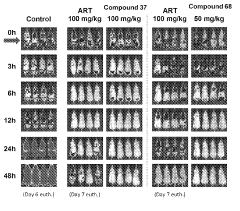
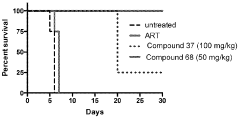
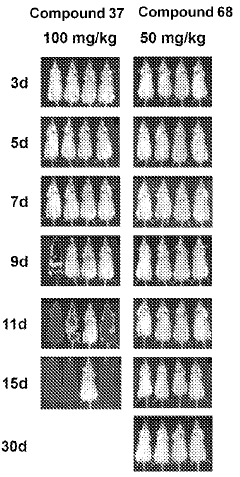
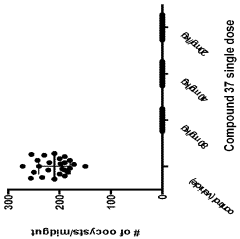
Influence of Geometric Isomers on the Antioxidant Pathways
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Geometric Isomers and Antioxidant Mechanisms
Geometric isomers play a crucial role in the antioxidant mechanisms of various compounds. These structural variations, characterized by the same molecular formula but different spatial arrangements of atoms, can significantly influence the antioxidant properties and pathways of molecules. The study of geometric isomers in relation to antioxidant activity has gained considerable attention in recent years due to its potential applications in pharmaceuticals, food science, and materials engineering.
The antioxidant mechanisms of geometric isomers primarily involve electron or hydrogen atom transfer processes. These processes are heavily influenced by the spatial configuration of the molecule, which affects its ability to interact with free radicals and other reactive species. For instance, in the case of carotenoids, the cis and trans isomers exhibit different antioxidant capacities due to their varying molecular geometries. The trans isomers generally demonstrate higher antioxidant activity compared to their cis counterparts, attributed to their extended conjugated system and enhanced ability to quench singlet oxygen.
The influence of geometric isomerism on antioxidant pathways is particularly evident in the case of phenolic compounds. The spatial arrangement of hydroxyl groups in these molecules can significantly impact their ability to donate hydrogen atoms or electrons to free radicals. For example, in the case of resveratrol, the trans isomer has been found to exhibit superior antioxidant properties compared to the cis isomer. This difference is attributed to the more planar structure of the trans isomer, which allows for better electron delocalization and enhanced radical scavenging ability.
Furthermore, geometric isomerism can affect the bioavailability and cellular uptake of antioxidant compounds, thereby influencing their overall efficacy. The spatial configuration of a molecule can determine its ability to cross cell membranes, interact with cellular receptors, and reach specific cellular compartments where antioxidant activity is most needed. For instance, the cis isomers of certain carotenoids have been shown to have improved bioavailability compared to their trans counterparts, potentially leading to enhanced antioxidant effects in vivo.
The study of geometric isomers and their influence on antioxidant pathways has also led to the development of novel antioxidant strategies. Researchers are exploring the possibility of designing synthetic antioxidants with specific geometric configurations to optimize their antioxidant activity and target specific cellular processes. This approach holds promise for the development of more effective and targeted antioxidant therapies for various oxidative stress-related conditions.
In conclusion, the influence of geometric isomers on antioxidant pathways is a complex and multifaceted phenomenon that encompasses structural, electronic, and biological aspects. Understanding these relationships is crucial for the rational design of antioxidant compounds and the optimization of their therapeutic potential. As research in this field continues to advance, it is likely to yield new insights into the fundamental mechanisms of antioxidant activity and pave the way for innovative applications across multiple industries.
The antioxidant mechanisms of geometric isomers primarily involve electron or hydrogen atom transfer processes. These processes are heavily influenced by the spatial configuration of the molecule, which affects its ability to interact with free radicals and other reactive species. For instance, in the case of carotenoids, the cis and trans isomers exhibit different antioxidant capacities due to their varying molecular geometries. The trans isomers generally demonstrate higher antioxidant activity compared to their cis counterparts, attributed to their extended conjugated system and enhanced ability to quench singlet oxygen.
