Role of Geometric Isomers in Chiral Drug Formulations
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Chiral Drug Isomers: Background and Objectives
Chiral drug isomers have been a focal point in pharmaceutical research and development for several decades. The concept of chirality, derived from the Greek word for "hand," refers to molecules that are mirror images of each other but non-superimposable, much like left and right hands. In the context of drug formulations, these mirror-image molecules, known as enantiomers, can exhibit significantly different biological activities, pharmacokinetics, and toxicological profiles.
The historical background of chiral drug isomers traces back to the 1960s, with the thalidomide tragedy serving as a stark reminder of the importance of stereochemistry in drug development. This event catalyzed a paradigm shift in the pharmaceutical industry, leading to increased scrutiny of the role of geometric isomers in drug formulations and their potential impact on therapeutic outcomes.
Over the years, advancements in synthetic chemistry, analytical techniques, and regulatory guidelines have propelled the field of chiral drug development forward. The ability to synthesize and isolate single enantiomers has become a critical aspect of modern drug discovery and development processes. This evolution has been driven by the recognition that single-enantiomer drugs often offer improved efficacy, reduced side effects, and more predictable pharmacokinetic profiles compared to their racemic counterparts.
The objectives of studying geometric isomers in chiral drug formulations are multifaceted. Primarily, researchers aim to elucidate the specific biological activities of individual enantiomers, understanding how their three-dimensional structures interact with target receptors and enzymes in the body. This knowledge is crucial for optimizing drug efficacy and minimizing adverse effects.
Another key objective is to develop efficient and cost-effective methods for the synthesis and purification of single enantiomers. This includes exploring novel catalytic systems, asymmetric synthesis techniques, and chromatographic separation methods. The goal is to streamline the production of enantiopure drugs, making them more accessible and economically viable for widespread use.
Furthermore, researchers seek to understand the potential for chiral switching – the development of single-enantiomer versions of existing racemic drugs. This strategy has the potential to extend patent life, improve drug profiles, and address unmet medical needs. The objectives also extend to investigating the impact of chirality on drug formulation, stability, and delivery systems, as these factors can significantly influence the overall performance of a pharmaceutical product.
In the broader context, the study of geometric isomers in chiral drug formulations aims to contribute to the development of more precise, effective, and safer medications. This aligns with the growing trend towards personalized medicine, where understanding the nuanced interactions between drug molecules and individual patient biology is paramount.
The historical background of chiral drug isomers traces back to the 1960s, with the thalidomide tragedy serving as a stark reminder of the importance of stereochemistry in drug development. This event catalyzed a paradigm shift in the pharmaceutical industry, leading to increased scrutiny of the role of geometric isomers in drug formulations and their potential impact on therapeutic outcomes.
Over the years, advancements in synthetic chemistry, analytical techniques, and regulatory guidelines have propelled the field of chiral drug development forward. The ability to synthesize and isolate single enantiomers has become a critical aspect of modern drug discovery and development processes. This evolution has been driven by the recognition that single-enantiomer drugs often offer improved efficacy, reduced side effects, and more predictable pharmacokinetic profiles compared to their racemic counterparts.
The objectives of studying geometric isomers in chiral drug formulations are multifaceted. Primarily, researchers aim to elucidate the specific biological activities of individual enantiomers, understanding how their three-dimensional structures interact with target receptors and enzymes in the body. This knowledge is crucial for optimizing drug efficacy and minimizing adverse effects.
Another key objective is to develop efficient and cost-effective methods for the synthesis and purification of single enantiomers. This includes exploring novel catalytic systems, asymmetric synthesis techniques, and chromatographic separation methods. The goal is to streamline the production of enantiopure drugs, making them more accessible and economically viable for widespread use.
Furthermore, researchers seek to understand the potential for chiral switching – the development of single-enantiomer versions of existing racemic drugs. This strategy has the potential to extend patent life, improve drug profiles, and address unmet medical needs. The objectives also extend to investigating the impact of chirality on drug formulation, stability, and delivery systems, as these factors can significantly influence the overall performance of a pharmaceutical product.
In the broader context, the study of geometric isomers in chiral drug formulations aims to contribute to the development of more precise, effective, and safer medications. This aligns with the growing trend towards personalized medicine, where understanding the nuanced interactions between drug molecules and individual patient biology is paramount.
