Tautomerization in Fuel Cell Electrolytes: Efficiency Impacts
Tautomerization Fundamentals and Fuel Cell Goals
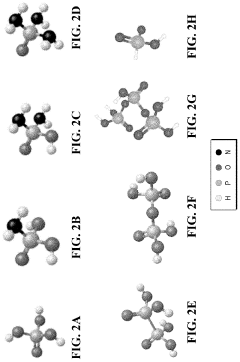
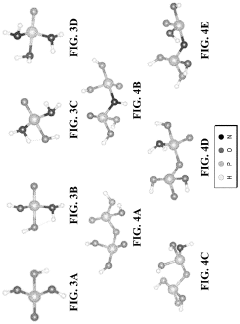
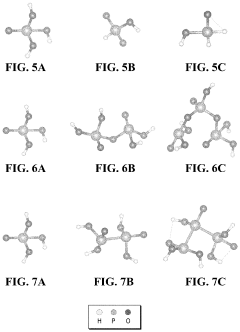
Tautomerization, a fundamental chemical process, plays a crucial role in the efficiency of fuel cell electrolytes. This phenomenon involves the rapid interconversion between structural isomers, known as tautomers, which can significantly impact the properties and performance of electrolyte materials. Understanding tautomerization is essential for optimizing fuel cell efficiency and advancing clean energy technologies.
The evolution of fuel cell technology has been driven by the need for more efficient and sustainable energy sources. As global efforts to reduce carbon emissions intensify, fuel cells have emerged as a promising alternative to traditional combustion engines. The primary goal in fuel cell development is to maximize energy conversion efficiency while minimizing losses and environmental impact.
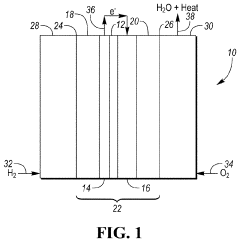
Tautomerization in fuel cell electrolytes directly influences proton conductivity, a critical factor in overall cell performance. The rapid exchange of protons between tautomeric forms can enhance or hinder the electrolyte's ability to facilitate charge transport. This process is particularly relevant in proton exchange membrane fuel cells (PEMFCs), where the electrolyte must efficiently conduct protons from the anode to the cathode.
Recent advancements in fuel cell technology have focused on developing electrolytes with improved tautomerization characteristics. Researchers aim to design materials that promote beneficial tautomeric shifts while suppressing those that may impede proton transport. This targeted approach has led to the exploration of novel polymer structures and composite materials with optimized tautomerization properties.
The impact of tautomerization extends beyond proton conductivity. It also affects the chemical stability of the electrolyte, its resistance to degradation, and its ability to maintain performance under varying operating conditions. As fuel cells are expected to function reliably in diverse environments, understanding and controlling tautomerization becomes crucial for ensuring long-term durability and consistent efficiency.
In the context of fuel cell goals, tautomerization research aims to address several key objectives. These include enhancing power density, improving cold-start capabilities, and extending the operational lifespan of fuel cells. By fine-tuning the tautomerization behavior of electrolytes, scientists and engineers strive to overcome current limitations and push the boundaries of fuel cell performance.
The study of tautomerization in fuel cell electrolytes represents a convergence of fundamental chemistry and applied energy research. As the field progresses, it promises to unlock new possibilities for high-efficiency fuel cells, contributing to the broader transition towards sustainable energy systems and a reduced carbon footprint in transportation and stationary power generation sectors.
Market Analysis for Advanced Fuel Cell Technologies
The market for advanced fuel cell technologies is experiencing significant growth, driven by increasing demand for clean energy solutions and the push towards decarbonization across various sectors. Fuel cells, particularly those utilizing advanced electrolytes, are gaining traction in both stationary and mobile applications.
In the automotive sector, fuel cell electric vehicles (FCEVs) are emerging as a viable alternative to battery electric vehicles, especially for long-range and heavy-duty applications. Major automakers are investing heavily in fuel cell technology, with several commercial models already available in select markets. The FCEV market is projected to grow substantially over the next decade, particularly in regions with well-developed hydrogen infrastructure.
The stationary power generation sector is another key market for advanced fuel cell technologies. Fuel cells are increasingly being adopted for backup power, combined heat and power (CHP) systems, and distributed generation applications. This market segment is driven by the need for reliable, efficient, and clean power sources, particularly in areas with unreliable grid infrastructure or stringent emissions regulations.