The influence of geometric isomerism on antioxidant pathways is particularly evident in the case of phenolic compounds. The spatial arrangement of hydroxyl groups in these molecules can significantly impact their ability to donate hydrogen atoms or electrons to free radicals. For example, in the case of resveratrol, the trans isomer has been found to exhibit superior antioxidant properties compared to the cis isomer. This difference is attributed to the more planar structure of the trans isomer, which allows for better electron delocalization and enhanced radical scavenging ability.
Furthermore, geometric isomerism can affect the bioavailability and cellular uptake of antioxidant compounds, thereby influencing their overall efficacy. The spatial configuration of a molecule can determine its ability to cross cell membranes, interact with cellular receptors, and reach specific cellular compartments where antioxidant activity is most needed. For instance, the cis isomers of certain carotenoids have been shown to have improved bioavailability compared to their trans counterparts, potentially leading to enhanced antioxidant effects in vivo.
The study of geometric isomers and their influence on antioxidant pathways has also led to the development of novel antioxidant strategies. Researchers are exploring the possibility of designing synthetic antioxidants with specific geometric configurations to optimize their antioxidant activity and target specific cellular processes. This approach holds promise for the development of more effective and targeted antioxidant therapies for various oxidative stress-related conditions.
In conclusion, the influence of geometric isomers on antioxidant pathways is a complex and multifaceted phenomenon that encompasses structural, electronic, and biological aspects. Understanding these relationships is crucial for the rational design of antioxidant compounds and the optimization of their therapeutic potential. As research in this field continues to advance, it is likely to yield new insights into the fundamental mechanisms of antioxidant activity and pave the way for innovative applications across multiple industries.
Market Demand for Antioxidant Research
The market demand for antioxidant research has been steadily growing, driven by increasing consumer awareness of health benefits and the expanding applications across various industries. The global antioxidant market is projected to reach significant value in the coming years, with a compound annual growth rate (CAGR) that reflects the rising interest in these compounds.
In the food and beverage industry, antioxidants play a crucial role in preserving product quality and extending shelf life. Consumers are increasingly seeking natural antioxidants in their diets, leading to a surge in demand for antioxidant-rich foods and supplements. This trend is particularly evident in the functional food sector, where products fortified with antioxidants are gaining popularity.
The cosmetics and personal care industry has also witnessed a substantial increase in the use of antioxidants. Anti-aging products, in particular, heavily rely on antioxidants to combat free radicals and reduce oxidative stress on the skin. This has led to a growing market for skincare products featuring antioxidant ingredients.
In the pharmaceutical sector, antioxidant research is pivotal in developing new therapies for various diseases associated with oxidative stress. The potential of antioxidants in preventing or treating conditions such as cardiovascular diseases, cancer, and neurodegenerative disorders has fueled extensive research and development efforts.
The nutraceutical industry has experienced significant growth, with antioxidant supplements becoming a staple in many consumers' health regimens. This has created a robust market for antioxidant formulations targeting specific health concerns and age groups.
Industrial applications of antioxidants, particularly in plastics, rubber, and fuel additives, represent another substantial market segment. The demand for antioxidants in these sectors is driven by the need to enhance product durability and performance.
The influence of geometric isomers on antioxidant pathways has emerged as a critical area of research, attracting attention from both academia and industry. This focus stems from the recognition that the spatial arrangement of molecules can significantly impact their antioxidant properties and efficacy.
As consumers become more educated about the molecular basis of antioxidant activity, there is a growing demand for products that leverage advanced scientific understanding, including the role of geometric isomers. This trend is likely to drive further research and innovation in antioxidant development, potentially leading to more effective and targeted antioxidant solutions.
The market demand for antioxidant research is also influenced by regulatory trends and safety concerns. As health authorities worldwide scrutinize the use of synthetic antioxidants, there is an increasing emphasis on identifying and developing natural antioxidants with proven safety profiles.
In the food and beverage industry, antioxidants play a crucial role in preserving product quality and extending shelf life. Consumers are increasingly seeking natural antioxidants in their diets, leading to a surge in demand for antioxidant-rich foods and supplements. This trend is particularly evident in the functional food sector, where products fortified with antioxidants are gaining popularity.
