Detection of Geometric Isomers in Pharmaceuticals Using NMR Spectroscopy
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
NMR Spectroscopy in Pharmaceutical Isomer Detection
Nuclear Magnetic Resonance (NMR) spectroscopy has emerged as a powerful analytical tool in the pharmaceutical industry, particularly for the detection and characterization of geometric isomers. This technique exploits the magnetic properties of certain atomic nuclei to provide detailed information about molecular structure and composition. In the context of pharmaceutical isomer detection, NMR spectroscopy offers unparalleled resolution and specificity.
The fundamental principle of NMR spectroscopy relies on the behavior of atomic nuclei with non-zero spin when placed in a strong magnetic field. These nuclei absorb and re-emit electromagnetic radiation at specific frequencies, which are influenced by their chemical environment. This phenomenon allows for the precise identification and quantification of different molecular structures, including geometric isomers.
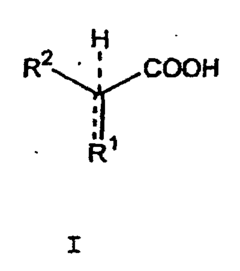
Geometric isomers, also known as cis-trans isomers, are compounds with the same molecular formula but different spatial arrangements of atoms. In pharmaceuticals, these isomers can exhibit significantly different biological activities, making their detection and characterization crucial for drug development and quality control. NMR spectroscopy provides a non-destructive method to distinguish between these isomers based on their unique spectral signatures.
One of the key advantages of NMR spectroscopy in isomer detection is its ability to provide structural information at the atomic level. Through various NMR experiments, such as 1D and 2D techniques, researchers can obtain detailed information about the connectivity, spatial relationships, and conformational properties of molecules. This level of detail is particularly valuable when dealing with complex pharmaceutical compounds.
Moreover, NMR spectroscopy offers quantitative analysis capabilities, allowing for the determination of isomer ratios in mixtures. This is essential for assessing the purity of pharmaceutical products and monitoring the efficiency of synthetic processes. The technique's high reproducibility and minimal sample preparation requirements further enhance its utility in routine quality control applications.
Recent advancements in NMR technology, including higher field strengths and improved probe designs, have significantly enhanced the sensitivity and resolution of the technique. These developments have expanded the range of applications in pharmaceutical analysis, enabling the detection of trace amounts of isomeric impurities and the study of more complex molecular systems.
In conclusion, NMR spectroscopy stands as a cornerstone technology in the detection of geometric isomers in pharmaceuticals. Its unique ability to provide detailed structural information, coupled with its quantitative capabilities and non-destructive nature, makes it an indispensable tool in drug discovery, development, and quality assurance processes.
The fundamental principle of NMR spectroscopy relies on the behavior of atomic nuclei with non-zero spin when placed in a strong magnetic field. These nuclei absorb and re-emit electromagnetic radiation at specific frequencies, which are influenced by their chemical environment. This phenomenon allows for the precise identification and quantification of different molecular structures, including geometric isomers.
Geometric isomers, also known as cis-trans isomers, are compounds with the same molecular formula but different spatial arrangements of atoms. In pharmaceuticals, these isomers can exhibit significantly different biological activities, making their detection and characterization crucial for drug development and quality control. NMR spectroscopy provides a non-destructive method to distinguish between these isomers based on their unique spectral signatures.
One of the key advantages of NMR spectroscopy in isomer detection is its ability to provide structural information at the atomic level. Through various NMR experiments, such as 1D and 2D techniques, researchers can obtain detailed information about the connectivity, spatial relationships, and conformational properties of molecules. This level of detail is particularly valuable when dealing with complex pharmaceutical compounds.
Moreover, NMR spectroscopy offers quantitative analysis capabilities, allowing for the determination of isomer ratios in mixtures. This is essential for assessing the purity of pharmaceutical products and monitoring the efficiency of synthetic processes. The technique's high reproducibility and minimal sample preparation requirements further enhance its utility in routine quality control applications.