Market Analysis of Chiral Pharmaceuticals
The chiral pharmaceuticals market has experienced significant growth in recent years, driven by the increasing demand for more effective and safer drugs. This market segment is characterized by the development and production of drugs that contain specific stereoisomers, which can have vastly different pharmacological properties compared to their mirror images. The global chiral technology market, which includes pharmaceuticals, was valued at approximately $7.8 billion in 2020 and is projected to reach $11.2 billion by 2027, growing at a CAGR of 5.3% during the forecast period.
The pharmaceutical industry has been the primary driver of this market, accounting for over 60% of the total chiral technology market share. This dominance is attributed to the growing recognition of the importance of chirality in drug efficacy and safety. Many blockbuster drugs, such as esomeprazole (Nexium) and escitalopram (Lexapro), are chiral formulations that have demonstrated superior therapeutic profiles compared to their racemic counterparts.
Geographically, North America leads the chiral pharmaceuticals market, followed by Europe and Asia-Pacific. The United States, in particular, has been at the forefront of chiral drug development, with the FDA encouraging pharmaceutical companies to develop single-enantiomer drugs. This regulatory support has been a significant factor in market growth.
The market for chiral pharmaceuticals is highly competitive, with major players including Pfizer, Johnson & Johnson, Merck, and Sanofi. These companies have invested heavily in chiral separation technologies and asymmetric synthesis methods to develop enantiopure drugs. Additionally, there has been a rise in collaborations between pharmaceutical companies and contract research organizations specializing in chiral technologies.
Looking ahead, the chiral pharmaceuticals market is expected to continue its growth trajectory. Factors contributing to this growth include the increasing prevalence of chronic diseases, the rising demand for personalized medicine, and advancements in chiral separation technologies. Moreover, the expiration of patents for several blockbuster racemic drugs is creating opportunities for the development of single-enantiomer versions with improved efficacy and reduced side effects.
However, challenges remain in the chiral pharmaceuticals market. The high costs associated with chiral drug development and production, as well as the complexity of chiral separation processes, can be barriers to market entry for smaller pharmaceutical companies. Additionally, regulatory hurdles and the need for extensive clinical trials to demonstrate the superiority of chiral formulations over existing racemic drugs can prolong the development timeline and increase costs.
The pharmaceutical industry has been the primary driver of this market, accounting for over 60% of the total chiral technology market share. This dominance is attributed to the growing recognition of the importance of chirality in drug efficacy and safety. Many blockbuster drugs, such as esomeprazole (Nexium) and escitalopram (Lexapro), are chiral formulations that have demonstrated superior therapeutic profiles compared to their racemic counterparts.
Geographically, North America leads the chiral pharmaceuticals market, followed by Europe and Asia-Pacific. The United States, in particular, has been at the forefront of chiral drug development, with the FDA encouraging pharmaceutical companies to develop single-enantiomer drugs. This regulatory support has been a significant factor in market growth.
The market for chiral pharmaceuticals is highly competitive, with major players including Pfizer, Johnson & Johnson, Merck, and Sanofi. These companies have invested heavily in chiral separation technologies and asymmetric synthesis methods to develop enantiopure drugs. Additionally, there has been a rise in collaborations between pharmaceutical companies and contract research organizations specializing in chiral technologies.
Looking ahead, the chiral pharmaceuticals market is expected to continue its growth trajectory. Factors contributing to this growth include the increasing prevalence of chronic diseases, the rising demand for personalized medicine, and advancements in chiral separation technologies. Moreover, the expiration of patents for several blockbuster racemic drugs is creating opportunities for the development of single-enantiomer versions with improved efficacy and reduced side effects.
However, challenges remain in the chiral pharmaceuticals market. The high costs associated with chiral drug development and production, as well as the complexity of chiral separation processes, can be barriers to market entry for smaller pharmaceutical companies. Additionally, regulatory hurdles and the need for extensive clinical trials to demonstrate the superiority of chiral formulations over existing racemic drugs can prolong the development timeline and increase costs.
Current Challenges in Geometric Isomer Separation
The separation of geometric isomers in chiral drug formulations presents several significant challenges that continue to impact the pharmaceutical industry. One of the primary difficulties lies in the structural similarity between geometric isomers, which often possess nearly identical physical and chemical properties. This similarity makes traditional separation techniques, such as crystallization or distillation, ineffective or impractical for large-scale production.
Chromatographic methods, while widely used, face limitations in terms of scalability and cost-effectiveness. High-performance liquid chromatography (HPLC) and supercritical fluid chromatography (SFC) are commonly employed but struggle with throughput and solvent consumption issues when scaled up to industrial levels. The development of suitable chiral stationary phases for these techniques remains an ongoing challenge, particularly for novel drug compounds.
