscores the importance of gaining a deeper understanding of their kinetics.
As we look to the future, the study of tautomerization kinetics in unstable molecular systems is poised to yield insights that could revolutionize multiple areas of chemistry and related fields. The ongoing development of more sensitive and time-resolved experimental techniques, coupled with advances in computational methods, promises to unlock new frontiers in this exciting area of research.
The historical development of tautomerization research can be traced back to the late 19th century, with early observations of keto-enol equilibria. However, it was not until the mid-20th century that significant progress was made in understanding the kinetics of these processes. The advent of advanced spectroscopic techniques, such as ultrafast laser spectroscopy and NMR, has revolutionized our ability to probe these rapid transformations.
In recent years, the focus has shifted towards understanding tautomerization in increasingly complex and unstable molecular systems. This shift is driven by the recognition of tautomerization's importance in areas such as drug design, materials science, and astrochemistry. The behavior of unstable tautomers in these contexts can significantly influence molecular properties and reactivity, making their study both challenging and essential.
The primary objective of current research in this field is to elucidate the mechanisms and factors governing tautomerization kinetics in unstable systems. This includes investigating the influence of molecular structure, environmental conditions, and external stimuli on tautomerization rates and equilibria. Additionally, there is a growing interest in developing predictive models that can accurately describe tautomerization behavior in diverse chemical environments.
Another key goal is to bridge the gap between theoretical predictions and experimental observations. Computational chemistry has made significant strides in modeling tautomerization processes, but validating these models against experimental data for unstable systems remains a considerable challenge. Overcoming this hurdle is crucial for advancing our understanding and predictive capabilities in this field.
Furthermore, researchers aim to exploit the unique properties of unstable tautomers for practical applications. This includes designing molecular switches, developing new catalytic systems, and creating responsive materials. The potential for harnessing tautomerization in unstable systems for these applications underscores the importance of gaining a deeper understanding of their kinetics.
As we look to the future, the study of tautomerization kinetics in unstable molecular systems is poised to yield insights that could revolutionize multiple areas of chemistry and related fields. The ongoing development of more sensitive and time-resolved experimental techniques, coupled with advances in computational methods, promises to unlock new frontiers in this exciting area of research.
Applications in Pharmaceutical and Materials Science
The kinetics of tautomerization in unstable molecular systems has significant implications for pharmaceutical and materials science applications. In drug discovery and development, understanding tautomeric equilibria is crucial for predicting drug-target interactions and optimizing drug efficacy. Tautomers can exhibit different binding affinities to target proteins, affecting the pharmacological activity of potential drug candidates. This knowledge enables pharmaceutical researchers to design more effective and selective drugs by exploiting the tautomeric preferences of lead compounds.
In the field of materials science, tautomerization kinetics play a vital role in the development of smart materials and molecular switches. The ability to control and manipulate tautomeric equilibria can lead to the creation of responsive materials that change their properties based on external stimuli such as light, temperature, or pH. These materials have potential applications in sensors, data storage devices, and adaptive coatings.
The study of tautomerization kinetics in unstable molecular systems also contributes to the development of novel catalysts for chemical synthesis. By understanding the mechanisms and rates of tautomeric interconversions, researchers can design catalysts that selectively stabilize specific tautomeric forms, leading to more efficient and selective chemical transformations. This has implications for the production of pharmaceuticals and fine chemicals, potentially reducing costs and improving yields in industrial processes.
In the realm of crystal engineering, tautomerization kinetics influence the formation and stability of crystal structures. The ability to control tautomeric equilibria can be leveraged to design materials with specific physical properties, such as improved solubility, enhanced thermal stability, or unique optical characteristics. This has applications in the development of advanced pharmaceutical formulations and functional materials for electronic and optoelectronic devices.
Furthermore, the study of tautomerization kinetics in unstable molecular systems contributes to the field of supramolecular chemistry. Understanding how tautomeric equilibria affect molecular recognition and self-assembly processes can lead to the design of novel supramolecular architectures with tailored properties. These structures have potential applications in drug delivery systems, molecular machines, and advanced materials for environmental remediation.