The cosmetics and personal care industry has also witnessed a substantial increase in the use of antioxidants. Anti-aging products, in particular, heavily rely on antioxidants to combat free radicals and reduce oxidative stress on the skin. This has led to a growing market for skincare products featuring antioxidant ingredients.
In the pharmaceutical sector, antioxidant research is pivotal in developing new therapies for various diseases associated with oxidative stress. The potential of antioxidants in preventing or treating conditions such as cardiovascular diseases, cancer, and neurodegenerative disorders has fueled extensive research and development efforts.
The nutraceutical industry has experienced significant growth, with antioxidant supplements becoming a staple in many consumers' health regimens. This has created a robust market for antioxidant formulations targeting specific health concerns and age groups.
Industrial applications of antioxidants, particularly in plastics, rubber, and fuel additives, represent another substantial market segment. The demand for antioxidants in these sectors is driven by the need to enhance product durability and performance.
The influence of geometric isomers on antioxidant pathways has emerged as a critical area of research, attracting attention from both academia and industry. This focus stems from the recognition that the spatial arrangement of molecules can significantly impact their antioxidant properties and efficacy.
As consumers become more educated about the molecular basis of antioxidant activity, there is a growing demand for products that leverage advanced scientific understanding, including the role of geometric isomers. This trend is likely to drive further research and innovation in antioxidant development, potentially leading to more effective and targeted antioxidant solutions.
The market demand for antioxidant research is also influenced by regulatory trends and safety concerns. As health authorities worldwide scrutinize the use of synthetic antioxidants, there is an increasing emphasis on identifying and developing natural antioxidants with proven safety profiles.
Current Challenges in Isomer-Specific Antioxidant Studies
The field of isomer-specific antioxidant studies faces several significant challenges that hinder progress and limit our understanding of the influence of geometric isomers on antioxidant pathways. One of the primary obstacles is the complexity of isolating and characterizing individual isomers. Geometric isomers often have similar physical and chemical properties, making their separation and purification a demanding task. Traditional chromatographic techniques may not always provide sufficient resolution, leading to potential cross-contamination and ambiguous results.
Another major challenge lies in the development of analytical methods capable of accurately quantifying and distinguishing between different geometric isomers in complex biological matrices. The subtle structural differences between isomers can result in similar spectroscopic profiles, complicating their identification and measurement. This limitation often necessitates the use of advanced analytical techniques, such as high-resolution mass spectrometry or nuclear magnetic resonance spectroscopy, which may not be readily available or cost-effective for all research groups.
The dynamic nature of isomerization processes in biological systems presents an additional layer of complexity. Geometric isomers can interconvert under physiological conditions, making it difficult to attribute observed antioxidant effects to specific isomeric forms. This interconversion can be influenced by various factors, including pH, temperature, and the presence of catalysts or enzymes, further complicating the study of isomer-specific antioxidant pathways.
Furthermore, the lack of standardized protocols for isomer-specific antioxidant assays poses a significant challenge to the field. The variability in experimental conditions and methodologies across different studies makes it challenging to compare and validate results, hindering the establishment of a comprehensive understanding of isomer-specific antioxidant mechanisms.
The biological relevance of isomer-specific effects also presents a challenge in translating in vitro findings to in vivo systems. The complex interactions between isomers and biological molecules, as well as the potential for metabolic transformations, can lead to discrepancies between laboratory observations and physiological outcomes. This gap underscores the need for more sophisticated in vivo models and clinical studies to elucidate the true impact of geometric isomers on antioxidant pathways in living organisms.
Lastly, the limited availability of pure isomeric standards for many compounds of interest impedes progress in this field. The synthesis and characterization of these standards can be time-consuming and costly, often requiring specialized expertise and resources. This scarcity of reference materials hampers the development of reliable quantification methods and the validation of experimental results, ultimately slowing the pace of research in isomer-specific antioxidant studies.