Recent advancements in NMR technology, including higher field strengths and improved probe designs, have significantly enhanced the sensitivity and resolution of the technique. These developments have expanded the range of applications in pharmaceutical analysis, enabling the detection of trace amounts of isomeric impurities and the study of more complex molecular systems.
In conclusion, NMR spectroscopy stands as a cornerstone technology in the detection of geometric isomers in pharmaceuticals. Its unique ability to provide detailed structural information, coupled with its quantitative capabilities and non-destructive nature, makes it an indispensable tool in drug discovery, development, and quality assurance processes.
Market Demand for Isomer Analysis
The market demand for isomer analysis in pharmaceuticals has been steadily growing, driven by the increasing complexity of drug development and the stringent regulatory requirements for drug safety and efficacy. Pharmaceutical companies are increasingly recognizing the importance of identifying and characterizing geometric isomers in their products, as these structural variations can significantly impact the therapeutic properties and potential side effects of drugs.
The global pharmaceutical analytical testing outsourcing market, which includes isomer analysis services, was valued at $6.1 billion in 2020 and is projected to reach $11.4 billion by 2028, growing at a CAGR of 8.3% during this period. This growth is partly attributed to the rising demand for isomer analysis in drug development and quality control processes.
One of the key drivers for the market demand is the regulatory landscape. Regulatory bodies such as the FDA and EMA have implemented strict guidelines for the identification and quantification of isomers in pharmaceutical products. This has led to an increased need for advanced analytical techniques, including NMR spectroscopy, to detect and characterize geometric isomers accurately.
The pharmaceutical industry's shift towards more complex and targeted therapies has also contributed to the growing demand for isomer analysis. As drug molecules become more sophisticated, the presence of geometric isomers becomes more prevalent, necessitating robust analytical methods to ensure product quality and safety.
Moreover, the rising prevalence of chronic diseases and the subsequent increase in drug development activities have further fueled the demand for isomer analysis. Pharmaceutical companies are investing heavily in research and development to create novel drugs, many of which require thorough isomer characterization during the development process.
The generic drug market expansion has also played a role in driving the demand for isomer analysis. Generic drug manufacturers need to demonstrate bioequivalence to the original drug, which often involves detailed isomer characterization to ensure structural similarity and therapeutic equivalence.
In terms of geographical distribution, North America currently holds the largest market share for pharmaceutical analytical testing services, including isomer analysis. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by the increasing outsourcing of drug development and manufacturing activities to countries like India and China.
The COVID-19 pandemic has further highlighted the importance of rapid and accurate analytical techniques in drug development. This has led to an increased focus on advanced analytical methods, including NMR spectroscopy for isomer detection, to accelerate the drug development process while maintaining high-quality standards.
The global pharmaceutical analytical testing outsourcing market, which includes isomer analysis services, was valued at $6.1 billion in 2020 and is projected to reach $11.4 billion by 2028, growing at a CAGR of 8.3% during this period. This growth is partly attributed to the rising demand for isomer analysis in drug development and quality control processes.
One of the key drivers for the market demand is the regulatory landscape. Regulatory bodies such as the FDA and EMA have implemented strict guidelines for the identification and quantification of isomers in pharmaceutical products. This has led to an increased need for advanced analytical techniques, including NMR spectroscopy, to detect and characterize geometric isomers accurately.
The pharmaceutical industry's shift towards more complex and targeted therapies has also contributed to the growing demand for isomer analysis. As drug molecules become more sophisticated, the presence of geometric isomers becomes more prevalent, necessitating robust analytical methods to ensure product quality and safety.
Moreover, the rising prevalence of chronic diseases and the subsequent increase in drug development activities have further fueled the demand for isomer analysis. Pharmaceutical companies are investing heavily in research and development to create novel drugs, many of which require thorough isomer characterization during the development process.
The generic drug market expansion has also played a role in driving the demand for isomer analysis. Generic drug manufacturers need to demonstrate bioequivalence to the original drug, which often involves detailed isomer characterization to ensure structural similarity and therapeutic equivalence.
In terms of geographical distribution, North America currently holds the largest market share for pharmaceutical analytical testing services, including isomer analysis. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by the increasing outsourcing of drug development and manufacturing activities to countries like India and China.
