How Geometric Isomers Influence Metalloprotein Active Sites
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Metalloprotein Isomers
Metalloprotein isomers play a crucial role in the functionality and efficiency of metalloprotein active sites. These isomers are structural variants of the same metalloprotein that differ in the spatial arrangement of atoms or groups within the molecule, particularly around the metal-binding site. The presence of different geometric isomers can significantly influence the properties and behavior of metalloprotein active sites, affecting their catalytic activity, substrate binding, and overall function.
Geometric isomerism in metalloproteins can arise from various sources, including the coordination geometry of the metal ion, the arrangement of ligands, and the conformation of the protein backbone. Common types of geometric isomers observed in metalloprotein active sites include cis-trans isomers, facial-meridional isomers, and conformational isomers. These isomeric forms can exhibit distinct chemical and physical properties, leading to variations in the metalloprotein's reactivity and specificity.
The influence of geometric isomers on metalloprotein active sites extends to several key aspects of their function. Firstly, the spatial arrangement of ligands around the metal center can affect the electronic properties of the metal ion, altering its redox potential and catalytic capabilities. This can result in differences in reaction rates, substrate preferences, and product distributions among isomeric forms of the same metalloprotein.
Furthermore, geometric isomerism can impact the accessibility of the active site to substrates and cofactors. The orientation of ligands and protein residues in different isomers may create varying degrees of steric hindrance or favorable interactions, influencing the binding affinity and selectivity of the metalloprotein. This, in turn, can affect the enzyme's substrate specificity and overall catalytic efficiency.
The interconversion between geometric isomers can also play a role in the regulation of metalloprotein function. Some metalloproteins exhibit dynamic isomerization, allowing them to switch between different conformations in response to environmental cues or substrate binding. This conformational flexibility can be essential for the protein's biological function, enabling it to adapt to different cellular conditions or participate in complex regulatory mechanisms.
Understanding the influence of geometric isomers on metalloprotein active sites is crucial for elucidating structure-function relationships and designing improved biocatalysts. Researchers employ various techniques, including X-ray crystallography, NMR spectroscopy, and computational modeling, to investigate the structural details and dynamic behavior of metalloprotein isomers. These studies provide valuable insights into the mechanisms of metalloenzyme catalysis and guide efforts to engineer metalloproteins with enhanced properties for biotechnological applications.
Geometric isomerism in metalloproteins can arise from various sources, including the coordination geometry of the metal ion, the arrangement of ligands, and the conformation of the protein backbone. Common types of geometric isomers observed in metalloprotein active sites include cis-trans isomers, facial-meridional isomers, and conformational isomers. These isomeric forms can exhibit distinct chemical and physical properties, leading to variations in the metalloprotein's reactivity and specificity.
The influence of geometric isomers on metalloprotein active sites extends to several key aspects of their function. Firstly, the spatial arrangement of ligands around the metal center can affect the electronic properties of the metal ion, altering its redox potential and catalytic capabilities. This can result in differences in reaction rates, substrate preferences, and product distributions among isomeric forms of the same metalloprotein.
Furthermore, geometric isomerism can impact the accessibility of the active site to substrates and cofactors. The orientation of ligands and protein residues in different isomers may create varying degrees of steric hindrance or favorable interactions, influencing the binding affinity and selectivity of the metalloprotein. This, in turn, can affect the enzyme's substrate specificity and overall catalytic efficiency.
The interconversion between geometric isomers can also play a role in the regulation of metalloprotein function. Some metalloproteins exhibit dynamic isomerization, allowing them to switch between different conformations in response to environmental cues or substrate binding. This conformational flexibility can be essential for the protein's biological function, enabling it to adapt to different cellular conditions or participate in complex regulatory mechanisms.
Understanding the influence of geometric isomers on metalloprotein active sites is crucial for elucidating structure-function relationships and designing improved biocatalysts. Researchers employ various techniques, including X-ray crystallography, NMR spectroscopy, and computational modeling, to investigate the structural details and dynamic behavior of metalloprotein isomers. These studies provide valuable insights into the mechanisms of metalloenzyme catalysis and guide efforts to engineer metalloproteins with enhanced properties for biotechnological applications.
