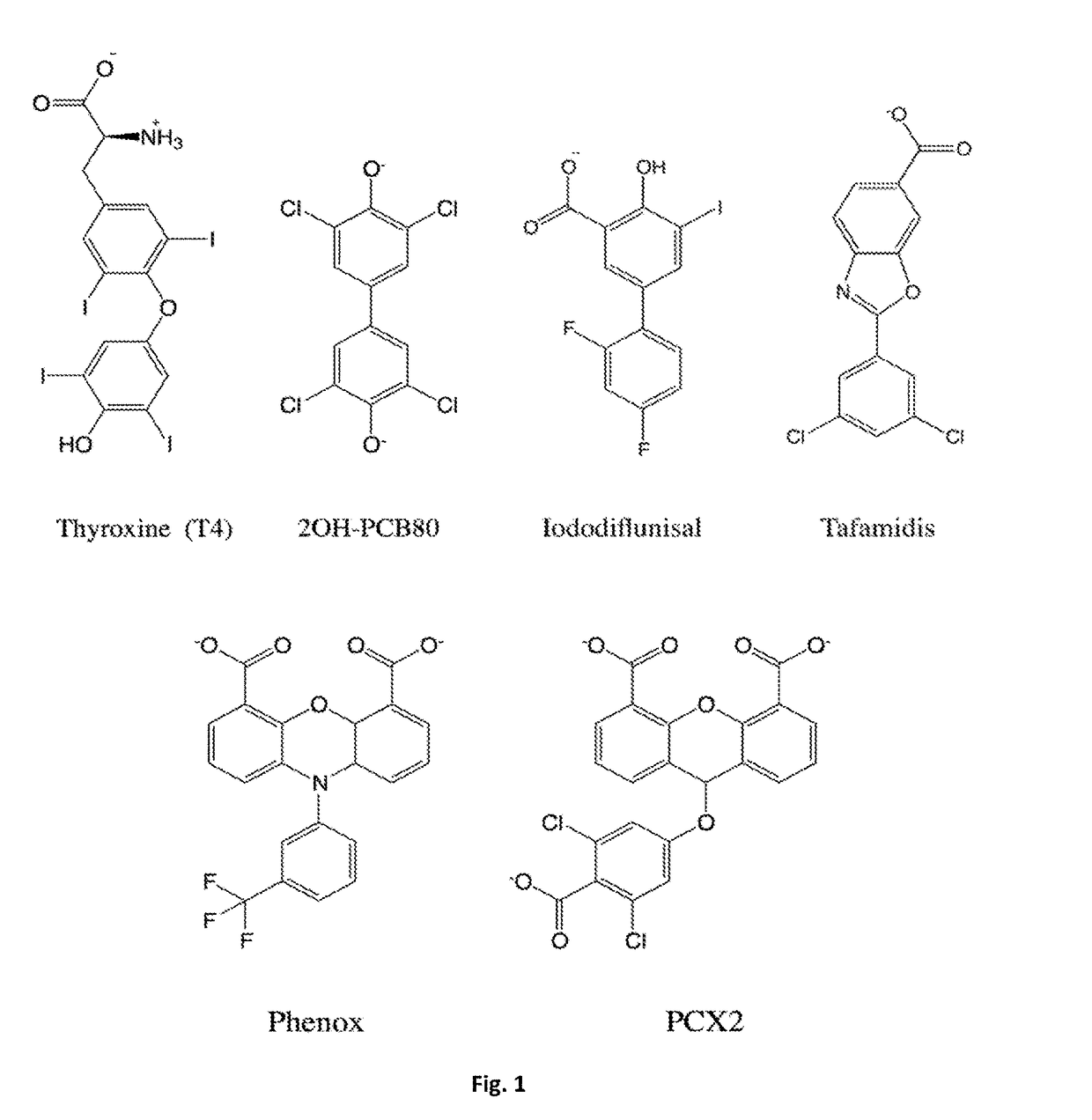
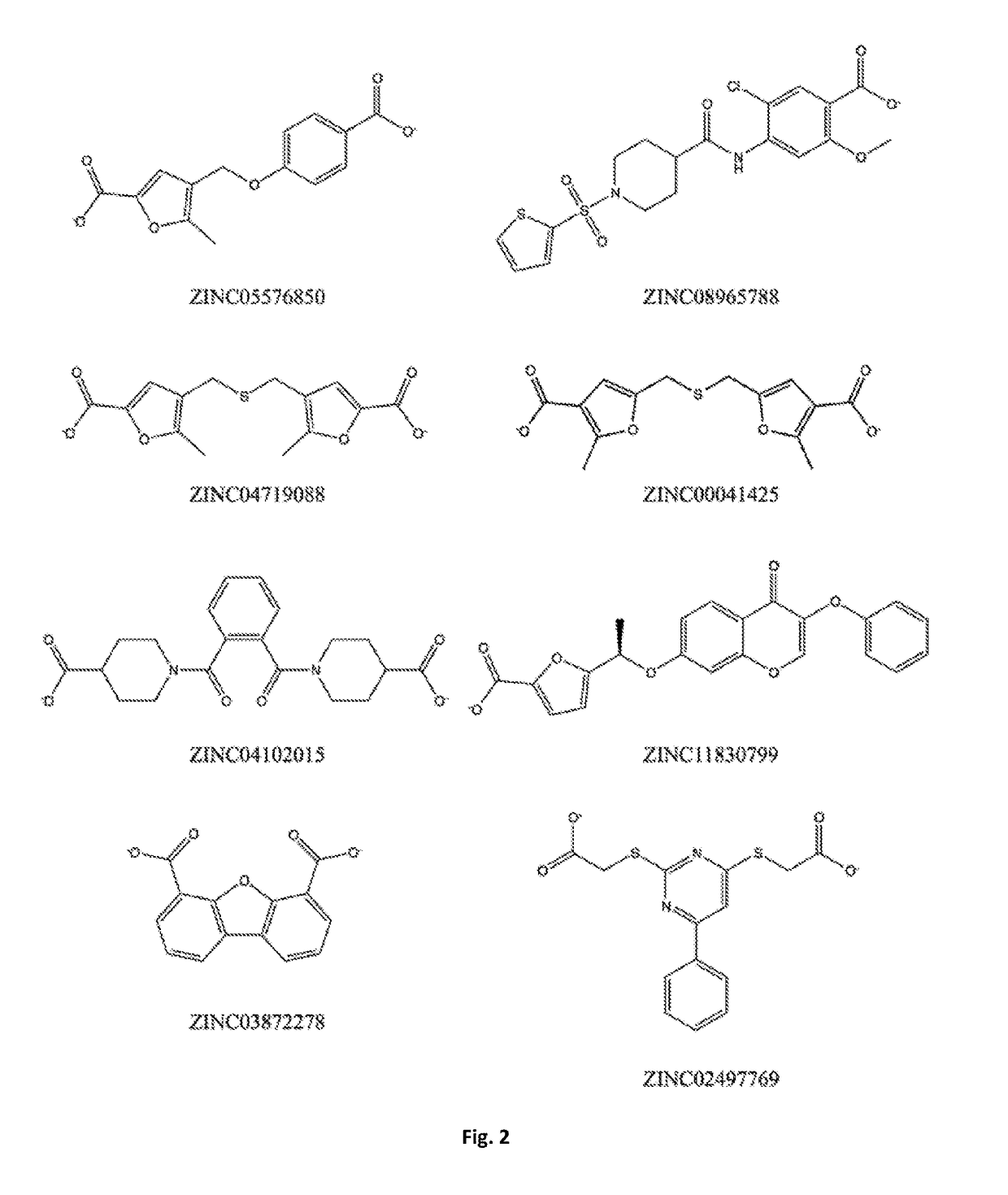
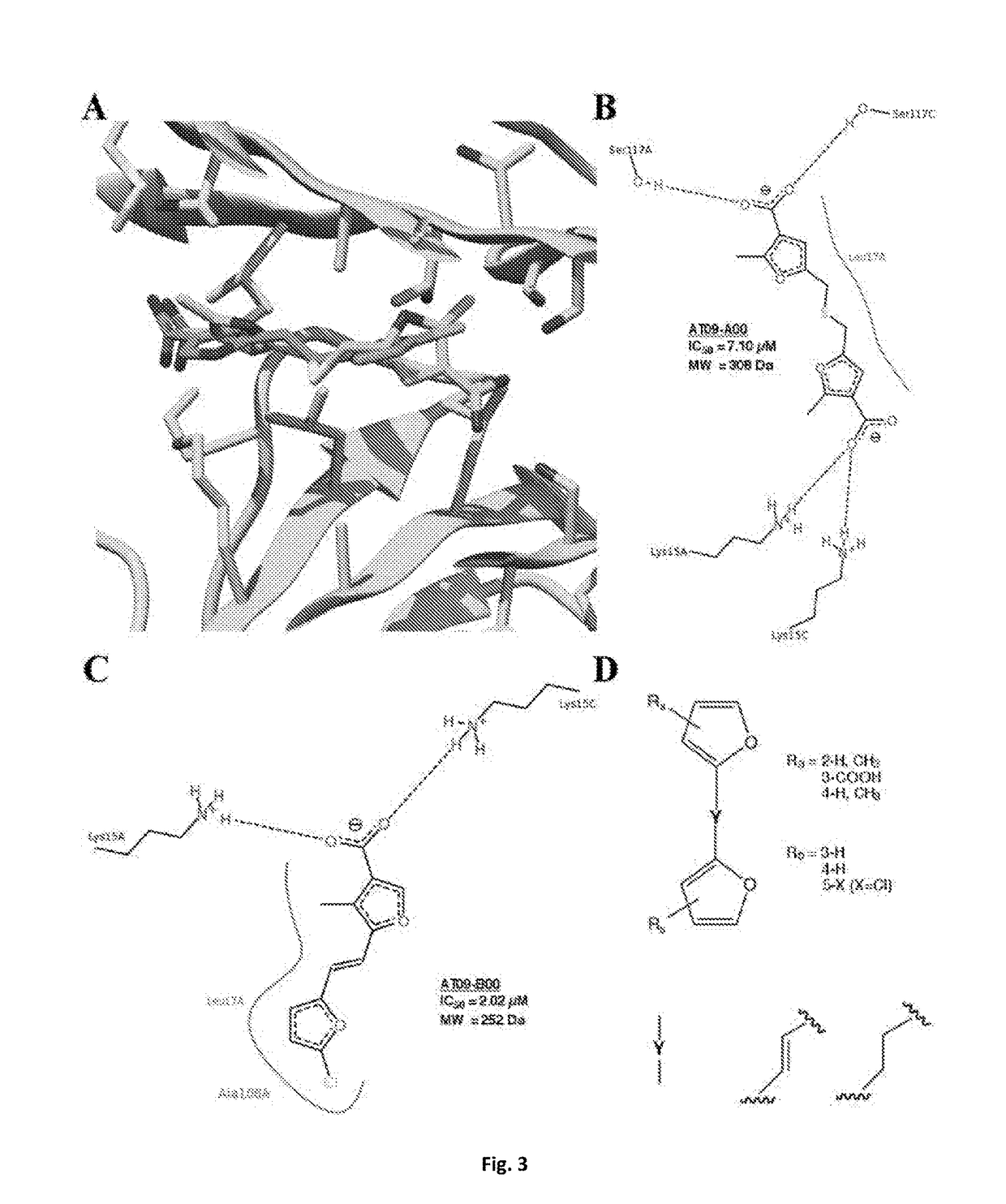
Does Tautomerization Affect Medicinal Chemistry Outcomes?
JUL 29, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Tautomerization in Drug Design: Background and Objectives
Tautomerization, a fundamental concept in organic chemistry, plays a crucial role in drug design and medicinal chemistry. This phenomenon involves the rapid interconversion between structural isomers, known as tautomers, which differ only in the position of a proton and a π bond. The study of tautomerization in drug design has gained significant attention over the past decades due to its potential impact on various aspects of drug discovery and development.
The historical context of tautomerization in medicinal chemistry dates back to the early 20th century when researchers first recognized its importance in biological systems. However, it wasn't until the advent of modern computational chemistry and advanced analytical techniques that scientists could fully appreciate the complexities and implications of tautomeric equilibria in drug-target interactions.