Another significant hurdle is the dynamic interconversion between geometric isomers under certain conditions. This phenomenon, known as isomerization, can occur during the separation process itself, leading to reduced purity and yield. Controlling reaction conditions to minimize isomerization while maintaining separation efficiency is a delicate balance that researchers continue to optimize.
The increasing complexity of drug molecules, often containing multiple chiral centers, further complicates the separation process. These compounds may form complex mixtures of stereoisomers, requiring sophisticated multi-step separation strategies. The development of such strategies is time-consuming and resource-intensive, potentially delaying drug development timelines.
Regulatory requirements for enantiomeric purity in pharmaceutical products have become increasingly stringent, demanding higher levels of separation efficiency and purity. Meeting these standards while maintaining cost-effectiveness and scalability poses a significant challenge for drug manufacturers.
Emerging technologies, such as simulated moving bed (SMB) chromatography and membrane-based separations, show promise in addressing some of these challenges. However, their widespread adoption is hindered by factors such as high initial investment costs, complex process optimization, and the need for specialized expertise.
The environmental impact of separation processes is also a growing concern. Many current methods rely heavily on organic solvents, which can be harmful to the environment and pose safety risks. Developing greener separation technologies that reduce solvent use and energy consumption while maintaining separation efficiency is an ongoing area of research and development in the field.
Chromatographic methods, while widely used, face limitations in terms of scalability and cost-effectiveness. High-performance liquid chromatography (HPLC) and supercritical fluid chromatography (SFC) are commonly employed but struggle with throughput and solvent consumption issues when scaled up to industrial levels. The development of suitable chiral stationary phases for these techniques remains an ongoing challenge, particularly for novel drug compounds.
Another significant hurdle is the dynamic interconversion between geometric isomers under certain conditions. This phenomenon, known as isomerization, can occur during the separation process itself, leading to reduced purity and yield. Controlling reaction conditions to minimize isomerization while maintaining separation efficiency is a delicate balance that researchers continue to optimize.
The increasing complexity of drug molecules, often containing multiple chiral centers, further complicates the separation process. These compounds may form complex mixtures of stereoisomers, requiring sophisticated multi-step separation strategies. The development of such strategies is time-consuming and resource-intensive, potentially delaying drug development timelines.
Regulatory requirements for enantiomeric purity in pharmaceutical products have become increasingly stringent, demanding higher levels of separation efficiency and purity. Meeting these standards while maintaining cost-effectiveness and scalability poses a significant challenge for drug manufacturers.
Emerging technologies, such as simulated moving bed (SMB) chromatography and membrane-based separations, show promise in addressing some of these challenges. However, their widespread adoption is hindered by factors such as high initial investment costs, complex process optimization, and the need for specialized expertise.
The environmental impact of separation processes is also a growing concern. Many current methods rely heavily on organic solvents, which can be harmful to the environment and pose safety risks. Developing greener separation technologies that reduce solvent use and energy consumption while maintaining separation efficiency is an ongoing area of research and development in the field.
Current Methodologies for Geometric Isomer Formulation
01 Synthesis and separation of geometric isomers
Methods for synthesizing and separating geometric isomers, including techniques for controlling the formation of specific isomers during chemical reactions and processes for isolating desired isomers from mixtures. These methods may involve catalysts, reaction conditions, or chromatographic techniques to achieve selective synthesis or separation.- Synthesis and separation of geometric isomers: Methods for synthesizing and separating geometric isomers, including techniques for controlling the formation of specific isomers during chemical reactions and purification processes to isolate desired isomers from mixtures.
- Characterization and analysis of geometric isomers: Techniques for identifying and analyzing geometric isomers, including spectroscopic methods, chromatography, and computational modeling to determine molecular structures and properties of different isomeric forms.
- Applications of geometric isomers in pharmaceuticals: Utilization of geometric isomers in drug development and pharmaceutical formulations, focusing on the different biological activities and therapeutic effects of various isomeric forms of active compounds.
- Geometric isomers in materials science and engineering: Exploration of geometric isomers in the development of advanced materials, including their role in determining physical properties, optical characteristics, and performance in various applications such as electronics and energy storage.
- Computational methods for studying geometric isomers: Development and application of computational techniques for modeling, predicting, and visualizing geometric isomers, including quantum chemical calculations, molecular dynamics simulations, and machine learning approaches.