The materials handling sector has also shown significant adoption of fuel cell technology, particularly in warehouse operations. Fuel cell-powered forklifts offer advantages over traditional battery-powered units, including faster refueling times and consistent power output throughout shifts.
Geographically, Asia-Pacific, particularly Japan and South Korea, leads in fuel cell adoption and market development. These countries have implemented supportive policies and invested heavily in hydrogen infrastructure. Europe and North America are also seeing growing interest and investment in fuel cell technologies, driven by ambitious climate targets and increasing focus on renewable energy integration.
The market for advanced fuel cell electrolytes, including those addressing tautomerization issues, is closely tied to the overall fuel cell market growth. Improved electrolyte performance can significantly enhance fuel cell efficiency and durability, making it a critical area of focus for manufacturers and researchers.
However, challenges remain in terms of cost reduction, infrastructure development, and public awareness. The high cost of fuel cell systems compared to conventional technologies remains a significant barrier to widespread adoption. Additionally, the limited availability of hydrogen refueling infrastructure in many regions hinders market growth, particularly for mobile applications.
Despite these challenges, the market outlook for advanced fuel cell technologies remains positive. Ongoing research and development efforts, including those focused on electrolyte optimization and addressing issues like tautomerization, are expected to drive further improvements in fuel cell performance and cost-effectiveness, potentially accelerating market adoption across various sectors.
Current Challenges in Electrolyte Tautomerization
Tautomerization in fuel cell electrolytes presents significant challenges that impact the overall efficiency and performance of fuel cell systems. One of the primary issues is the dynamic nature of tautomeric equilibria, which can lead to fluctuations in electrolyte properties. These fluctuations affect proton conductivity, a critical factor in fuel cell operation. The constant interconversion between tautomeric forms can result in inconsistent proton transfer rates, potentially causing voltage instabilities and reduced power output.
Another challenge lies in the temperature dependence of tautomerization processes. Fuel cells often operate at elevated temperatures, which can shift tautomeric equilibria and alter the distribution of different molecular forms. This temperature sensitivity complicates the design of electrolytes that maintain optimal performance across a wide range of operating conditions. Engineers must account for these thermal effects when developing electrolyte materials, adding an extra layer of complexity to fuel cell design.
The presence of multiple tautomeric species also impacts the long-term stability of electrolyte materials. Some tautomeric forms may be more prone to degradation or side reactions, leading to a gradual decline in fuel cell performance over time. This degradation can manifest as reduced proton conductivity, increased ohmic resistance, or the formation of unwanted byproducts that interfere with electrode reactions.
Furthermore, the interaction between tautomeric electrolytes and electrode materials poses a significant challenge. Different tautomeric forms may exhibit varying affinities for electrode surfaces, potentially leading to non-uniform current distribution and localized hotspots. These interactions can accelerate electrode degradation and reduce the overall lifespan of fuel cell components.
The complexity of tautomerization processes also complicates the modeling and prediction of fuel cell behavior. Traditional electrochemical models often struggle to accurately capture the dynamic nature of tautomeric systems, making it difficult to optimize fuel cell designs through simulation. This limitation hampers the development of more efficient and reliable fuel cell technologies.
Lastly, the challenge of controlling and manipulating tautomerization to enhance fuel cell performance remains a significant hurdle. While tautomerization can potentially be leveraged to improve proton transport, achieving precise control over these molecular transformations in a practical fuel cell environment is extremely challenging. Researchers continue to explore novel approaches to harness the benefits of tautomerization while mitigating its negative impacts on fuel cell efficiency.
Existing Strategies for Tautomerization Control
01 Polymer electrolyte membranes
Polymer electrolyte membranes are crucial for fuel cell efficiency. These membranes, often made of materials like Nafion, provide high proton conductivity while acting as a barrier to fuel crossover. Improvements in membrane composition and structure can lead to enhanced fuel cell performance and durability.- Polymer electrolyte membranes: Polymer electrolyte membranes are crucial for fuel cell efficiency. These membranes, often made of materials like Nafion, provide high proton conductivity while acting as a barrier to fuel crossover. Improvements in membrane composition and structure can lead to enhanced fuel cell performance and durability.