In the field of materials science, tautomerization kinetics play a vital role in the development of smart materials and molecular switches. The ability to control and manipulate tautomeric equilibria can lead to the creation of responsive materials that change their properties based on external stimuli such as light, temperature, or pH. These materials have potential applications in sensors, data storage devices, and adaptive coatings.
The study of tautomerization kinetics in unstable molecular systems also contributes to the development of novel catalysts for chemical synthesis. By understanding the mechanisms and rates of tautomeric interconversions, researchers can design catalysts that selectively stabilize specific tautomeric forms, leading to more efficient and selective chemical transformations. This has implications for the production of pharmaceuticals and fine chemicals, potentially reducing costs and improving yields in industrial processes.
In the realm of crystal engineering, tautomerization kinetics influence the formation and stability of crystal structures. The ability to control tautomeric equilibria can be leveraged to design materials with specific physical properties, such as improved solubility, enhanced thermal stability, or unique optical characteristics. This has applications in the development of advanced pharmaceutical formulations and functional materials for electronic and optoelectronic devices.
Furthermore, the study of tautomerization kinetics in unstable molecular systems contributes to the field of supramolecular chemistry. Understanding how tautomeric equilibria affect molecular recognition and self-assembly processes can lead to the design of novel supramolecular architectures with tailored properties. These structures have potential applications in drug delivery systems, molecular machines, and advanced materials for environmental remediation.
Current Challenges in Studying Unstable Molecular Systems
The study of unstable molecular systems presents several significant challenges that hinder our understanding of tautomerization kinetics. One of the primary difficulties lies in the rapid interconversion between tautomeric forms, which often occurs on timescales faster than conventional spectroscopic techniques can capture. This temporal limitation makes it challenging to isolate and characterize individual tautomers, leading to uncertainties in kinetic measurements.
Another major obstacle is the environmental sensitivity of unstable molecular systems. Tautomerization processes can be heavily influenced by factors such as solvent polarity, pH, temperature, and pressure. These external variables can dramatically alter the equilibrium between tautomeric forms and affect the kinetics of interconversion. Consequently, researchers must carefully control and account for these parameters to obtain reliable and reproducible results.
The inherent instability of these molecular systems also poses significant experimental challenges. Many unstable tautomers have short lifetimes, making their isolation and direct observation extremely difficult. This instability often necessitates the use of advanced spectroscopic techniques, such as ultrafast laser spectroscopy or cryogenic matrix isolation, which require specialized equipment and expertise.
Computational modeling of tautomerization kinetics in unstable systems is another area fraught with challenges. The complexity of these systems, including the need to account for quantum effects and multiple reaction pathways, often pushes the limits of current computational methods. Accurately predicting energy barriers and transition states for tautomerization reactions remains a significant challenge, particularly for larger and more complex molecular systems.
Furthermore, the study of tautomerization kinetics is complicated by the potential for concurrent reactions or competing pathways. In many unstable molecular systems, tautomerization may occur alongside other processes such as isomerization, decomposition, or intermolecular interactions. Disentangling these various processes and isolating the kinetics of tautomerization alone can be extremely challenging and requires careful experimental design and data analysis.
Lastly, the development of standardized methodologies for studying tautomerization kinetics in unstable systems remains an ongoing challenge. The diversity of molecular systems and the range of experimental techniques employed make it difficult to compare results across different studies. Establishing robust, widely applicable protocols for measuring and reporting tautomerization kinetics is crucial for advancing our understanding of these complex systems.
Another major obstacle is the environmental sensitivity of unstable molecular systems. Tautomerization processes can be heavily influenced by factors such as solvent polarity, pH, temperature, and pressure. These external variables can dramatically alter the equilibrium between tautomeric forms and affect the kinetics of interconversion. Consequently, researchers must carefully control and account for these parameters to obtain reliable and reproducible results.