The COVID-19 pandemic has further highlighted the importance of rapid and accurate analytical techniques in drug development. This has led to an increased focus on advanced analytical methods, including NMR spectroscopy for isomer detection, to accelerate the drug development process while maintaining high-quality standards.
Current Challenges in Geometric Isomer Detection
The detection of geometric isomers in pharmaceuticals using NMR spectroscopy faces several significant challenges that hinder its widespread application and reliability. One of the primary obstacles is the complexity of spectral interpretation, particularly in cases where the isomers have similar chemical structures. The subtle differences in chemical shifts and coupling patterns between geometric isomers can be difficult to discern, especially in complex molecular structures.
Another challenge lies in the sensitivity of NMR spectroscopy when dealing with low concentrations of geometric isomers. In pharmaceutical formulations, the presence of impurities or minor isomeric forms may be at trace levels, pushing the limits of NMR detection capabilities. This issue is compounded by potential signal overlap from other components in the drug formulation, making it challenging to isolate and quantify specific isomeric signals.
The need for high-resolution NMR spectrometers presents both a technical and economic challenge. While higher field strengths can improve spectral resolution and sensitivity, they also increase the cost and complexity of the instrumentation. This can limit the accessibility of advanced NMR techniques in some pharmaceutical research and quality control settings.
Sample preparation is another critical factor that can impact the accuracy of geometric isomer detection. Ensuring consistent and reproducible sample conditions, including concentration, pH, and solvent effects, is crucial for reliable NMR analysis. Any variations in these parameters can lead to shifts in spectral peaks, potentially masking or misrepresenting the presence of geometric isomers.
The development of robust and standardized methodologies for data analysis and interpretation remains a challenge. The complexity of NMR data often requires sophisticated software and skilled analysts to accurately identify and quantify geometric isomers. This dependency on expert interpretation can introduce variability and potential errors in the analysis process.
Furthermore, the time-consuming nature of NMR experiments, especially for complex mixtures or when multiple nuclei need to be analyzed, can be a limiting factor in high-throughput pharmaceutical testing environments. This challenge is particularly relevant in quality control processes where rapid and efficient analysis is crucial.
Lastly, the differentiation between geometric isomers that have very similar NMR spectra continues to be a significant challenge. In some cases, additional analytical techniques may be required to complement NMR data, adding complexity and time to the analysis process. Overcoming these challenges is crucial for enhancing the reliability and applicability of NMR spectroscopy in the detection of geometric isomers in pharmaceuticals.
Another challenge lies in the sensitivity of NMR spectroscopy when dealing with low concentrations of geometric isomers. In pharmaceutical formulations, the presence of impurities or minor isomeric forms may be at trace levels, pushing the limits of NMR detection capabilities. This issue is compounded by potential signal overlap from other components in the drug formulation, making it challenging to isolate and quantify specific isomeric signals.
The need for high-resolution NMR spectrometers presents both a technical and economic challenge. While higher field strengths can improve spectral resolution and sensitivity, they also increase the cost and complexity of the instrumentation. This can limit the accessibility of advanced NMR techniques in some pharmaceutical research and quality control settings.
Sample preparation is another critical factor that can impact the accuracy of geometric isomer detection. Ensuring consistent and reproducible sample conditions, including concentration, pH, and solvent effects, is crucial for reliable NMR analysis. Any variations in these parameters can lead to shifts in spectral peaks, potentially masking or misrepresenting the presence of geometric isomers.
The development of robust and standardized methodologies for data analysis and interpretation remains a challenge. The complexity of NMR data often requires sophisticated software and skilled analysts to accurately identify and quantify geometric isomers. This dependency on expert interpretation can introduce variability and potential errors in the analysis process.
Furthermore, the time-consuming nature of NMR experiments, especially for complex mixtures or when multiple nuclei need to be analyzed, can be a limiting factor in high-throughput pharmaceutical testing environments. This challenge is particularly relevant in quality control processes where rapid and efficient analysis is crucial.