Another major challenge lies in the development of analytical methods capable of accurately quantifying and distinguishing between different geometric isomers in complex biological matrices. The subtle structural differences between isomers can result in similar spectroscopic profiles, complicating their identification and measurement. This limitation often necessitates the use of advanced analytical techniques, such as high-resolution mass spectrometry or nuclear magnetic resonance spectroscopy, which may not be readily available or cost-effective for all research groups.
The dynamic nature of isomerization processes in biological systems presents an additional layer of complexity. Geometric isomers can interconvert under physiological conditions, making it difficult to attribute observed antioxidant effects to specific isomeric forms. This interconversion can be influenced by various factors, including pH, temperature, and the presence of catalysts or enzymes, further complicating the study of isomer-specific antioxidant pathways.
Furthermore, the lack of standardized protocols for isomer-specific antioxidant assays poses a significant challenge to the field. The variability in experimental conditions and methodologies across different studies makes it challenging to compare and validate results, hindering the establishment of a comprehensive understanding of isomer-specific antioxidant mechanisms.
The biological relevance of isomer-specific effects also presents a challenge in translating in vitro findings to in vivo systems. The complex interactions between isomers and biological molecules, as well as the potential for metabolic transformations, can lead to discrepancies between laboratory observations and physiological outcomes. This gap underscores the need for more sophisticated in vivo models and clinical studies to elucidate the true impact of geometric isomers on antioxidant pathways in living organisms.
Lastly, the limited availability of pure isomeric standards for many compounds of interest impedes progress in this field. The synthesis and characterization of these standards can be time-consuming and costly, often requiring specialized expertise and resources. This scarcity of reference materials hampers the development of reliable quantification methods and the validation of experimental results, ultimately slowing the pace of research in isomer-specific antioxidant studies.
Existing Methods for Studying Geometric Isomers
01 Synthesis of geometric isomers with antioxidant properties
Methods for synthesizing geometric isomers that exhibit antioxidant properties. These processes involve creating compounds with specific spatial arrangements that contribute to their antioxidant activity. The synthesized isomers can be used in various applications where antioxidant properties are desired.- Synthesis of geometric isomers with antioxidant properties: Methods for synthesizing geometric isomers that exhibit antioxidant properties. These processes involve creating compounds with specific spatial arrangements that contribute to their ability to neutralize free radicals and protect against oxidative stress. The synthesized isomers can be used in various applications, including pharmaceuticals and food additives.
- Identification and characterization of antioxidant pathways in geometric isomers: Techniques for identifying and characterizing the specific antioxidant pathways utilized by geometric isomers. This involves studying the molecular mechanisms by which these compounds interact with reactive oxygen species and other oxidants. Understanding these pathways can lead to the development of more effective antioxidant strategies and targeted interventions.
- Formulation of antioxidant compositions using geometric isomers: Development of antioxidant compositions that incorporate geometric isomers as active ingredients. These formulations may include combinations of different isomers or blends with other antioxidant compounds to achieve synergistic effects. The compositions can be tailored for specific applications in cosmetics, nutraceuticals, or industrial products.
- Analytical methods for studying geometric isomers in antioxidant pathways: Advanced analytical techniques for studying the behavior and efficacy of geometric isomers in antioxidant pathways. These methods may include spectroscopic analysis, chromatography, and computational modeling to elucidate the structure-activity relationships and quantify the antioxidant capacity of different isomers.
- Applications of geometric isomers in enhancing antioxidant defenses: Practical applications of geometric isomers in enhancing antioxidant defenses in various contexts. This includes their use in preventive medicine, anti-aging products, food preservation, and environmental protection. The isomers can be incorporated into different delivery systems to maximize their effectiveness in specific target areas.