The inherent instability of these molecular systems also poses significant experimental challenges. Many unstable tautomers have short lifetimes, making their isolation and direct observation extremely difficult. This instability often necessitates the use of advanced spectroscopic techniques, such as ultrafast laser spectroscopy or cryogenic matrix isolation, which require specialized equipment and expertise.
Computational modeling of tautomerization kinetics in unstable systems is another area fraught with challenges. The complexity of these systems, including the need to account for quantum effects and multiple reaction pathways, often pushes the limits of current computational methods. Accurately predicting energy barriers and transition states for tautomerization reactions remains a significant challenge, particularly for larger and more complex molecular systems.
Furthermore, the study of tautomerization kinetics is complicated by the potential for concurrent reactions or competing pathways. In many unstable molecular systems, tautomerization may occur alongside other processes such as isomerization, decomposition, or intermolecular interactions. Disentangling these various processes and isolating the kinetics of tautomerization alone can be extremely challenging and requires careful experimental design and data analysis.
Lastly, the development of standardized methodologies for studying tautomerization kinetics in unstable systems remains an ongoing challenge. The diversity of molecular systems and the range of experimental techniques employed make it difficult to compare results across different studies. Establishing robust, widely applicable protocols for measuring and reporting tautomerization kinetics is crucial for advancing our understanding of these complex systems.
Experimental Techniques for Tautomerization Analysis
01 Tautomerization kinetics measurement techniques
Various techniques are employed to measure tautomerization kinetics in unstable molecular systems. These may include spectroscopic methods, such as NMR or UV-Vis spectroscopy, as well as computational approaches. These techniques allow researchers to observe and quantify the rate of tautomeric interconversion in real-time, providing valuable insights into the stability and behavior of these molecular systems.- Tautomerization kinetics measurement techniques: Various techniques are employed to measure tautomerization kinetics in unstable molecular systems. These may include spectroscopic methods, such as NMR or UV-Vis spectroscopy, as well as computational approaches. These techniques allow researchers to observe and quantify the rate of tautomeric interconversion in real-time, providing valuable insights into the stability and behavior of these molecular systems.
- Factors influencing tautomerization rates: Several factors can affect the rate of tautomerization in unstable molecular systems. These may include temperature, pH, solvent effects, and the presence of catalysts or other chemical species. Understanding these factors is crucial for predicting and controlling tautomerization processes in various applications, such as drug design and materials science.
- Computational modeling of tautomerization: Advanced computational methods are used to model and predict tautomerization kinetics in unstable molecular systems. These may include quantum mechanical calculations, molecular dynamics simulations, and machine learning approaches. Such models help researchers understand the energetics and mechanisms of tautomerization processes at the molecular level.
- Applications of tautomerization kinetics: Understanding tautomerization kinetics has important applications in various fields. These may include the development of new pharmaceuticals, design of molecular switches and sensors, and optimization of chemical processes. By controlling tautomerization rates, researchers can manipulate the properties and functions of molecular systems for specific purposes.
- Stabilization strategies for unstable tautomers: Researchers employ various strategies to stabilize unstable tautomers and control tautomerization kinetics. These may include chemical modifications, encapsulation techniques, or the use of specific environmental conditions. Such approaches are crucial for harnessing the properties of unstable molecular systems in practical applications.