- Composite electrolytes: Composite electrolytes combine different materials to improve overall fuel cell efficiency. These may include polymer-ceramic hybrids or multi-layer structures that enhance proton conductivity, mechanical strength, and thermal stability. Such composites can address limitations of single-component electrolytes and optimize fuel cell performance.
- Ionic liquid-based electrolytes: Ionic liquids are being explored as alternative electrolytes for fuel cells due to their high ionic conductivity and thermal stability. These non-volatile, non-flammable liquids can operate at higher temperatures and potentially improve fuel cell efficiency and safety compared to traditional aqueous electrolytes.
- Nanostructured electrolytes: Nanostructured electrolytes incorporate nanomaterials or nanostructures to enhance fuel cell efficiency. These can include nanoparticles, nanofibers, or nanoporous structures that increase the active surface area, improve ion transport, and enhance the overall electrochemical performance of the fuel cell.
- Solid oxide electrolytes: Solid oxide electrolytes are used in high-temperature fuel cells to improve efficiency. These ceramic-based electrolytes, such as yttria-stabilized zirconia, offer high oxygen ion conductivity at elevated temperatures. Research focuses on developing new materials and optimizing existing ones to lower operating temperatures while maintaining high efficiency.
02 Composite electrolytes
Composite electrolytes combine different materials to improve overall fuel cell efficiency. These may include polymer-ceramic composites or hybrid organic-inorganic materials. Such composites can offer benefits like improved mechanical strength, higher conductivity, and better water retention, leading to enhanced fuel cell performance across various operating conditions.Expand Specific Solutions03 Ionic liquid-based electrolytes
Ionic liquids are being explored as alternative electrolytes for fuel cells due to their unique properties. These include high ionic conductivity, low volatility, and wide electrochemical stability window. Incorporating ionic liquids into fuel cell electrolytes can potentially improve efficiency and expand the operating temperature range of fuel cells.Expand Specific Solutions04 Nanostructured electrolytes
Nanostructured electrolytes utilize nanomaterials to enhance fuel cell efficiency. These may include nanoparticle additives, nanofibers, or nanocomposites. The high surface area and unique properties of nanomaterials can lead to improved proton conductivity, better water management, and enhanced mechanical properties of the electrolyte.Expand Specific Solutions05 High-temperature electrolytes
Developing electrolytes for high-temperature fuel cells is crucial for improving overall system efficiency. These electrolytes must maintain stability and conductivity at elevated temperatures, often above 150°C. Materials such as phosphoric acid-doped polybenzimidazole (PBI) membranes or ceramic electrolytes are being investigated for this purpose, offering potential advantages in terms of fuel flexibility and heat management.Expand Specific Solutions
Key Players in Fuel Cell Electrolyte Research
The tautomerization in fuel cell electrolytes presents a complex competitive landscape in the energy sector. The industry is in a growth phase, with increasing market size driven by the global push for clean energy solutions. The technology's maturity is advancing, with key players like Toyota Motor Corp., Nissan Motor Co., and General Motors LLC leading research and development efforts. These automotive giants are investing heavily in fuel cell technology, particularly for electric vehicles. Additionally, companies such as Samsung SDI Co., Ltd. and Panasonic Holdings Corp. are contributing to the field through their expertise in battery and electronic materials. The involvement of diverse players, including chemical companies like DuPont de Nemours, Inc., indicates a multifaceted approach to addressing efficiency challenges in fuel cell electrolytes.
Toyota Motor Corp.
GM Global Technology Operations LLC
Innovative Approaches to Electrolyte Stability
- Development of particles capable of catalyzing oxygen reduction or hydrogen oxidation, functionalized with proton-conducting fluorinated polymers through ATRP polymerization, which are grafted onto the surface of catalysts to enhance electrochemical performance and prevent flooding.
- A nitrogen-doped phosphate tetrahedral network electrolyte is developed, comprising compounds such as H3+xPO4−xNx and H3+x−2yPO4−yNx, which include phosphoramidate, diamidophosphate, and phosphoramide, with optional substitution of phosphorus atoms with Si, B, and/or Al, forming a corner-sharing network that enhances proton conductivity and reduces electrostatic interactions.