As the field of medicinal chemistry has evolved, so too has our understanding of how tautomerization can influence drug efficacy, bioavailability, and toxicity. The ability of a molecule to exist in multiple tautomeric forms can significantly affect its physicochemical properties, including solubility, lipophilicity, and pKa values. These properties, in turn, can impact a drug's absorption, distribution, metabolism, and excretion (ADME) profile.
The primary objective of studying tautomerization in drug design is to enhance the predictability and success rate of drug discovery efforts. By understanding the tautomeric behavior of potential drug candidates, researchers aim to optimize molecular structures for improved target binding, reduced off-target effects, and enhanced pharmacokinetic properties. This knowledge is particularly crucial in the early stages of drug development, where accurate predictions can save substantial time and resources.
Recent technological advancements have enabled more sophisticated approaches to analyzing and predicting tautomeric behavior. High-throughput screening methods, coupled with in silico modeling, have allowed researchers to explore vast chemical spaces and identify promising lead compounds while considering tautomeric effects. Moreover, the integration of artificial intelligence and machine learning algorithms has further enhanced our ability to predict and manipulate tautomeric equilibria in drug design.
As we delve deeper into the intricacies of tautomerization, it becomes evident that this phenomenon is not merely an academic curiosity but a critical factor in the successful development of new therapeutic agents. The ongoing research in this field aims to bridge the gap between theoretical understanding and practical application, ultimately leading to more effective and safer drugs for a wide range of diseases and conditions.
The historical context of tautomerization in medicinal chemistry dates back to the early 20th century when researchers first recognized its importance in biological systems. However, it wasn't until the advent of modern computational chemistry and advanced analytical techniques that scientists could fully appreciate the complexities and implications of tautomeric equilibria in drug-target interactions.