02 Identification and characterization of antioxidant pathways in geometric isomers
Techniques for identifying and characterizing the antioxidant pathways in geometric isomers. This involves studying the molecular mechanisms by which these isomers neutralize free radicals or prevent oxidative stress. Understanding these pathways can lead to the development of more effective antioxidant compounds.Expand Specific Solutions03 Formulation of antioxidant compositions using geometric isomers
Methods for formulating antioxidant compositions that incorporate geometric isomers. These formulations may include combinations of different isomers or other antioxidant compounds to enhance overall antioxidant activity. The compositions can be tailored for specific applications in industries such as food, cosmetics, or pharmaceuticals.Expand Specific Solutions04 Analytical methods for studying geometric isomers in antioxidant pathways
Development of analytical techniques to study geometric isomers and their role in antioxidant pathways. These methods may include spectroscopic techniques, chromatography, or computational modeling to elucidate the structure-activity relationships of geometric isomers and their antioxidant mechanisms.Expand Specific Solutions05 Applications of geometric isomers in antioxidant-based therapies
Exploration of therapeutic applications utilizing geometric isomers with antioxidant properties. This includes the development of novel drugs or treatments that leverage the antioxidant pathways of these isomers to combat oxidative stress-related diseases or conditions. Research in this area may involve in vitro and in vivo studies to assess efficacy and safety.Expand Specific Solutions
Key Players in Antioxidant and Isomer Research
The competitive landscape for research on "Influence of Geometric Isomers on the Antioxidant Pathways" is in an early development stage, with a growing market potential as antioxidants play a crucial role in various industries. The market size is expanding due to increasing applications in pharmaceuticals, food, and materials science. Technologically, the field is still evolving, with companies like Polnox Corp. and AbbVie, Inc. leading in antioxidant research. Academic institutions such as The Rockefeller University and Tufts University are contributing significantly to fundamental research. Collaboration between industry and academia is driving innovation, with companies like Novartis AG and Bayer CropScience LP leveraging their resources to advance the understanding of geometric isomers' impact on antioxidant mechanisms.
AbbVie, Inc.
Technical Solution: AbbVie, Inc. has pioneered research into the impact of geometric isomers on antioxidant pathways, particularly in the context of inflammatory diseases. Their approach combines structural biology and medicinal chemistry to design isomer-specific antioxidant molecules. AbbVie's scientists have developed a proprietary platform that allows for the rapid synthesis and evaluation of geometric isomers with tailored antioxidant properties[4]. They have successfully identified several lead compounds where specific geometric configurations significantly enhance the compounds' ability to modulate key antioxidant pathways, such as Nrf2 activation[5]. AbbVie's research has also extended to studying the pharmacokinetics and metabolism of these isomers, ensuring optimal therapeutic efficacy.
Strengths: Strong pipeline in immunology and oncology, experienced in bringing drugs to market. Weaknesses: Dependence on a few key products, potential patent expirations.
Trustees of the University of Pennsylvania
Technical Solution: The University of Pennsylvania's research on geometric isomers and antioxidant pathways is notable for its focus on translational medicine. Their teams have developed advanced in vitro and in vivo models to study the effects of geometric isomerization on antioxidant efficacy. Penn researchers have made significant progress in understanding how geometric isomers influence the bioavailability and tissue distribution of antioxidant compounds[10]. They have also explored the potential of geometric isomerization as a strategy to enhance the therapeutic index of existing antioxidant drugs. Penn's work has led to several promising lead compounds, particularly in the areas of cardiovascular and neurodegenerative diseases.
Strengths: Strong track record in translational research, extensive clinical trial network. Weaknesses: Potential intellectual property complexities in academic-industry partnerships.
Core Innovations in Isomer-Antioxidant Interactions
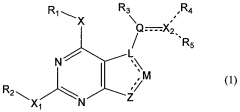
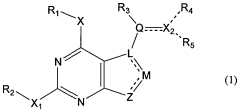
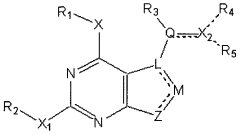
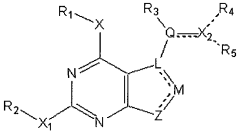
Methods of preparation and resolution of e/z isomers of vinylfuro[2,3-d] pyrimidine and their biological activities
PatentWO2007109661A2
Innovation
- Development of methods for the preparation, resolution, and isolation of individual E- and Z-isomers of 2,4-substituted-5-vinylfuro[2,3-d]pyrimidine compounds using optimized reaction conditions, catalysis, chromatography, and crystallization to separate and purify these isomers, allowing for the production of pharmaceutically acceptable salts and prodrugs, which can act as receptor tyrosine kinase inhibitors and anti-angiogenic agents.