Market Applications
The influence of geometric isomers on metalloprotein active sites has significant market applications across various industries, particularly in biotechnology, pharmaceuticals, and materials science. In the biotechnology sector, understanding the role of geometric isomers in metalloprotein active sites is crucial for enzyme engineering and the development of novel biocatalysts. These engineered enzymes can be utilized in industrial processes for the production of fine chemicals, pharmaceuticals, and biofuels, offering more sustainable and efficient alternatives to traditional chemical synthesis methods.
In the pharmaceutical industry, the impact of geometric isomers on metalloprotein active sites is of paramount importance in drug discovery and development. Many drug targets are metalloproteins, and the spatial arrangement of ligands in their active sites can significantly affect drug efficacy and selectivity. By leveraging knowledge of geometric isomers' influence, pharmaceutical companies can design more potent and specific drugs, potentially leading to improved treatments for various diseases, including cancer, cardiovascular disorders, and neurodegenerative conditions.
The materials science sector also benefits from insights into how geometric isomers affect metalloprotein active sites. This knowledge can be applied to the development of biomimetic materials and catalysts that mimic the function of natural metalloproteins. Such materials have applications in environmental remediation, energy storage, and conversion technologies. For instance, understanding the geometric isomerism in the active sites of metalloproteins involved in photosynthesis can inspire the design of more efficient solar cells and artificial photosynthetic systems.
In the field of nanotechnology, the influence of geometric isomers on metalloprotein active sites is being explored for the development of nanoscale devices and sensors. By manipulating the spatial arrangement of metal centers and ligands, researchers can create highly sensitive and selective biosensors for detecting various analytes, including environmental pollutants, biomarkers, and pathogens.
The food and beverage industry is another sector that can benefit from this research. Understanding how geometric isomers affect metalloprotein active sites can lead to improvements in food processing enzymes, enhancing their stability, activity, and specificity. This knowledge can be applied to develop more efficient and cost-effective methods for food production, preservation, and quality control.
In the agricultural sector, insights into geometric isomerism in metalloprotein active sites can contribute to the development of more effective and environmentally friendly pesticides and herbicides. By targeting specific metalloprotein active sites in pests or weeds, while minimizing effects on beneficial organisms, researchers can create more sustainable crop protection solutions.
In the pharmaceutical industry, the impact of geometric isomers on metalloprotein active sites is of paramount importance in drug discovery and development. Many drug targets are metalloproteins, and the spatial arrangement of ligands in their active sites can significantly affect drug efficacy and selectivity. By leveraging knowledge of geometric isomers' influence, pharmaceutical companies can design more potent and specific drugs, potentially leading to improved treatments for various diseases, including cancer, cardiovascular disorders, and neurodegenerative conditions.
The materials science sector also benefits from insights into how geometric isomers affect metalloprotein active sites. This knowledge can be applied to the development of biomimetic materials and catalysts that mimic the function of natural metalloproteins. Such materials have applications in environmental remediation, energy storage, and conversion technologies. For instance, understanding the geometric isomerism in the active sites of metalloproteins involved in photosynthesis can inspire the design of more efficient solar cells and artificial photosynthetic systems.
In the field of nanotechnology, the influence of geometric isomers on metalloprotein active sites is being explored for the development of nanoscale devices and sensors. By manipulating the spatial arrangement of metal centers and ligands, researchers can create highly sensitive and selective biosensors for detecting various analytes, including environmental pollutants, biomarkers, and pathogens.
The food and beverage industry is another sector that can benefit from this research. Understanding how geometric isomers affect metalloprotein active sites can lead to improvements in food processing enzymes, enhancing their stability, activity, and specificity. This knowledge can be applied to develop more efficient and cost-effective methods for food production, preservation, and quality control.
In the agricultural sector, insights into geometric isomerism in metalloprotein active sites can contribute to the development of more effective and environmentally friendly pesticides and herbicides. By targeting specific metalloprotein active sites in pests or weeds, while minimizing effects on beneficial organisms, researchers can create more sustainable crop protection solutions.
Current Challenges
The field of metalloprotein active sites faces several significant challenges in understanding and manipulating geometric isomers. One of the primary obstacles is the complexity of protein structures and their dynamic nature. Metalloproteins often have intricate three-dimensional configurations that can change in response to various factors, making it difficult to isolate and study specific geometric isomers.
Another challenge lies in the precise control and characterization of geometric isomers within metalloprotein active sites. Current analytical techniques may not always provide sufficient resolution to distinguish between subtle differences in isomeric structures, especially in complex biological environments. This limitation hinders our ability to fully understand the relationship between geometric isomerism and protein function.