As the field of medicinal chemistry has evolved, so too has our understanding of how tautomerization can influence drug efficacy, bioavailability, and toxicity. The ability of a molecule to exist in multiple tautomeric forms can significantly affect its physicochemical properties, including solubility, lipophilicity, and pKa values. These properties, in turn, can impact a drug's absorption, distribution, metabolism, and excretion (ADME) profile.
The primary objective of studying tautomerization in drug design is to enhance the predictability and success rate of drug discovery efforts. By understanding the tautomeric behavior of potential drug candidates, researchers aim to optimize molecular structures for improved target binding, reduced off-target effects, and enhanced pharmacokinetic properties. This knowledge is particularly crucial in the early stages of drug development, where accurate predictions can save substantial time and resources.
Recent technological advancements have enabled more sophisticated approaches to analyzing and predicting tautomeric behavior. High-throughput screening methods, coupled with in silico modeling, have allowed researchers to explore vast chemical spaces and identify promising lead compounds while considering tautomeric effects. Moreover, the integration of artificial intelligence and machine learning algorithms has further enhanced our ability to predict and manipulate tautomeric equilibria in drug design.
As we delve deeper into the intricacies of tautomerization, it becomes evident that this phenomenon is not merely an academic curiosity but a critical factor in the successful development of new therapeutic agents. The ongoing research in this field aims to bridge the gap between theoretical understanding and practical application, ultimately leading to more effective and safer drugs for a wide range of diseases and conditions.
Market Demand for Tautomer-Aware Drug Discovery
The market demand for tautomer-aware drug discovery has been steadily increasing in recent years, driven by the growing recognition of tautomerism's impact on medicinal chemistry outcomes. Pharmaceutical companies and research institutions are increasingly seeking tools and methodologies that can accurately predict and account for tautomeric forms of drug candidates. This demand stems from the realization that overlooking tautomerism can lead to significant errors in predicting drug-target interactions, pharmacokinetics, and overall drug efficacy.
The global drug discovery market, valued at approximately $68 billion in 2020, is expected to grow at a CAGR of 8.9% through 2028. Within this market, there is a growing segment specifically focused on computational drug discovery and molecular modeling, where tautomer-aware technologies play a crucial role. This sub-segment is estimated to be worth several hundred million dollars and is experiencing rapid growth due to the increasing adoption of AI and machine learning in drug discovery processes.
Pharmaceutical companies are particularly interested in tautomer-aware drug discovery solutions to optimize their lead compound selection and reduce late-stage failures in clinical trials. The cost of bringing a new drug to market can exceed $2.6 billion, with a significant portion attributed to failed candidates. By incorporating tautomer awareness early in the drug discovery pipeline, companies aim to improve success rates and reduce overall development costs.
Academic research institutions and biotechnology startups are also contributing to the market demand. These entities are actively developing and seeking advanced computational tools that can accurately model tautomeric equilibria and predict their effects on drug-target interactions. The publication of research highlighting the importance of tautomerism in drug discovery has further fueled this demand, with an increasing number of scientific papers addressing the topic in recent years.
The market is also seeing a rise in collaborations between pharmaceutical companies and software developers specializing in molecular modeling and cheminformatics. These partnerships aim to create more sophisticated, tautomer-aware drug discovery platforms that integrate seamlessly into existing workflows. Such collaborations are indicative of the industry's recognition of the critical role tautomerism plays in drug development and the need for specialized tools to address it.
As the field of personalized medicine continues to advance, there is a growing demand for tautomer-aware technologies that can account for genetic variations affecting drug metabolism and efficacy. This trend is expected to further drive the market for sophisticated drug discovery tools that can predict tautomeric behavior across diverse patient populations.
The global drug discovery market, valued at approximately $68 billion in 2020, is expected to grow at a CAGR of 8.9% through 2028. Within this market, there is a growing segment specifically focused on computational drug discovery and molecular modeling, where tautomer-aware technologies play a crucial role. This sub-segment is estimated to be worth several hundred million dollars and is experiencing rapid growth due to the increasing adoption of AI and machine learning in drug discovery processes.
Pharmaceutical companies are particularly interested in tautomer-aware drug discovery solutions to optimize their lead compound selection and reduce late-stage failures in clinical trials. The cost of bringing a new drug to market can exceed $2.6 billion, with a significant portion attributed to failed candidates. By incorporating tautomer awareness early in the drug discovery pipeline, companies aim to improve success rates and reduce overall development costs.
Academic research institutions and biotechnology startups are also contributing to the market demand. These entities are actively developing and seeking advanced computational tools that can accurately model tautomeric equilibria and predict their effects on drug-target interactions. The publication of research highlighting the importance of tautomerism in drug discovery has further fueled this demand, with an increasing number of scientific papers addressing the topic in recent years.