Compounds and methods for the treatment of malaria
PatentInactiveIN202118043692A
Innovation
- Development of specific compounds, such as those represented by Formula I and listed in Table 1, which offer new structural features and functional groups to target malaria parasites effectively, including those resistant to existing drugs.
Regulatory Aspects of Antioxidant Claims
The regulatory landscape surrounding antioxidant claims is complex and varies significantly across different regions and jurisdictions. In the United States, the Food and Drug Administration (FDA) oversees the regulation of antioxidant claims on food and dietary supplement labels. The FDA requires that antioxidant claims be based on scientific evidence and that the specific nutrient responsible for the antioxidant effect be identified.
The European Food Safety Authority (EFSA) has established strict guidelines for antioxidant claims in the European Union. EFSA requires substantial scientific evidence to support any health claims related to antioxidant properties. This includes demonstrating a cause-and-effect relationship between the consumption of the food or ingredient and the claimed health benefit.
In the context of geometric isomers and their influence on antioxidant pathways, regulatory bodies are increasingly focusing on the specificity of antioxidant claims. The molecular structure of geometric isomers can significantly impact their antioxidant properties, and regulators are beginning to recognize the importance of distinguishing between different isomeric forms when evaluating antioxidant claims.
For example, the FDA has started to consider the specific isomeric composition of compounds when reviewing antioxidant-related health claims. This is particularly relevant for natural products and supplements that may contain mixtures of geometric isomers with varying antioxidant potencies.
Regulatory agencies are also paying closer attention to the methods used to measure antioxidant activity. Traditional assays like ORAC (Oxygen Radical Absorbance Capacity) are no longer considered sufficient evidence for antioxidant claims by many regulatory bodies. Instead, there is a growing emphasis on in vivo studies that demonstrate the bioavailability and physiological effects of antioxidants, taking into account the potential differences between geometric isomers.
The regulatory landscape is evolving to address the complexities introduced by geometric isomerism in antioxidant research. Manufacturers and researchers must now provide more detailed information about the isomeric composition of their products and demonstrate how different isomers may contribute to the overall antioxidant effect. This shift towards more precise regulation reflects the growing understanding of the intricate relationship between molecular structure and antioxidant function.
The European Food Safety Authority (EFSA) has established strict guidelines for antioxidant claims in the European Union. EFSA requires substantial scientific evidence to support any health claims related to antioxidant properties. This includes demonstrating a cause-and-effect relationship between the consumption of the food or ingredient and the claimed health benefit.
In the context of geometric isomers and their influence on antioxidant pathways, regulatory bodies are increasingly focusing on the specificity of antioxidant claims. The molecular structure of geometric isomers can significantly impact their antioxidant properties, and regulators are beginning to recognize the importance of distinguishing between different isomeric forms when evaluating antioxidant claims.
For example, the FDA has started to consider the specific isomeric composition of compounds when reviewing antioxidant-related health claims. This is particularly relevant for natural products and supplements that may contain mixtures of geometric isomers with varying antioxidant potencies.
Regulatory agencies are also paying closer attention to the methods used to measure antioxidant activity. Traditional assays like ORAC (Oxygen Radical Absorbance Capacity) are no longer considered sufficient evidence for antioxidant claims by many regulatory bodies. Instead, there is a growing emphasis on in vivo studies that demonstrate the bioavailability and physiological effects of antioxidants, taking into account the potential differences between geometric isomers.
The regulatory landscape is evolving to address the complexities introduced by geometric isomerism in antioxidant research. Manufacturers and researchers must now provide more detailed information about the isomeric composition of their products and demonstrate how different isomers may contribute to the overall antioxidant effect. This shift towards more precise regulation reflects the growing understanding of the intricate relationship between molecular structure and antioxidant function.