Lastly, the differentiation between geometric isomers that have very similar NMR spectra continues to be a significant challenge. In some cases, additional analytical techniques may be required to complement NMR data, adding complexity and time to the analysis process. Overcoming these challenges is crucial for enhancing the reliability and applicability of NMR spectroscopy in the detection of geometric isomers in pharmaceuticals.
Existing NMR Methods for Isomer Identification
01 Advanced NMR detection techniques
This category focuses on innovative NMR detection methods that enhance sensitivity and resolution. These techniques may include novel pulse sequences, advanced signal processing algorithms, or specialized hardware configurations designed to improve the quality of NMR spectroscopy data.- Advanced NMR detection techniques: Various advanced techniques have been developed to enhance NMR spectroscopy detection. These include improved pulse sequences, signal processing methods, and specialized hardware components. Such advancements allow for increased sensitivity, better resolution, and the ability to detect and analyze complex molecular structures more effectively.
- NMR-based molecular imaging and diagnostics: NMR spectroscopy is increasingly used for molecular imaging and diagnostic applications. This includes the development of contrast agents, targeted molecular probes, and methods for non-invasive detection of diseases or metabolic processes. These techniques often combine NMR with other imaging modalities to provide comprehensive diagnostic information.
- Portable and miniaturized NMR systems: Efforts have been made to develop portable and miniaturized NMR systems for on-site or point-of-care applications. These systems often involve innovative magnet designs, compact electronics, and specialized probes to achieve NMR capabilities in smaller, more accessible formats. Such developments expand the potential applications of NMR spectroscopy beyond traditional laboratory settings.
- Hyperpolarization techniques for NMR signal enhancement: Hyperpolarization methods have been developed to dramatically increase NMR signal intensity. These techniques involve creating non-equilibrium spin states that can enhance NMR sensitivity by several orders of magnitude. Various approaches, including dynamic nuclear polarization and parahydrogen-induced polarization, are being explored to improve the detection of low-concentration analytes and accelerate data acquisition.
- NMR-based chemical and biological sensors: NMR spectroscopy is being utilized to develop highly sensitive and selective chemical and biological sensors. These sensors often incorporate functionalized nanoparticles, specialized probe designs, or microfluidic systems to detect specific analytes or biological markers. Such NMR-based sensors offer advantages in terms of sensitivity, specificity, and the ability to perform label-free detection in complex sample matrices.
02 NMR spectroscopy for biological samples
This area covers the application of NMR spectroscopy in analyzing biological samples, such as proteins, metabolites, and other biomolecules. It includes methods for sample preparation, data acquisition, and interpretation specifically tailored for biological specimens.Expand Specific Solutions03 Portable and miniaturized NMR systems
This category encompasses the development of compact, portable NMR spectrometers and detectors. These systems aim to bring NMR technology out of the laboratory and into field applications, enabling on-site analysis in various industries and research settings.Expand Specific Solutions04 Hyperpolarization techniques for NMR
This point covers methods to enhance NMR signal intensity through hyperpolarization of nuclear spins. Techniques such as dynamic nuclear polarization (DNP) and parahydrogen-induced polarization (PHIP) are used to dramatically increase sensitivity and enable detection of low-concentration samples.Expand Specific Solutions05 NMR data processing and analysis software
This category focuses on software tools and algorithms for processing and analyzing NMR spectroscopy data. It includes advanced signal processing techniques, automated peak assignment, and integration with other analytical methods to extract meaningful information from complex NMR spectra.Expand Specific Solutions
Key Players in NMR Spectroscopy
The detection of geometric isomers in pharmaceuticals using NMR spectroscopy is a critical area in the pharmaceutical industry, currently in a mature stage of development. The market for this technology is substantial, driven by the increasing demand for high-quality drug manufacturing and quality control. Companies like AbbVie, Laboratorios Farmaceúticos Rovi, and GlaxoSmithKline are actively utilizing this technology, while specialized firms such as Bruker BioSpin Corp. and Valisure LLC are at the forefront of developing advanced NMR spectroscopy solutions. The technology's maturity is evident in its widespread adoption across the pharmaceutical sector, with ongoing research focused on enhancing sensitivity and resolution for more complex molecular structures.