The market is also seeing a rise in collaborations between pharmaceutical companies and software developers specializing in molecular modeling and cheminformatics. These partnerships aim to create more sophisticated, tautomer-aware drug discovery platforms that integrate seamlessly into existing workflows. Such collaborations are indicative of the industry's recognition of the critical role tautomerism plays in drug development and the need for specialized tools to address it.
As the field of personalized medicine continues to advance, there is a growing demand for tautomer-aware technologies that can account for genetic variations affecting drug metabolism and efficacy. This trend is expected to further drive the market for sophisticated drug discovery tools that can predict tautomeric behavior across diverse patient populations.
Current Challenges in Predicting Tautomeric Effects
Predicting the effects of tautomerization on medicinal chemistry outcomes remains a significant challenge in drug discovery and development. Tautomers, which are structural isomers that readily interconvert, can exhibit different physicochemical properties, potentially affecting drug-target interactions and pharmacokinetics. The current challenges in accurately predicting tautomeric effects stem from several factors, including the complexity of tautomeric equilibria in biological systems and limitations in computational methods.
One of the primary challenges is the dynamic nature of tautomerization in physiological conditions. The relative stability of tautomers can vary significantly depending on the environment, such as pH, temperature, and solvent effects. This variability makes it difficult to determine which tautomeric form is predominant or active at the target site. Moreover, the interconversion rates between tautomers can be rapid, further complicating the prediction of which form is responsible for the observed biological activity.
Computational methods, while advancing, still face limitations in accurately modeling tautomeric equilibria. Quantum mechanical calculations can provide insights into tautomer stability, but they are computationally expensive and often impractical for large-scale drug discovery efforts. Force field-based methods, which are more efficient, may not capture the subtle electronic effects that influence tautomerization. This gap in computational capabilities hinders the accurate prediction of tautomeric effects on binding affinities and ADME properties.
Another challenge lies in the lack of comprehensive experimental data on tautomeric equilibria for diverse chemical structures. While databases of tautomers exist, they are often limited in scope and may not cover the full range of chemical space relevant to drug discovery. This scarcity of empirical data makes it difficult to validate and improve computational models, creating a cycle of uncertainty in predictions.
The impact of tautomerization on protein-ligand interactions adds another layer of complexity. Different tautomeric forms can interact with target proteins in distinct ways, potentially leading to varied binding modes and affinities. Current docking and scoring methods often struggle to account for these tautomeric effects, potentially leading to inaccurate predictions of drug-target interactions and false positives or negatives in virtual screening campaigns.
Furthermore, the influence of tautomerization on ADME properties, such as solubility, permeability, and metabolic stability, is not fully understood. These properties are crucial for drug efficacy and safety, yet current predictive models often fail to adequately account for tautomeric effects, potentially leading to suboptimal candidate selection in early-stage drug discovery.
Addressing these challenges requires a multifaceted approach, combining advances in computational methods, experimental techniques, and data integration. Improved algorithms for tautomer enumeration and stability prediction, coupled with more accurate force fields and quantum mechanical methods, are needed. Additionally, expanding experimental databases of tautomeric equilibria and developing high-throughput methods for measuring tautomerization in biologically relevant conditions will be crucial for validating and refining predictive models.
One of the primary challenges is the dynamic nature of tautomerization in physiological conditions. The relative stability of tautomers can vary significantly depending on the environment, such as pH, temperature, and solvent effects. This variability makes it difficult to determine which tautomeric form is predominant or active at the target site. Moreover, the interconversion rates between tautomers can be rapid, further complicating the prediction of which form is responsible for the observed biological activity.
Computational methods, while advancing, still face limitations in accurately modeling tautomeric equilibria. Quantum mechanical calculations can provide insights into tautomer stability, but they are computationally expensive and often impractical for large-scale drug discovery efforts. Force field-based methods, which are more efficient, may not capture the subtle electronic effects that influence tautomerization. This gap in computational capabilities hinders the accurate prediction of tautomeric effects on binding affinities and ADME properties.
Another challenge lies in the lack of comprehensive experimental data on tautomeric equilibria for diverse chemical structures. While databases of tautomers exist, they are often limited in scope and may not cover the full range of chemical space relevant to drug discovery. This scarcity of empirical data makes it difficult to validate and improve computational models, creating a cycle of uncertainty in predictions.
The impact of tautomerization on protein-ligand interactions adds another layer of complexity. Different tautomeric forms can interact with target proteins in distinct ways, potentially leading to varied binding modes and affinities. Current docking and scoring methods often struggle to account for these tautomeric effects, potentially leading to inaccurate predictions of drug-target interactions and false positives or negatives in virtual screening campaigns.
Furthermore, the influence of tautomerization on ADME properties, such as solubility, permeability, and metabolic stability, is not fully understood. These properties are crucial for drug efficacy and safety, yet current predictive models often fail to adequately account for tautomeric effects, potentially leading to suboptimal candidate selection in early-stage drug discovery.