02 Factors influencing tautomerization rates
Several factors can affect the rate of tautomerization in unstable molecular systems. These may include temperature, pH, solvent effects, and the presence of catalysts or other reactive species. Understanding these factors is crucial for predicting and controlling tautomerization processes in various applications, such as drug design and materials science.Expand Specific Solutions03 Computational modeling of tautomerization
Advanced computational methods are used to model and predict tautomerization kinetics in unstable molecular systems. These may include quantum mechanical calculations, molecular dynamics simulations, and machine learning approaches. Such models help researchers understand the energetics and mechanisms of tautomerization processes at the molecular level.Expand Specific Solutions04 Applications of tautomerization kinetics
Understanding tautomerization kinetics has important applications in various fields. These may include the development of new pharmaceuticals, design of molecular switches and sensors, and optimization of chemical processes. By controlling tautomerization rates, researchers can tune the properties and reactivity of molecular systems for specific applications.Expand Specific Solutions05 Stabilization strategies for tautomeric systems
Various strategies are employed to stabilize tautomeric systems and control their interconversion rates. These may include chemical modifications, such as introducing substituents or forming complexes, as well as environmental control methods like temperature regulation or solvent selection. These approaches allow researchers to manipulate the stability and behavior of tautomeric molecular systems for desired outcomes.Expand Specific Solutions
Leading Research Groups and Institutions
The field of tautomerization kinetics in unstable molecular systems is in a nascent stage of development, with a growing market driven by pharmaceutical and chemical industries. The technology's maturity is still evolving, as evidenced by the diverse range of institutions involved, including academic powerhouses like the University of Maryland and Anhui University, research organizations such as CNRS, and pharmaceutical companies like Sunshine Lake Pharma and Humanwell Healthcare. This mix of players suggests a collaborative ecosystem where fundamental research is gradually transitioning towards practical applications. The involvement of both established firms and newer entrants indicates a competitive landscape with potential for significant advancements in understanding and controlling molecular behavior in unstable systems.
University of Maryland
Technical Solution: The University of Maryland has pioneered experimental techniques to directly observe tautomerization in unstable molecular systems using ultrafast spectroscopy. Their approach employs femtosecond laser pulses to initiate and probe tautomerization reactions with unprecedented time resolution[4]. They have developed novel sample preparation methods to stabilize reactive intermediates, allowing for the study of short-lived tautomers[5]. The Maryland team has also integrated their experimental setup with microfluidic devices, enabling the investigation of tautomerization kinetics under a wide range of conditions, including extreme temperatures and pressures[6].
Strengths: State-of-the-art experimental facilities, ability to directly observe fast chemical processes. Weaknesses: Limited to systems amenable to spectroscopic analysis, potential artifacts from intense laser pulses.
Centre National de la Recherche Scientifique
Technical Solution: The Centre National de la Recherche Scientifique (CNRS) has developed advanced computational methods to study the kinetics of tautomerization in unstable molecular systems. Their approach combines quantum mechanical calculations with molecular dynamics simulations to accurately predict tautomerization rates and mechanisms[1]. They have implemented a multi-scale modeling framework that bridges electronic structure calculations with coarse-grained models, allowing for the simulation of tautomerization processes across different time and length scales[2]. The CNRS team has also developed machine learning algorithms to accelerate the prediction of tautomerization pathways in complex molecular environments, enabling the study of these processes in biologically relevant systems[3].
Strengths: Cutting-edge computational methods, multidisciplinary approach combining theory and experiment. Weaknesses: High computational cost, potential limitations in modeling highly unstable systems.