Revvity Health Sciences, Inc.
Technical Solution: Revvity Health Sciences, Inc. has developed an innovative NMR-based approach for the detection of geometric isomers in pharmaceuticals, focusing on high-throughput screening and quality control applications. Their method utilizes benchtop NMR spectrometers combined with advanced pulse sequences and data analysis algorithms [13]. This approach allows for rapid and cost-effective isomer detection in pharmaceutical manufacturing settings. Revvity has also implemented flow-through NMR techniques, enabling real-time monitoring of isomerization processes during drug synthesis [14]. To enhance the sensitivity and specificity of their NMR-based isomer detection, they have developed novel NMR probe designs optimized for pharmaceutical samples, including microcoil and cryogenic probes [15].
Strengths: Rapid analysis, cost-effective for routine quality control, real-time monitoring capabilities. Weaknesses: Lower spectral resolution compared to high-field NMR systems, may be less suitable for complex structural elucidation.
Glaxo Group Ltd.
Technical Solution: Glaxo Group Ltd. has implemented a comprehensive NMR-based approach for detecting geometric isomers in their pharmaceutical products. Their method combines traditional 1D 1H and 13C NMR spectroscopy with advanced 2D techniques such as COSY, HSQC, and NOESY [4]. This multi-dimensional approach allows for unambiguous identification and quantification of geometric isomers, even in complex drug formulations. Glaxo has also developed proprietary software algorithms for automated spectral analysis and isomer quantification, significantly reducing analysis time and improving reproducibility [5]. Additionally, they have integrated their NMR-based isomer detection methods with other analytical techniques like HPLC and mass spectrometry for a more robust characterization of pharmaceutical compounds [6].
Strengths: Comprehensive multi-dimensional approach, proprietary data analysis software, integration with other analytical techniques. Weaknesses: Potentially longer analysis times for complex samples, requires high-end NMR instrumentation.
Innovations in NMR for Geometric Isomers
Site-specific isotopically-labeled proteins, amino acids, and biochemical precursors therefor
PatentInactiveEP1169282B1
Innovation
- Site-specific isotopic labeling of amino acids like leucine, isoleucine, and valine with 13C or 14C at non-adjacent carbon atoms, allowing for the production of proteins and polypeptides that minimize spin-spin interactions, enhancing NMR signal clarity and reducing production costs.
Method for verifying the correct spatial structure of molecules by means of NMR spectroscopy
PatentWO2005052626A1
Innovation
- A method utilizing standardized NMR spectroscopy to compare the three-dimensional structure of test molecules with a reference molecule, involving protocol-defined sample preparation and data processing to quantify the proportion of molecules with the correct structure, allowing for automatic discrimination of samples based on structural integrity.
Regulatory Aspects of Pharmaceutical Isomer Detection
The regulatory landscape surrounding the detection of geometric isomers in pharmaceuticals is complex and evolving. Regulatory bodies worldwide, including the FDA, EMA, and ICH, have established guidelines and requirements for the identification and characterization of isomers in drug substances and products. These regulations aim to ensure the safety, efficacy, and quality of pharmaceutical products.
One of the primary regulatory considerations is the need for accurate and reliable methods to detect and quantify geometric isomers. NMR spectroscopy has emerged as a powerful tool in this regard, offering high specificity and sensitivity. Regulatory agencies often require pharmaceutical companies to demonstrate the capability of their analytical methods to distinguish between geometric isomers and accurately determine their relative proportions.
The ICH Q3A(R2) guideline on impurities in new drug substances addresses the reporting, identification, and qualification thresholds for isomers. This guideline stipulates that geometric isomers should be considered as impurities when they are not part of the desired product. Consequently, pharmaceutical companies must develop and validate analytical methods, such as NMR spectroscopy, to detect and quantify these isomers at levels below the specified thresholds.