The influence of the protein environment on metal coordination geometry presents an additional hurdle. The protein scaffold can exert significant constraints on the metal center, potentially favoring certain geometric isomers over others. Disentangling the effects of the protein matrix from the inherent properties of the metal complex remains a formidable task.
Furthermore, the dynamic interconversion between geometric isomers in metalloprotein active sites poses a challenge for both experimental and computational studies. The timescales of these transitions can vary widely, from picoseconds to seconds, making it difficult to capture and analyze all relevant conformational states.
The development of synthetic models that accurately mimic the geometric isomerism observed in natural metalloproteins is another area of ongoing difficulty. While significant progress has been made in creating artificial metalloenzymes, replicating the subtle geometric control exerted by natural systems remains elusive.
Computational modeling of geometric isomers in metalloprotein active sites also faces challenges. The large size of protein systems, coupled with the need for accurate quantum mechanical treatment of the metal center, strains current computational resources and methodologies. Balancing accuracy with computational efficiency is an ongoing struggle in this field.
Lastly, translating fundamental knowledge about geometric isomers in metalloprotein active sites into practical applications, such as the design of novel catalysts or therapeutic interventions, remains a significant challenge. Bridging the gap between basic research and applied technologies requires overcoming numerous technical and conceptual hurdles.
Another challenge lies in the precise control and characterization of geometric isomers within metalloprotein active sites. Current analytical techniques may not always provide sufficient resolution to distinguish between subtle differences in isomeric structures, especially in complex biological environments. This limitation hinders our ability to fully understand the relationship between geometric isomerism and protein function.
The influence of the protein environment on metal coordination geometry presents an additional hurdle. The protein scaffold can exert significant constraints on the metal center, potentially favoring certain geometric isomers over others. Disentangling the effects of the protein matrix from the inherent properties of the metal complex remains a formidable task.
Furthermore, the dynamic interconversion between geometric isomers in metalloprotein active sites poses a challenge for both experimental and computational studies. The timescales of these transitions can vary widely, from picoseconds to seconds, making it difficult to capture and analyze all relevant conformational states.
The development of synthetic models that accurately mimic the geometric isomerism observed in natural metalloproteins is another area of ongoing difficulty. While significant progress has been made in creating artificial metalloenzymes, replicating the subtle geometric control exerted by natural systems remains elusive.
Computational modeling of geometric isomers in metalloprotein active sites also faces challenges. The large size of protein systems, coupled with the need for accurate quantum mechanical treatment of the metal center, strains current computational resources and methodologies. Balancing accuracy with computational efficiency is an ongoing struggle in this field.
Lastly, translating fundamental knowledge about geometric isomers in metalloprotein active sites into practical applications, such as the design of novel catalysts or therapeutic interventions, remains a significant challenge. Bridging the gap between basic research and applied technologies requires overcoming numerous technical and conceptual hurdles.
Analytical Techniques
01 Identification and characterization of geometric isomers
Methods for identifying and characterizing geometric isomers, particularly in complex molecular structures. This includes techniques for determining the spatial arrangement of atoms or groups around double bonds or rings, which can significantly affect the properties and reactivity of compounds.- Identification and characterization of geometric isomers: Methods for identifying and characterizing geometric isomers, particularly in relation to their active sites. This includes techniques for determining the spatial arrangement of atoms or groups within molecules and how these arrangements affect the molecule's properties and interactions at active sites.
- Synthesis and separation of geometric isomers: Techniques for synthesizing specific geometric isomers and methods for separating mixtures of geometric isomers. This is crucial for isolating the desired isomer with the most effective active site configuration for a particular application.
- Active site modeling and prediction for geometric isomers: Computational methods and models used to predict the behavior of active sites in different geometric isomers. This includes molecular modeling, structure-activity relationship studies, and quantum mechanical calculations to understand how geometric differences affect active site properties.
- Application of geometric isomers in drug design: Utilization of geometric isomerism in pharmaceutical research and drug design. This involves studying how different geometric configurations affect drug-target interactions at active sites, potentially leading to more effective or selective medications.
- Influence of geometric isomerism on catalytic activity: Investigation of how geometric isomerism affects the catalytic activity of molecules, particularly at active sites. This includes studies on how the spatial arrangement of atoms in catalysts influences their efficiency, selectivity, and overall performance in chemical reactions.