Key Theoretical Models for Tautomerization Kinetics
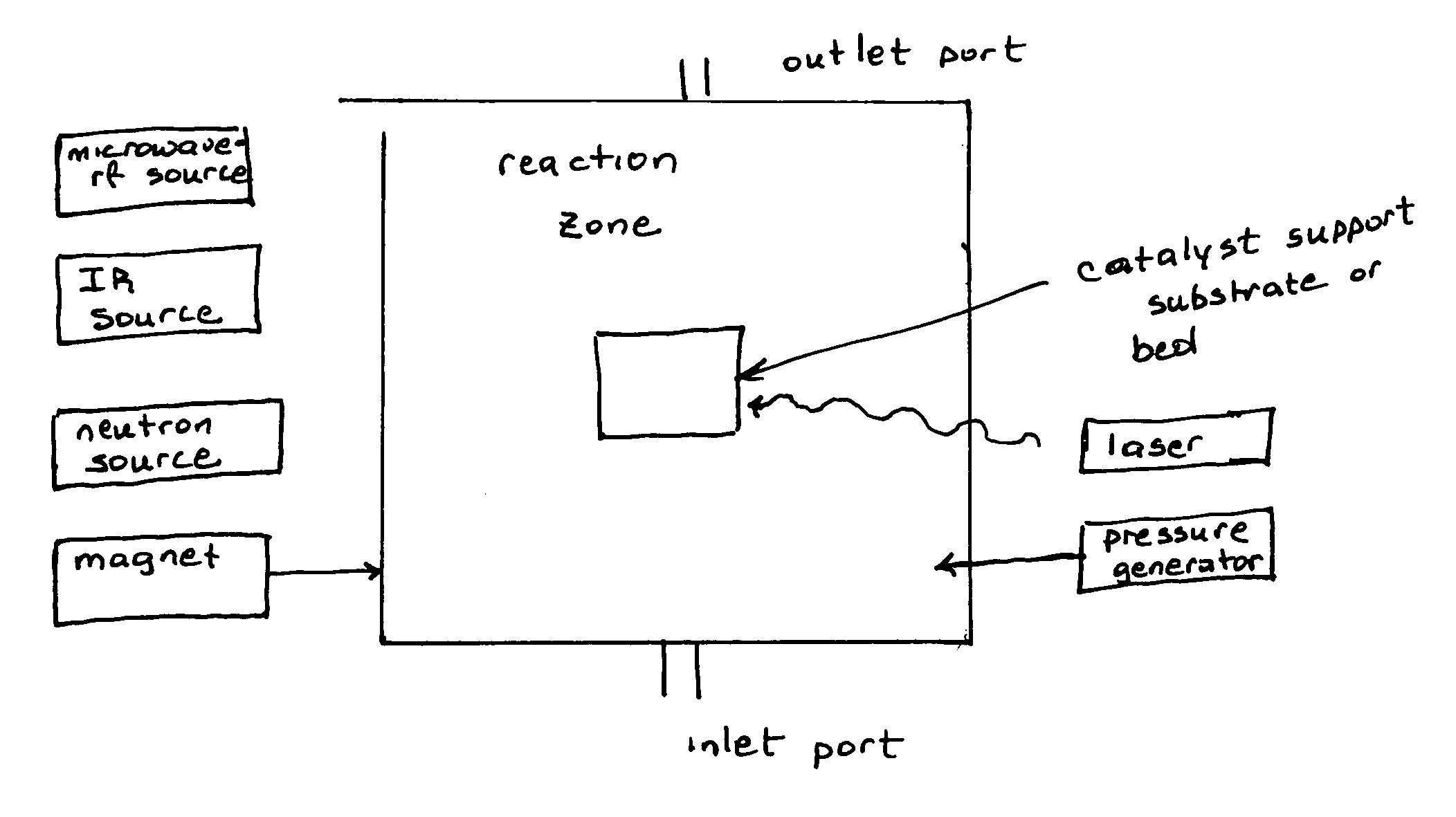
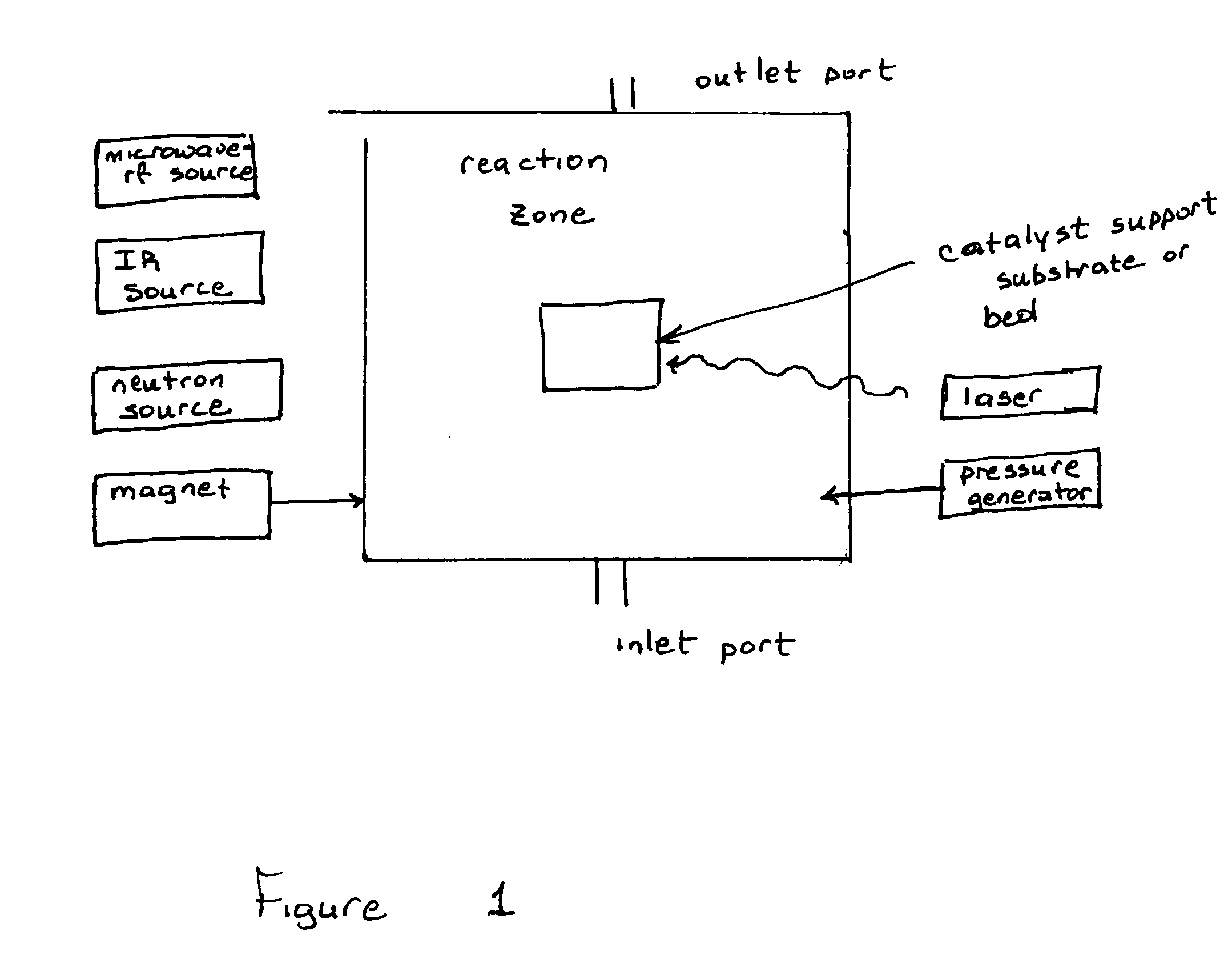
Magnetic stimulated catalytic chemical conversion of second series elemental compounds: combination, decomposition rearrangement and/or reformation magneto chemistry
PatentInactiveUS20060233703A1
Innovation
- The use of intense static and dynamic magnetic fields in conjunction with IR and laser heating to enhance catalytic processes, allowing for the selective formation of these compounds with reduced energy input and elimination of harsh conditions, utilizing a reaction chamber with a heating element, magnetic field generator, and laser radiation to control electronic states and spin dynamics.
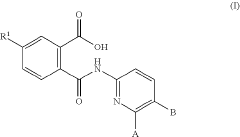
Pyridine derivatives as modulators of sortilin activity
PatentPendingUS20240132514A1
Innovation
- Development of compounds of formula (I) that inhibit or antagonize sortilin, disrupting its interaction with pro-neurotrophins or p75NTR, thereby preventing the formation of harmful trimeric complexes associated with these conditions.
Computational Methods for Unstable Molecular Systems
Computational methods play a crucial role in studying the kinetics of tautomerization in unstable molecular systems. These methods provide powerful tools for simulating and analyzing complex molecular behaviors that are challenging to observe experimentally due to their transient nature.