Regulatory bodies also emphasize the importance of understanding the potential impact of geometric isomers on drug safety and efficacy. The FDA's guidance on stereoisomeric drugs highlights the need for comprehensive characterization of isomeric composition and its potential effects on pharmacological activity. This requirement has led to increased scrutiny of isomeric purity in drug development and manufacturing processes.
In the context of NMR spectroscopy for isomer detection, regulatory agencies expect validated methods that demonstrate specificity, accuracy, precision, and robustness. Method validation protocols must adhere to guidelines such as ICH Q2(R1) on analytical method validation. Companies are required to provide detailed documentation of their NMR-based methods, including sample preparation procedures, instrument parameters, and data analysis techniques.
The regulatory landscape also encompasses stability testing requirements. ICH Q1A(R2) guideline on stability testing of new drug substances and products mandates the evaluation of isomeric composition throughout the product's shelf life. NMR spectroscopy plays a crucial role in monitoring potential isomerization during storage and establishing appropriate expiration dates.
As the field of pharmaceutical isomer detection continues to advance, regulatory agencies are likely to update their guidelines to incorporate new technologies and methodologies. Companies engaged in drug development and manufacturing must stay abreast of these regulatory developments and ensure their analytical approaches, including NMR spectroscopy-based methods, remain compliant with evolving standards.
One of the primary regulatory considerations is the need for accurate and reliable methods to detect and quantify geometric isomers. NMR spectroscopy has emerged as a powerful tool in this regard, offering high specificity and sensitivity. Regulatory agencies often require pharmaceutical companies to demonstrate the capability of their analytical methods to distinguish between geometric isomers and accurately determine their relative proportions.
The ICH Q3A(R2) guideline on impurities in new drug substances addresses the reporting, identification, and qualification thresholds for isomers. This guideline stipulates that geometric isomers should be considered as impurities when they are not part of the desired product. Consequently, pharmaceutical companies must develop and validate analytical methods, such as NMR spectroscopy, to detect and quantify these isomers at levels below the specified thresholds.
Regulatory bodies also emphasize the importance of understanding the potential impact of geometric isomers on drug safety and efficacy. The FDA's guidance on stereoisomeric drugs highlights the need for comprehensive characterization of isomeric composition and its potential effects on pharmacological activity. This requirement has led to increased scrutiny of isomeric purity in drug development and manufacturing processes.
In the context of NMR spectroscopy for isomer detection, regulatory agencies expect validated methods that demonstrate specificity, accuracy, precision, and robustness. Method validation protocols must adhere to guidelines such as ICH Q2(R1) on analytical method validation. Companies are required to provide detailed documentation of their NMR-based methods, including sample preparation procedures, instrument parameters, and data analysis techniques.
The regulatory landscape also encompasses stability testing requirements. ICH Q1A(R2) guideline on stability testing of new drug substances and products mandates the evaluation of isomeric composition throughout the product's shelf life. NMR spectroscopy plays a crucial role in monitoring potential isomerization during storage and establishing appropriate expiration dates.
As the field of pharmaceutical isomer detection continues to advance, regulatory agencies are likely to update their guidelines to incorporate new technologies and methodologies. Companies engaged in drug development and manufacturing must stay abreast of these regulatory developments and ensure their analytical approaches, including NMR spectroscopy-based methods, remain compliant with evolving standards.
Economic Impact of Isomer Differentiation
The economic impact of isomer differentiation in pharmaceuticals is substantial and multifaceted. Accurate detection and characterization of geometric isomers using NMR spectroscopy can significantly influence drug development, manufacturing processes, and regulatory compliance, ultimately affecting the pharmaceutical industry's bottom line.
In drug development, the ability to distinguish between geometric isomers is crucial for ensuring the efficacy and safety of new compounds. NMR spectroscopy enables researchers to identify and quantify different isomers early in the development process, potentially saving millions of dollars in research and development costs. By identifying unfavorable isomers early, companies can avoid investing resources in candidates that may fail in later stages of clinical trials due to unexpected side effects or reduced efficacy.