02 Characterization and analysis of geometric isomers
Techniques for identifying and analyzing geometric isomers, including spectroscopic methods, X-ray crystallography, and computational modeling. These approaches help determine the structure, properties, and relative abundance of different isomers in a sample, which is crucial for quality control and research purposes.Expand Specific Solutions03 Applications of geometric isomers in pharmaceuticals
The use of specific geometric isomers in drug development and formulation, exploiting differences in biological activity between isomers. This includes the development of single-isomer drugs, which can offer improved efficacy and reduced side effects compared to racemic mixtures.Expand Specific Solutions04 Geometric isomers in materials science and engineering
Exploration of geometric isomers in the development of new materials with specific properties, such as liquid crystals, polymers, and nanomaterials. The arrangement of atoms in these isomers can significantly influence the physical and chemical properties of the resulting materials.Expand Specific Solutions05 Computational methods for studying geometric isomers
Development and application of computational techniques for predicting, modeling, and visualizing geometric isomers. These methods include molecular dynamics simulations, quantum chemical calculations, and machine learning approaches to understand isomer behavior and properties.Expand Specific Solutions
Key Players in Chiral Drug Manufacturing
The competitive landscape for geometric isomers in chiral drug formulations is evolving rapidly, with the market in a growth phase. The global chiral technology market is expanding, driven by increasing demand for enantiopure drugs. Major players like Pfizer, AstraZeneca, and GlaxoSmithKline are investing heavily in chiral drug development. Smaller biotechs such as Codexis and Poxel are also making significant contributions. The technology is maturing, with advanced separation and synthesis techniques being developed by companies like Xuanzhu Biopharmaceutical and Sun Pharmaceutical. Academic institutions like Vanderbilt University and National Tsing-Hua University are conducting cutting-edge research, further advancing the field.
AstraZeneca AB
Technical Solution: AstraZeneca has pioneered innovative approaches to leverage geometric isomers in chiral drug formulations. They have developed a proprietary chiral switching technology that allows for the systematic evaluation of individual enantiomers and their potential therapeutic benefits[2]. This process involves high-throughput screening of geometric isomers to identify those with optimal pharmacological profiles. AstraZeneca also utilizes advanced chromatographic techniques for large-scale separation of chiral compounds, ensuring high purity of the desired isomers[4]. Their formulation strategy includes the development of co-crystal forms with specific chiral excipients to enhance bioavailability and stability of the active isomer[6].
Strengths: Proprietary chiral switching technology, advanced separation techniques, and expertise in co-crystal formulations. Weaknesses: Increased development time for chiral drugs, potential for higher manufacturing costs.
Pfizer Inc.
Technical Solution: Pfizer has developed a comprehensive approach to chiral drug formulations, focusing on the role of geometric isomers. Their strategy involves using advanced stereochemical techniques to separate and purify individual isomers, ensuring the isolation of the most therapeutically active form. They employ asymmetric synthesis methods to produce single enantiomers directly, reducing the need for complex separation processes[1]. Pfizer has also invested in computational modeling to predict the behavior of different geometric isomers in biological systems, allowing for more targeted drug design[3]. Their formulation process includes stability testing of individual isomers and racemic mixtures to determine the optimal composition for drug efficacy and safety[5].
Strengths: Advanced stereochemical techniques, computational modeling capabilities, and extensive experience in drug development. Weaknesses: High costs associated with isomer separation and purification, potential regulatory challenges for novel chiral formulations.
Innovations in Chiral Separation Techniques
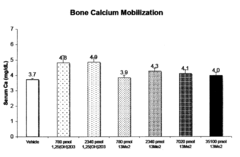
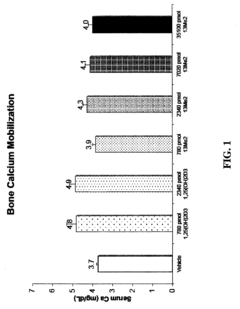
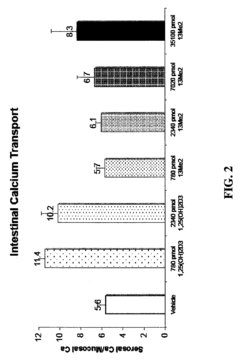
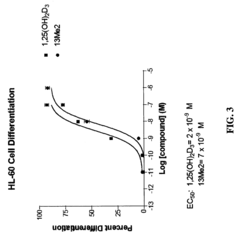
13,13-dimethyl-<i>des</i>-C,D analogs of 1α,25-dihydroxy-19-nor-vitamin D<sub>3 </sub>compounds and topical composition dosage forms and methods of treating skin conditions thereof
PatentInactiveUS8193171B2
Innovation
- Separation and isolation of geometric isomers of vitamin D analogs, specifically E and Z isomers of 13,13-dimethyl-des-C,D analogs of 1α,25-dihydroxy-19-nor-vitamin D3.