Addressing these challenges requires a multifaceted approach, combining advances in computational methods, experimental techniques, and data integration. Improved algorithms for tautomer enumeration and stability prediction, coupled with more accurate force fields and quantum mechanical methods, are needed. Additionally, expanding experimental databases of tautomeric equilibria and developing high-throughput methods for measuring tautomerization in biologically relevant conditions will be crucial for validating and refining predictive models.
Existing Methods for Tautomer Prediction and Analysis
01 Tautomerization in drug design and development
Tautomerization plays a crucial role in medicinal chemistry, affecting drug design and development. It can influence a compound's physicochemical properties, binding affinity, and biological activity. Understanding tautomeric equilibria is essential for predicting drug-target interactions and optimizing lead compounds.- Tautomerization in drug design and development: Tautomerization plays a crucial role in medicinal chemistry, affecting drug design and development. It can influence the physicochemical properties, binding affinity, and biological activity of drug molecules. Understanding tautomeric equilibria is essential for predicting drug-target interactions and optimizing lead compounds.
- Impact of tautomerization on drug metabolism: Tautomerization can significantly affect drug metabolism by altering the molecule's susceptibility to enzymatic reactions. This can lead to changes in bioavailability, half-life, and potential metabolic pathways of drugs. Consideration of tautomeric forms is crucial for predicting and optimizing drug metabolism profiles.
- Tautomerization in structure-based drug design: In structure-based drug design, accounting for tautomerization is essential for accurate molecular docking and virtual screening. Different tautomeric forms can exhibit varying binding modes and affinities to target proteins. Incorporating tautomeric considerations in computational models can improve the prediction of drug-target interactions and lead optimization.
- Analytical methods for tautomer characterization: Advanced analytical techniques are employed to characterize and quantify tautomeric forms of drug molecules. These methods include NMR spectroscopy, X-ray crystallography, and mass spectrometry. Accurate identification and measurement of tautomeric ratios are crucial for understanding structure-activity relationships and optimizing drug formulations.
- Tautomerization in prodrug design: Tautomerization can be exploited in prodrug design to improve drug delivery and efficacy. By designing molecules that undergo tautomerization under specific physiological conditions, it is possible to create prodrugs that are activated at the target site. This approach can enhance drug selectivity, reduce side effects, and improve overall therapeutic outcomes.
02 Impact of tautomerization on drug metabolism
Tautomerization can significantly affect drug metabolism by altering the compound's susceptibility to enzymatic reactions. This can lead to changes in bioavailability, half-life, and potential metabolic pathways. Consideration of tautomeric forms is crucial for predicting and optimizing drug pharmacokinetics.Expand Specific Solutions03 Tautomerization in structure-activity relationship studies
Tautomerization is an important factor in structure-activity relationship (SAR) studies. Different tautomeric forms can exhibit varying biological activities, affecting the interpretation of SAR data. Accurate representation and analysis of tautomers are essential for developing predictive models and designing more effective drugs.Expand Specific Solutions04 Computational methods for predicting tautomerization
Advanced computational methods have been developed to predict tautomerization in medicinal chemistry. These tools help researchers identify potential tautomeric forms, estimate their relative stabilities, and evaluate their impact on drug-like properties. Integration of these methods into drug discovery workflows can improve the efficiency of lead optimization.Expand Specific Solutions05 Tautomerization in drug formulation and stability
Tautomerization can affect drug formulation and stability, influencing factors such as solubility, crystal form, and shelf life. Understanding tautomeric behavior is crucial for developing stable pharmaceutical formulations and predicting potential issues in drug manufacturing and storage.Expand Specific Solutions
Key Players in Tautomer-Focused Drug Development
The tautomerization effect on medicinal chemistry outcomes is a complex and evolving field, currently in its growth phase. The market size is expanding as pharmaceutical companies increasingly recognize its importance in drug discovery and development. Technologically, it's progressing from basic research to practical applications, with varying levels of maturity across different aspects. Companies like Humanwell Healthcare, Sunshine Lake Pharma, and Galapagos NV are at the forefront, integrating tautomerization considerations into their drug design processes. Academic institutions such as MIT and Harvard, along with research centers like Dana-Farber Cancer Institute, are contributing significantly to advancing the fundamental understanding of tautomeric effects in medicinal chemistry.
President & Fellows of Harvard College
Technical Solution: Harvard researchers have developed a systems biology approach to assess the impact of tautomerization on medicinal chemistry outcomes. Their method integrates high-throughput screening, proteomics, and metabolomics to evaluate how tautomeric shifts affect drug-target interactions and metabolic profiles[14]. Harvard has also pioneered the use of cryo-EM techniques to visualize tautomeric states of drugs bound to their targets, providing unprecedented structural insights[15]. Additionally, they have developed machine learning algorithms to predict tautomer-dependent changes in ADME properties, enabling more accurate in silico prediction of drug efficacy and toxicity[16].