One of the primary computational approaches used in this field is molecular dynamics (MD) simulations. MD simulations allow researchers to model the time-dependent behavior of molecular systems, including the rapid interconversion between tautomeric forms. These simulations can provide insights into the energy barriers, transition states, and reaction pathways involved in tautomerization processes.
Quantum mechanical (QM) calculations are another essential computational method for studying unstable molecular systems. Density Functional Theory (DFT) and ab initio methods can be employed to calculate the electronic structure and energetics of different tautomeric forms with high accuracy. These calculations help in determining the relative stabilities of tautomers and the energy barriers for their interconversion.
Hybrid quantum mechanics/molecular mechanics (QM/MM) methods have gained popularity in recent years for studying tautomerization in complex environments, such as within proteins or in solution. These methods combine the accuracy of QM calculations for the reactive center with the computational efficiency of MM simulations for the surrounding environment.
Advanced sampling techniques, such as metadynamics and umbrella sampling, are often employed to enhance the exploration of configurational space and improve the accuracy of free energy calculations for tautomerization processes. These methods help overcome the limitations of traditional MD simulations in sampling rare events and crossing high energy barriers.
Machine learning (ML) approaches are increasingly being integrated into computational studies of unstable molecular systems. ML models can be trained on large datasets of molecular properties and used to predict tautomerization rates and equilibria for new compounds, potentially accelerating the discovery of molecules with desired properties.
Time-dependent density functional theory (TD-DFT) calculations are valuable for studying the excited-state properties of tautomeric systems, which can be particularly important for understanding photochemical processes and spectroscopic observations.
Continuum solvation models, such as the polarizable continuum model (PCM), are often used in conjunction with QM calculations to account for solvent effects on tautomerization kinetics. These models provide a computationally efficient way to simulate the influence of different solvents on the stability and interconversion of tautomers.
One of the primary computational approaches used in this field is molecular dynamics (MD) simulations. MD simulations allow researchers to model the time-dependent behavior of molecular systems, including the rapid interconversion between tautomeric forms. These simulations can provide insights into the energy barriers, transition states, and reaction pathways involved in tautomerization processes.
Quantum mechanical (QM) calculations are another essential computational method for studying unstable molecular systems. Density Functional Theory (DFT) and ab initio methods can be employed to calculate the electronic structure and energetics of different tautomeric forms with high accuracy. These calculations help in determining the relative stabilities of tautomers and the energy barriers for their interconversion.
Hybrid quantum mechanics/molecular mechanics (QM/MM) methods have gained popularity in recent years for studying tautomerization in complex environments, such as within proteins or in solution. These methods combine the accuracy of QM calculations for the reactive center with the computational efficiency of MM simulations for the surrounding environment.
Advanced sampling techniques, such as metadynamics and umbrella sampling, are often employed to enhance the exploration of configurational space and improve the accuracy of free energy calculations for tautomerization processes. These methods help overcome the limitations of traditional MD simulations in sampling rare events and crossing high energy barriers.
Machine learning (ML) approaches are increasingly being integrated into computational studies of unstable molecular systems. ML models can be trained on large datasets of molecular properties and used to predict tautomerization rates and equilibria for new compounds, potentially accelerating the discovery of molecules with desired properties.
Time-dependent density functional theory (TD-DFT) calculations are valuable for studying the excited-state properties of tautomeric systems, which can be particularly important for understanding photochemical processes and spectroscopic observations.
Continuum solvation models, such as the polarizable continuum model (PCM), are often used in conjunction with QM calculations to account for solvent effects on tautomerization kinetics. These models provide a computationally efficient way to simulate the influence of different solvents on the stability and interconversion of tautomers.
Environmental Factors Affecting Tautomerization Kinetics
The kinetics of tautomerization in unstable molecular systems are significantly influenced by various environmental factors. Temperature plays a crucial role in determining the rate of tautomerization, with higher temperatures generally accelerating the process. This is due to the increased thermal energy available to overcome the activation barrier between tautomeric forms. The Arrhenius equation provides a quantitative relationship between temperature and reaction rate, allowing researchers to predict tautomerization kinetics under different thermal conditions.