02 Active site analysis in geometric isomers
Techniques for analyzing and comparing active sites in geometric isomers. This involves studying how the spatial arrangement of atoms affects the binding properties and reactivity of molecules, particularly in biological systems or catalytic processes.Expand Specific Solutions03 Synthesis and separation of geometric isomers
Methods for synthesizing specific geometric isomers and techniques for separating mixtures of isomers. This includes stereoselective synthesis approaches and chromatographic or other separation methods to isolate desired isomeric forms.Expand Specific Solutions04 Computational modeling of geometric isomers and active sites
Use of computational methods to model and predict the properties of geometric isomers and their active sites. This involves molecular modeling, quantum chemical calculations, and structure-activity relationship studies to understand isomer behavior and reactivity.Expand Specific Solutions05 Applications of geometric isomers in drug design and catalysis
Exploration of how geometric isomerism affects drug efficacy and the design of catalysts. This includes studying how different isomeric forms can lead to varied biological activities or catalytic performances, and strategies for optimizing isomer selection in pharmaceutical and industrial applications.Expand Specific Solutions
Key Research Groups
The field of geometric isomers influencing metalloprotein active sites is in a developing stage, with growing market potential as researchers explore its implications for drug design and biochemical processes. The market size is expanding as pharmaceutical companies and research institutions invest in this area. Technologically, it's progressing from basic research to applied sciences, with companies like Amgen, Incyte, and Pfizer leading in translating findings into potential therapeutic applications. Academic institutions such as the University of Pennsylvania and Northwestern University are contributing fundamental research, while biotechnology firms like Arecor and Viamet Pharmaceuticals are exploring novel approaches to manipulate metalloprotein active sites for drug development.
Amgen, Inc.
Technical Solution: Amgen has developed a structure-based drug design approach that takes into account the influence of geometric isomers on metalloprotein active sites. Their method involves high-throughput screening of compound libraries against specific metalloprotein targets, followed by detailed structural analysis of protein-ligand complexes[4]. They use advanced computational modeling to predict how different geometric isomers of potential drug candidates might interact with the metal centers in target proteins. This approach has led to the development of several metalloprotein inhibitors that are in various stages of clinical trials[5]. Amgen has also invested in the development of novel metalloprotein engineering techniques, allowing them to create custom metalloproteins with specific geometric configurations in their active sites for therapeutic applications[6].
Strengths: Strong integration of computational and experimental approaches, direct application to drug discovery. Weaknesses: May be limited to specific therapeutic areas within Amgen's focus.
Trustees of the University of Pennsylvania
Technical Solution: The University of Pennsylvania has developed advanced spectroscopic techniques to study geometric isomers in metalloprotein active sites. They utilize a combination of X-ray crystallography and advanced NMR spectroscopy to elucidate the three-dimensional structures of metalloproteins and their ligand binding sites[1]. Their approach involves using paramagnetic NMR to probe the electronic structure of metal centers and their coordination geometry[2]. This allows for detailed mapping of how subtle changes in ligand geometry can influence the reactivity and function of metalloenzymes. The university has also pioneered the use of electron paramagnetic resonance (EPR) spectroscopy to investigate the electronic states of metal ions in protein active sites, providing insights into how geometric isomerism affects electron transfer processes[3].
Strengths: Cutting-edge spectroscopic techniques, multidisciplinary approach combining structural biology and bioinorganic chemistry. Weaknesses: Research may be more focused on fundamental understanding rather than direct industrial applications.