Strengths: Holistic systems biology approach; cutting-edge structural biology techniques for tautomer visualization. Weaknesses: High cost and complexity of integrated approach; may be challenging to implement in early-stage drug discovery.
Massachusetts Institute of Technology
Technical Solution: MIT researchers have developed a novel approach to address tautomerization in medicinal chemistry using dynamic covalent chemistry principles. Their method involves designing molecular scaffolds that can dynamically interconvert between tautomeric forms in response to specific cellular environments[8]. This approach allows for the creation of "smart" drugs that can adapt their tautomeric state to optimize target binding or improve pharmacokinetic properties[9]. MIT has also pioneered the use of time-resolved spectroscopy techniques to study tautomerization kinetics in biologically relevant conditions, providing crucial insights into the dynamic behavior of drug molecules in vivo[10].
Strengths: Innovative approach leveraging dynamic covalent chemistry; potential for environment-responsive drug design. Weaknesses: Complexity in predicting and controlling in vivo behavior; potential regulatory challenges for dynamic molecular entities.
Innovative Approaches to Tautomerization Modeling
Bis-furan derivatives as transthyretin (TTR) stabilizers and amyloid inhibitors for the treatment of familial amyloid polyneuropathy (FAP)
PatentActiveUS20180208570A1
Innovation
- Development of novel compounds, including bis-furan derivatives, that selectively bind to TTR, stabilize the native tetrameric form, and inhibit amyloid fibril formation, offering an alternative to existing treatments by enhancing TTR stability and reducing amyloid deposition.
Drug delivery compositions and uses thereof
PatentPendingAU2023266240A1
Innovation
- Development of targeted drug delivery systems using biomaterials like hydrogels, specifically hyaluronic acid, that combine immunomodulatory agents such as STING agonists, cytokines (e.g., IL-15 superagonist), and chemokines (e.g., CXCL9) to concentrate therapeutic agents at tumor sites, minimizing exposure to non-diseased tissues and enhancing antitumor immunity.
Regulatory Considerations for Tautomeric Drugs
Tautomerization presents unique challenges in the regulatory landscape of drug development and approval. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have specific guidelines and considerations for tautomeric drugs. These guidelines aim to ensure the safety, efficacy, and quality of pharmaceutical products that exhibit tautomerism.
One of the primary regulatory concerns is the accurate identification and characterization of all relevant tautomeric forms of a drug molecule. Manufacturers must provide comprehensive data on the tautomeric equilibrium, including the relative abundance of each tautomer under various physiological conditions. This information is crucial for assessing the drug's pharmacokinetic and pharmacodynamic properties, as different tautomers may exhibit varying biological activities.
Regulatory bodies also require detailed analytical methods for detecting and quantifying tautomers in drug substances and drug products. These methods must be validated to demonstrate their ability to accurately distinguish between tautomeric forms and measure their relative proportions. The stability of tautomeric equilibrium during manufacturing, storage, and administration is another critical aspect that regulators scrutinize.
In terms of drug labeling and nomenclature, regulatory agencies often require clear identification of the specific tautomeric form(s) present in the drug product. This information must be consistently represented across all regulatory documents, including the drug application, product labeling, and manufacturing specifications. In some cases, regulators may mandate the use of a specific tautomeric form or a defined mixture of tautomers to ensure batch-to-batch consistency and reproducible clinical outcomes.
The potential impact of tautomerization on drug-drug interactions and metabolism is another area of regulatory focus. Manufacturers must provide data on how tautomerization may affect the drug's interaction with metabolizing enzymes, transporters, and other drugs. This information is essential for assessing potential safety risks and determining appropriate dosing regimens.
Regulatory agencies also consider the intellectual property implications of tautomeric drugs. Patent applications and regulatory submissions must clearly define the tautomeric forms covered by the claims to avoid potential disputes and ensure proper protection of the drug's intellectual property.
In light of these regulatory considerations, pharmaceutical companies developing tautomeric drugs must adopt a proactive approach to address potential challenges early in the drug development process. This includes implementing robust analytical methods, conducting comprehensive tautomer characterization studies, and maintaining clear communication with regulatory authorities throughout the development and approval stages.
One of the primary regulatory concerns is the accurate identification and characterization of all relevant tautomeric forms of a drug molecule. Manufacturers must provide comprehensive data on the tautomeric equilibrium, including the relative abundance of each tautomer under various physiological conditions. This information is crucial for assessing the drug's pharmacokinetic and pharmacodynamic properties, as different tautomers may exhibit varying biological activities.