Solvent effects are another critical environmental factor affecting tautomerization kinetics. The polarity, hydrogen bonding capability, and dielectric constant of the solvent can dramatically alter the stability of different tautomeric forms and the transition state. Polar solvents may stabilize charged or highly polar tautomers, while non-polar solvents favor less polar forms. Additionally, protic solvents can participate in proton transfer processes, potentially catalyzing tautomerization reactions.
pH is a particularly important factor for tautomerization involving proton transfer. In aqueous solutions, the pH can significantly influence the equilibrium between tautomeric forms and the rate of interconversion. Acidic or basic conditions can catalyze tautomerization by facilitating proton transfer processes. The Henderson-Hasselbalch equation can be used to predict the distribution of tautomers at different pH values, providing insights into the kinetics of the system.
Pressure is another environmental variable that can affect tautomerization kinetics, especially in systems where there is a significant volume change between tautomeric forms or in the transition state. High-pressure conditions can favor tautomers with smaller molecular volumes or transition states that involve a decrease in volume.
The presence of catalysts or specific ions in the environment can also dramatically alter tautomerization kinetics. Metal ions, for example, can coordinate with tautomeric molecules, stabilizing certain forms or lowering the activation energy for interconversion. Enzymes in biological systems can catalyze tautomerization reactions with high specificity and efficiency, often under mild conditions.
Light exposure is an important consideration for photochemical tautomerization processes. The wavelength and intensity of light can induce electronic transitions that facilitate tautomerization, particularly in systems with conjugated π-electron structures. This factor is especially relevant in the design of photoswitchable materials and in understanding natural photobiological processes.
Lastly, the local chemical environment, including the presence of neighboring molecules or surfaces, can influence tautomerization kinetics through intermolecular interactions. These interactions may include hydrogen bonding, π-π stacking, or van der Waals forces, which can stabilize certain tautomeric forms or alter the energy landscape of the tautomerization process.
Solvent effects are another critical environmental factor affecting tautomerization kinetics. The polarity, hydrogen bonding capability, and dielectric constant of the solvent can dramatically alter the stability of different tautomeric forms and the transition state. Polar solvents may stabilize charged or highly polar tautomers, while non-polar solvents favor less polar forms. Additionally, protic solvents can participate in proton transfer processes, potentially catalyzing tautomerization reactions.
pH is a particularly important factor for tautomerization involving proton transfer. In aqueous solutions, the pH can significantly influence the equilibrium between tautomeric forms and the rate of interconversion. Acidic or basic conditions can catalyze tautomerization by facilitating proton transfer processes. The Henderson-Hasselbalch equation can be used to predict the distribution of tautomers at different pH values, providing insights into the kinetics of the system.
Pressure is another environmental variable that can affect tautomerization kinetics, especially in systems where there is a significant volume change between tautomeric forms or in the transition state. High-pressure conditions can favor tautomers with smaller molecular volumes or transition states that involve a decrease in volume.
The presence of catalysts or specific ions in the environment can also dramatically alter tautomerization kinetics. Metal ions, for example, can coordinate with tautomeric molecules, stabilizing certain forms or lowering the activation energy for interconversion. Enzymes in biological systems can catalyze tautomerization reactions with high specificity and efficiency, often under mild conditions.
Light exposure is an important consideration for photochemical tautomerization processes. The wavelength and intensity of light can induce electronic transitions that facilitate tautomerization, particularly in systems with conjugated π-electron structures. This factor is especially relevant in the design of photoswitchable materials and in understanding natural photobiological processes.
Lastly, the local chemical environment, including the presence of neighboring molecules or surfaces, can influence tautomerization kinetics through intermolecular interactions. These interactions may include hydrogen bonding, π-π stacking, or van der Waals forces, which can stabilize certain tautomeric forms or alter the energy landscape of the tautomerization process.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!