- Development of topical dosage forms containing separated geometric isomers of vitamin D analogs as active pharmaceutical ingredients.
- Utilization of a specific topical carrier system comprising 30-70% ethanol and 30-70% propylene glycol for the delivery of the separated geometric isomers.
Process for isomerizing one of the geometric isomers of an " ," -unsaturated aldehyde to its corresponding other geometric isomer
PatentInactiveUS4145366A
Innovation
- Isomerization of one geometric isomer of α,β-unsaturated aldehyde to the corresponding other geometric isomer at a temperature of 30°C to 400°C in the presence of an acid with a pKa of 1 to 7, allowing for high selectivity and separation of isomers.
Regulatory Framework for Chiral Pharmaceuticals
The regulatory framework for chiral pharmaceuticals has evolved significantly over the past few decades, reflecting the growing understanding of the importance of stereochemistry in drug development and efficacy. The United States Food and Drug Administration (FDA) has been at the forefront of establishing guidelines for the development and approval of chiral drugs.
In 1992, the FDA issued its first policy statement on the development of stereoisomeric drugs, emphasizing the need for manufacturers to characterize the stereochemical composition of their products and evaluate the pharmacological and toxicological properties of individual enantiomers. This policy marked a turning point in the regulatory approach to chiral drugs, encouraging the development of single-enantiomer formulations where appropriate.
The European Medicines Agency (EMA) followed suit in the late 1990s, implementing similar guidelines for the development and marketing of chiral drugs. These regulations require pharmaceutical companies to provide detailed information on the stereochemical properties of their drug candidates, including the rationale for developing a racemate or a single enantiomer.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has also adopted stringent regulations for chiral drugs, aligning with international standards while maintaining some unique requirements tailored to the Japanese market.
One of the key aspects of the regulatory framework is the requirement for manufacturers to demonstrate the safety and efficacy of each enantiomer separately, as well as the racemate, when developing chiral drugs. This approach ensures a comprehensive understanding of the drug's pharmacological profile and potential side effects.
The regulatory landscape also addresses the issue of chiral switching, where a single enantiomer is developed from an existing racemic drug. Regulatory agencies have established specific guidelines for these cases, requiring manufacturers to demonstrate the advantages of the single-enantiomer formulation over the existing racemic product.
In recent years, there has been an increased focus on the potential for chiral impurities in drug formulations. Regulatory bodies now require more stringent controls and analytical methods to detect and quantify these impurities, ensuring the quality and safety of chiral pharmaceuticals.
The global harmonization of regulatory requirements for chiral drugs has been an ongoing effort, with initiatives such as the International Conference on Harmonisation (ICH) playing a crucial role in aligning standards across different regions. This harmonization has facilitated the development and approval of chiral drugs on a global scale, benefiting both pharmaceutical companies and patients worldwide.
In 1992, the FDA issued its first policy statement on the development of stereoisomeric drugs, emphasizing the need for manufacturers to characterize the stereochemical composition of their products and evaluate the pharmacological and toxicological properties of individual enantiomers. This policy marked a turning point in the regulatory approach to chiral drugs, encouraging the development of single-enantiomer formulations where appropriate.
The European Medicines Agency (EMA) followed suit in the late 1990s, implementing similar guidelines for the development and marketing of chiral drugs. These regulations require pharmaceutical companies to provide detailed information on the stereochemical properties of their drug candidates, including the rationale for developing a racemate or a single enantiomer.
Japan's Pharmaceuticals and Medical Devices Agency (PMDA) has also adopted stringent regulations for chiral drugs, aligning with international standards while maintaining some unique requirements tailored to the Japanese market.
One of the key aspects of the regulatory framework is the requirement for manufacturers to demonstrate the safety and efficacy of each enantiomer separately, as well as the racemate, when developing chiral drugs. This approach ensures a comprehensive understanding of the drug's pharmacological profile and potential side effects.
The regulatory landscape also addresses the issue of chiral switching, where a single enantiomer is developed from an existing racemic drug. Regulatory agencies have established specific guidelines for these cases, requiring manufacturers to demonstrate the advantages of the single-enantiomer formulation over the existing racemic product.