Regulatory bodies also require detailed analytical methods for detecting and quantifying tautomers in drug substances and drug products. These methods must be validated to demonstrate their ability to accurately distinguish between tautomeric forms and measure their relative proportions. The stability of tautomeric equilibrium during manufacturing, storage, and administration is another critical aspect that regulators scrutinize.
In terms of drug labeling and nomenclature, regulatory agencies often require clear identification of the specific tautomeric form(s) present in the drug product. This information must be consistently represented across all regulatory documents, including the drug application, product labeling, and manufacturing specifications. In some cases, regulators may mandate the use of a specific tautomeric form or a defined mixture of tautomers to ensure batch-to-batch consistency and reproducible clinical outcomes.
The potential impact of tautomerization on drug-drug interactions and metabolism is another area of regulatory focus. Manufacturers must provide data on how tautomerization may affect the drug's interaction with metabolizing enzymes, transporters, and other drugs. This information is essential for assessing potential safety risks and determining appropriate dosing regimens.
Regulatory agencies also consider the intellectual property implications of tautomeric drugs. Patent applications and regulatory submissions must clearly define the tautomeric forms covered by the claims to avoid potential disputes and ensure proper protection of the drug's intellectual property.
In light of these regulatory considerations, pharmaceutical companies developing tautomeric drugs must adopt a proactive approach to address potential challenges early in the drug development process. This includes implementing robust analytical methods, conducting comprehensive tautomer characterization studies, and maintaining clear communication with regulatory authorities throughout the development and approval stages.
Impact of Tautomerization on ADME Properties
Tautomerization, a dynamic equilibrium between structural isomers, plays a crucial role in determining the ADME (Absorption, Distribution, Metabolism, and Excretion) properties of drug molecules. This phenomenon can significantly impact the pharmacokinetic profile of potential drug candidates, influencing their overall efficacy and safety.
The absorption of a drug is often affected by tautomerization, as different tautomeric forms may exhibit varying solubility and permeability characteristics. For instance, the keto-enol tautomerism of certain compounds can alter their lipophilicity, potentially affecting their ability to cross biological membranes. This can lead to unexpected changes in oral bioavailability or tissue distribution patterns.
Distribution of drugs within the body can also be influenced by tautomerization. The interconversion between tautomers may affect protein binding affinities, as different structural forms can interact differently with plasma proteins or tissue receptors. This can result in altered drug distribution profiles and potentially impact the drug's therapeutic index.
Metabolism of drug molecules is another area where tautomerization can have significant consequences. Certain tautomeric forms may be more susceptible to enzymatic reactions, leading to altered metabolic pathways or rates. This can affect the drug's half-life, clearance, and the formation of active or toxic metabolites, all of which are critical factors in drug development and safety assessment.
Excretion processes can be affected by tautomerization as well. Changes in the molecule's polarity or charge distribution due to tautomeric shifts may influence renal clearance or biliary excretion rates. This can lead to unexpected pharmacokinetic profiles and potentially impact the drug's duration of action or accumulation in specific tissues.
Furthermore, tautomerization can complicate in vitro ADME studies and in silico predictions. The dynamic nature of tautomeric equilibria may lead to discrepancies between experimental results and computational models, necessitating careful consideration in drug design and optimization processes. Accurate prediction and control of tautomerization effects on ADME properties are essential for successful drug development and can significantly impact the selection of lead compounds in medicinal chemistry programs.
The absorption of a drug is often affected by tautomerization, as different tautomeric forms may exhibit varying solubility and permeability characteristics. For instance, the keto-enol tautomerism of certain compounds can alter their lipophilicity, potentially affecting their ability to cross biological membranes. This can lead to unexpected changes in oral bioavailability or tissue distribution patterns.
Distribution of drugs within the body can also be influenced by tautomerization. The interconversion between tautomers may affect protein binding affinities, as different structural forms can interact differently with plasma proteins or tissue receptors. This can result in altered drug distribution profiles and potentially impact the drug's therapeutic index.
Metabolism of drug molecules is another area where tautomerization can have significant consequences. Certain tautomeric forms may be more susceptible to enzymatic reactions, leading to altered metabolic pathways or rates. This can affect the drug's half-life, clearance, and the formation of active or toxic metabolites, all of which are critical factors in drug development and safety assessment.
Excretion processes can be affected by tautomerization as well. Changes in the molecule's polarity or charge distribution due to tautomeric shifts may influence renal clearance or biliary excretion rates. This can lead to unexpected pharmacokinetic profiles and potentially impact the drug's duration of action or accumulation in specific tissues.
Furthermore, tautomerization can complicate in vitro ADME studies and in silico predictions. The dynamic nature of tautomeric equilibria may lead to discrepancies between experimental results and computational models, necessitating careful consideration in drug design and optimization processes. Accurate prediction and control of tautomerization effects on ADME properties are essential for successful drug development and can significantly impact the selection of lead compounds in medicinal chemistry programs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!