Environmental Impact of Fuel Cell Technologies
Fuel cell technologies have gained significant attention as a promising alternative to conventional energy sources due to their potential for clean and efficient power generation. However, the environmental impact of these technologies must be carefully considered to ensure their sustainability and long-term viability.
One of the primary environmental benefits of fuel cell technologies is their potential to reduce greenhouse gas emissions. Unlike traditional combustion-based power generation, fuel cells produce electricity through electrochemical reactions, resulting in significantly lower carbon dioxide emissions. This characteristic makes fuel cells an attractive option for mitigating climate change and meeting increasingly stringent environmental regulations.
Water management is another crucial aspect of fuel cell environmental impact. While fuel cells produce water as a byproduct, the amount and purity of this water can vary depending on the type of fuel cell and its operating conditions. In some cases, the water produced can be recycled or used for other purposes, potentially reducing overall water consumption. However, the production and disposal of fuel cell components may still have water-related environmental implications that need to be addressed.
The manufacturing process of fuel cells and their components also contributes to their overall environmental footprint. The production of catalysts, membranes, and other materials used in fuel cells can involve energy-intensive processes and the use of rare or precious metals. As the industry scales up, it is essential to develop more sustainable manufacturing techniques and explore alternative materials to minimize the environmental impact of fuel cell production.
End-of-life considerations for fuel cell systems are becoming increasingly important as the technology matures. The recyclability and proper disposal of fuel cell components, particularly those containing precious metals or potentially hazardous materials, must be carefully managed to prevent negative environmental consequences. Developing effective recycling and disposal strategies will be crucial for ensuring the long-term sustainability of fuel cell technologies.
The environmental impact of fuel production and distribution for fuel cells must also be taken into account. While hydrogen fuel cells have the potential to be entirely emission-free at the point of use, the production of hydrogen itself can have significant environmental implications depending on the method used. Efforts to develop and implement more sustainable hydrogen production methods, such as electrolysis powered by renewable energy sources, will be critical in maximizing the environmental benefits of fuel cell technologies.
Economic Feasibility of Tautomerization Solutions
The economic feasibility of tautomerization solutions in fuel cell electrolytes is a critical factor in determining their potential for widespread adoption and commercialization. To assess this aspect, it is essential to consider both the costs associated with implementing tautomerization-based technologies and the potential benefits they offer in terms of improved fuel cell efficiency and performance.
From a cost perspective, the development and implementation of tautomerization solutions may require significant initial investments in research and development. This includes expenses related to laboratory equipment, materials, and personnel with specialized expertise in electrochemistry and fuel cell technology. Additionally, the integration of tautomerization-optimized electrolytes into existing fuel cell systems may necessitate modifications to manufacturing processes and supply chains, potentially increasing production costs in the short term.
However, the potential benefits of tautomerization solutions could outweigh these initial costs in the long run. Improved fuel cell efficiency resulting from optimized tautomerization processes can lead to reduced fuel consumption and increased power output. This translates to lower operating costs for end-users and potentially longer lifespans for fuel cell systems, enhancing their overall economic value proposition.
Furthermore, the implementation of tautomerization solutions may open up new market opportunities for fuel cell technologies. Enhanced efficiency and performance could make fuel cells more competitive in applications where they currently face challenges, such as in the automotive sector or large-scale stationary power generation. This expanded market potential could drive economies of scale, further reducing production costs and improving the economic feasibility of tautomerization-based solutions.
It is also important to consider the potential impact of tautomerization solutions on the broader fuel cell supply chain. Suppliers of electrolyte materials and fuel cell components may need to adapt their products to accommodate optimized tautomerization processes, potentially creating new revenue streams and business opportunities within the industry.
The economic feasibility of tautomerization solutions will likely vary depending on the specific application and scale of implementation. Large-scale industrial applications may see more immediate economic benefits due to the potential for significant energy savings, while smaller-scale or niche applications may require a longer timeframe to achieve economic viability.
In conclusion, while the initial costs of developing and implementing tautomerization solutions may be substantial, the potential long-term economic benefits in terms of improved fuel cell efficiency, expanded market opportunities, and reduced operating costs suggest that these solutions could be economically feasible. However, further research and real-world testing will be necessary to fully quantify the economic impact and determine the optimal pathways for commercialization.