The manufacturing sector of the pharmaceutical industry also benefits greatly from precise isomer differentiation. NMR spectroscopy allows for the optimization of synthesis processes, ensuring that the desired isomer is produced in higher yields. This increased efficiency can lead to substantial cost savings in large-scale production, as well as reduce waste and environmental impact. Moreover, the ability to consistently produce the correct isomeric form is essential for maintaining product quality and meeting regulatory standards.
From a regulatory perspective, the economic implications of isomer differentiation are significant. Regulatory bodies such as the FDA require comprehensive characterization of drug substances, including their isomeric composition. NMR spectroscopy provides a robust method for meeting these requirements, potentially expediting the approval process and reducing the risk of costly delays or rejections. This can translate into faster time-to-market for new drugs, providing pharmaceutical companies with a competitive edge and extended patent protection periods.
The market value of drugs can also be directly influenced by isomer differentiation. In some cases, specific isomers may demonstrate superior therapeutic properties or fewer side effects. The ability to isolate and market these advantageous isomers as separate products can lead to premium pricing and extended market exclusivity. This strategy, known as "chiral switching," has been successfully employed by several pharmaceutical companies to extend the lifecycle of their products and generate additional revenue streams.
Furthermore, the economic impact extends to the realm of generic drug manufacturing. As patents expire on brand-name drugs, the ability to accurately reproduce the isomeric composition of the original product becomes critical for generic manufacturers. NMR spectroscopy plays a vital role in ensuring bioequivalence and can be a deciding factor in the success or failure of generic drug applications, potentially worth billions of dollars in market share.
In conclusion, the economic ramifications of isomer differentiation using NMR spectroscopy permeate throughout the pharmaceutical value chain, from initial research to market competition. The technology's ability to provide precise isomeric information not only drives innovation and efficiency but also ensures regulatory compliance and product quality, ultimately contributing to the industry's economic sustainability and growth.
In drug development, the ability to distinguish between geometric isomers is crucial for ensuring the efficacy and safety of new compounds. NMR spectroscopy enables researchers to identify and quantify different isomers early in the development process, potentially saving millions of dollars in research and development costs. By identifying unfavorable isomers early, companies can avoid investing resources in candidates that may fail in later stages of clinical trials due to unexpected side effects or reduced efficacy.
The manufacturing sector of the pharmaceutical industry also benefits greatly from precise isomer differentiation. NMR spectroscopy allows for the optimization of synthesis processes, ensuring that the desired isomer is produced in higher yields. This increased efficiency can lead to substantial cost savings in large-scale production, as well as reduce waste and environmental impact. Moreover, the ability to consistently produce the correct isomeric form is essential for maintaining product quality and meeting regulatory standards.
From a regulatory perspective, the economic implications of isomer differentiation are significant. Regulatory bodies such as the FDA require comprehensive characterization of drug substances, including their isomeric composition. NMR spectroscopy provides a robust method for meeting these requirements, potentially expediting the approval process and reducing the risk of costly delays or rejections. This can translate into faster time-to-market for new drugs, providing pharmaceutical companies with a competitive edge and extended patent protection periods.
The market value of drugs can also be directly influenced by isomer differentiation. In some cases, specific isomers may demonstrate superior therapeutic properties or fewer side effects. The ability to isolate and market these advantageous isomers as separate products can lead to premium pricing and extended market exclusivity. This strategy, known as "chiral switching," has been successfully employed by several pharmaceutical companies to extend the lifecycle of their products and generate additional revenue streams.
Furthermore, the economic impact extends to the realm of generic drug manufacturing. As patents expire on brand-name drugs, the ability to accurately reproduce the isomeric composition of the original product becomes critical for generic manufacturers. NMR spectroscopy plays a vital role in ensuring bioequivalence and can be a deciding factor in the success or failure of generic drug applications, potentially worth billions of dollars in market share.
In conclusion, the economic ramifications of isomer differentiation using NMR spectroscopy permeate throughout the pharmaceutical value chain, from initial research to market competition. The technology's ability to provide precise isomeric information not only drives innovation and efficiency but also ensures regulatory compliance and product quality, ultimately contributing to the industry's economic sustainability and growth.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!