In recent years, there has been an increased focus on the potential for chiral impurities in drug formulations. Regulatory bodies now require more stringent controls and analytical methods to detect and quantify these impurities, ensuring the quality and safety of chiral pharmaceuticals.
The global harmonization of regulatory requirements for chiral drugs has been an ongoing effort, with initiatives such as the International Conference on Harmonisation (ICH) playing a crucial role in aligning standards across different regions. This harmonization has facilitated the development and approval of chiral drugs on a global scale, benefiting both pharmaceutical companies and patients worldwide.
Environmental Impact of Chiral Drug Production
The production of chiral drugs has significant environmental implications that warrant careful consideration. The manufacturing processes involved in synthesizing chiral compounds often require complex chemical reactions and purification steps, which can generate substantial amounts of waste and consume significant energy resources. One of the primary environmental concerns is the use of organic solvents, which are frequently employed in large quantities during chiral drug production. These solvents, if not properly managed, can lead to air and water pollution, contributing to environmental degradation and potential health risks for surrounding communities.
Furthermore, the synthesis of chiral drugs often involves the use of heavy metals as catalysts, which can accumulate in the environment if not adequately removed from waste streams. This accumulation poses risks to ecosystems and human health, as heavy metals can persist in soil and water systems for extended periods. The production of chiral drugs also typically results in the generation of non-racemic mixtures, necessitating additional separation and purification steps. These processes not only increase energy consumption but also produce additional waste streams that require proper treatment and disposal.
Another environmental consideration is the potential for the release of active pharmaceutical ingredients (APIs) into the environment. Chiral drugs, due to their specific molecular configurations, may have unique environmental fates and effects compared to their achiral counterparts. The persistence and bioaccumulation of these compounds in aquatic environments can lead to unintended consequences for wildlife and potentially impact human health through contaminated water sources.
To address these environmental challenges, the pharmaceutical industry has been exploring greener approaches to chiral drug production. This includes the development of biocatalytic processes that utilize enzymes instead of traditional chemical catalysts, potentially reducing the need for harsh solvents and minimizing waste generation. Additionally, continuous flow chemistry techniques are being implemented to improve reaction efficiency and reduce solvent usage. Efforts are also being made to enhance recycling and recovery of solvents and catalysts, thereby minimizing the overall environmental footprint of chiral drug manufacturing.
The implementation of more sustainable practices in chiral drug production is not only environmentally beneficial but also economically advantageous in the long term. By reducing waste, improving energy efficiency, and minimizing the use of hazardous materials, pharmaceutical companies can lower production costs and mitigate environmental liabilities. Moreover, as regulatory bodies worldwide increasingly focus on the environmental impact of pharmaceutical manufacturing, adopting sustainable production methods for chiral drugs becomes crucial for compliance and maintaining a positive corporate image.
Furthermore, the synthesis of chiral drugs often involves the use of heavy metals as catalysts, which can accumulate in the environment if not adequately removed from waste streams. This accumulation poses risks to ecosystems and human health, as heavy metals can persist in soil and water systems for extended periods. The production of chiral drugs also typically results in the generation of non-racemic mixtures, necessitating additional separation and purification steps. These processes not only increase energy consumption but also produce additional waste streams that require proper treatment and disposal.
Another environmental consideration is the potential for the release of active pharmaceutical ingredients (APIs) into the environment. Chiral drugs, due to their specific molecular configurations, may have unique environmental fates and effects compared to their achiral counterparts. The persistence and bioaccumulation of these compounds in aquatic environments can lead to unintended consequences for wildlife and potentially impact human health through contaminated water sources.
To address these environmental challenges, the pharmaceutical industry has been exploring greener approaches to chiral drug production. This includes the development of biocatalytic processes that utilize enzymes instead of traditional chemical catalysts, potentially reducing the need for harsh solvents and minimizing waste generation. Additionally, continuous flow chemistry techniques are being implemented to improve reaction efficiency and reduce solvent usage. Efforts are also being made to enhance recycling and recovery of solvents and catalysts, thereby minimizing the overall environmental footprint of chiral drug manufacturing.
The implementation of more sustainable practices in chiral drug production is not only environmentally beneficial but also economically advantageous in the long term. By reducing waste, improving energy efficiency, and minimizing the use of hazardous materials, pharmaceutical companies can lower production costs and mitigate environmental liabilities. Moreover, as regulatory bodies worldwide increasingly focus on the environmental impact of pharmaceutical manufacturing, adopting sustainable production methods for chiral drugs becomes crucial for compliance and maintaining a positive corporate image.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!