Environmental Factors Affecting Isomer Stability
Environmental factors play a crucial role in determining the stability and interconversion of geometric isomers, which in turn significantly influences their antioxidant pathways. Temperature is one of the most critical factors affecting isomer stability. Higher temperatures generally increase the rate of isomerization, potentially altering the ratio of geometric isomers present in a system. This temperature-dependent isomerization can lead to changes in the overall antioxidant capacity of a mixture, as different isomers may exhibit varying degrees of antioxidant activity.
Light exposure is another key environmental factor that can impact isomer stability. Photoisomerization, particularly in compounds with carbon-carbon double bonds, can occur upon exposure to specific wavelengths of light. This process can result in the conversion between cis and trans isomers, potentially modifying their antioxidant properties. For instance, the photoisomerization of certain carotenoids can alter their ability to quench singlet oxygen, a potent oxidizing agent.
pH levels in the surrounding environment can also significantly affect isomer stability. Changes in pH can promote or inhibit isomerization reactions, particularly in compounds with ionizable groups. This pH-dependent isomerization can lead to shifts in the predominant isomeric form, potentially altering the overall antioxidant efficacy of the compound. Some antioxidants may exhibit enhanced stability and activity at specific pH ranges, while others may be more susceptible to degradation or isomerization under certain pH conditions.
The presence of metal ions in the environment can catalyze isomerization reactions, affecting the stability of geometric isomers. Transition metals, in particular, can facilitate electron transfer processes that promote isomerization. This metal-catalyzed isomerization can impact the antioxidant pathways by altering the distribution of isomers and potentially generating reactive intermediates that may participate in oxidation or reduction reactions.
Solvent polarity is another environmental factor that can influence isomer stability and, consequently, antioxidant pathways. The polarity of the surrounding medium can affect the relative stability of different geometric isomers, potentially favoring one form over another. This solvent-dependent isomerization can lead to changes in the overall antioxidant capacity of a system, as the predominant isomeric form may exhibit different antioxidant properties compared to its counterparts.
Understanding these environmental factors and their impact on isomer stability is crucial for optimizing antioxidant formulations and predicting their efficacy in various applications. By carefully controlling environmental conditions, it may be possible to manipulate the isomeric distribution of antioxidants to enhance their overall performance and stability in different systems.
Light exposure is another key environmental factor that can impact isomer stability. Photoisomerization, particularly in compounds with carbon-carbon double bonds, can occur upon exposure to specific wavelengths of light. This process can result in the conversion between cis and trans isomers, potentially modifying their antioxidant properties. For instance, the photoisomerization of certain carotenoids can alter their ability to quench singlet oxygen, a potent oxidizing agent.
pH levels in the surrounding environment can also significantly affect isomer stability. Changes in pH can promote or inhibit isomerization reactions, particularly in compounds with ionizable groups. This pH-dependent isomerization can lead to shifts in the predominant isomeric form, potentially altering the overall antioxidant efficacy of the compound. Some antioxidants may exhibit enhanced stability and activity at specific pH ranges, while others may be more susceptible to degradation or isomerization under certain pH conditions.
The presence of metal ions in the environment can catalyze isomerization reactions, affecting the stability of geometric isomers. Transition metals, in particular, can facilitate electron transfer processes that promote isomerization. This metal-catalyzed isomerization can impact the antioxidant pathways by altering the distribution of isomers and potentially generating reactive intermediates that may participate in oxidation or reduction reactions.
Solvent polarity is another environmental factor that can influence isomer stability and, consequently, antioxidant pathways. The polarity of the surrounding medium can affect the relative stability of different geometric isomers, potentially favoring one form over another. This solvent-dependent isomerization can lead to changes in the overall antioxidant capacity of a system, as the predominant isomeric form may exhibit different antioxidant properties compared to its counterparts.
Understanding these environmental factors and their impact on isomer stability is crucial for optimizing antioxidant formulations and predicting their efficacy in various applications. By carefully controlling environmental conditions, it may be possible to manipulate the isomeric distribution of antioxidants to enhance their overall performance and stability in different systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!