Structural Insights
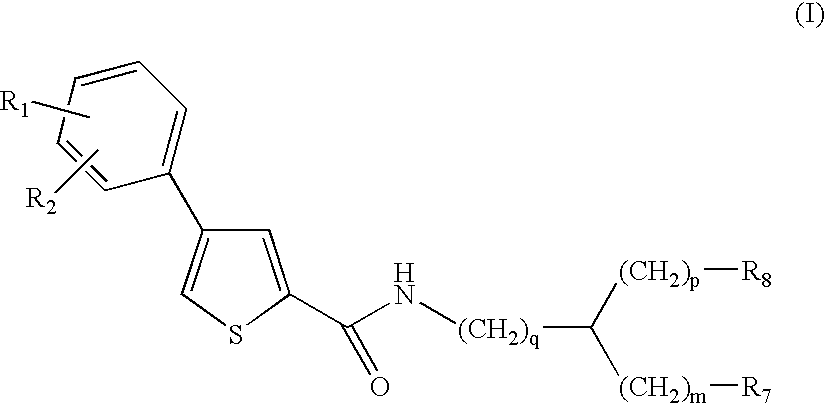
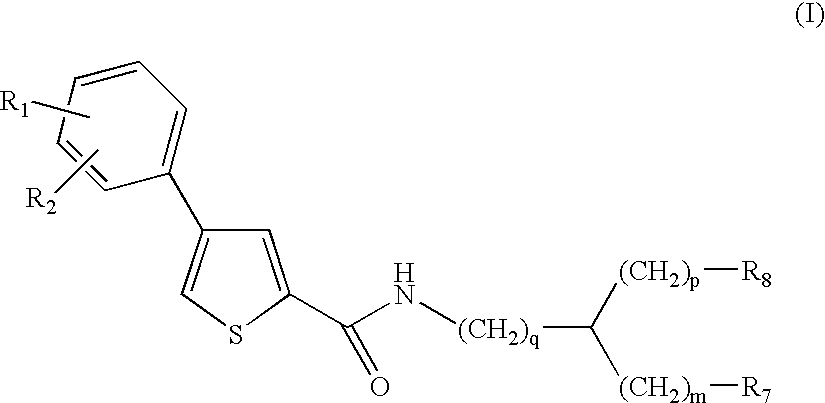
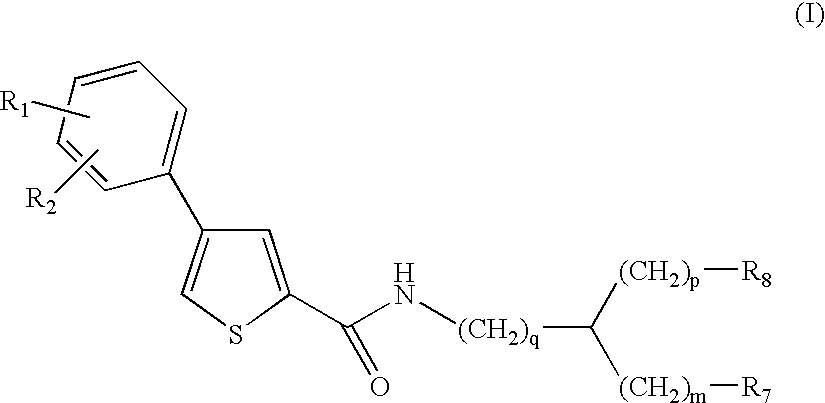
Compounds and methods for the treatment of malaria
PatentInactiveIN202118043692A
Innovation
- Development of specific compounds, such as those represented by Formula I and listed in Table 1, which offer new structural features and functional groups to target malaria parasites effectively, including those resistant to existing drugs.
Thiophene amino acid derivatives, process for preparing them and pharmaceutical compositions containing them
PatentInactiveUS20050014816A1
Innovation
- Development of novel thiophene amino acid derivatives that selectively inhibit MMP-12 and MMP-13, providing a pharmaceutical composition for treating respiratory pathologies such as COPD, emphysema, and inflammatory conditions like arthritis, with reduced toxicity.
Computational Methods
Computational methods play a crucial role in understanding how geometric isomers influence metalloprotein active sites. These methods provide valuable insights into the structural and electronic properties of metalloproteins, allowing researchers to investigate the relationship between geometric isomerism and protein function at the molecular level.
Quantum mechanical (QM) calculations are widely employed to study the electronic structure of metalloprotein active sites. Density Functional Theory (DFT) is particularly popular due to its balance between accuracy and computational efficiency. DFT calculations can reveal how different geometric isomers affect the electronic configuration of metal centers, ligand binding affinities, and catalytic mechanisms. Advanced QM methods, such as coupled cluster theory, are used for higher accuracy in smaller model systems.
Molecular dynamics (MD) simulations are essential for exploring the dynamic behavior of metalloproteins and their active sites. These simulations can reveal how geometric isomers influence protein flexibility, ligand binding, and conformational changes. Enhanced sampling techniques, like metadynamics or replica exchange, are often employed to overcome energy barriers and explore rare events that may be crucial for understanding isomer-dependent protein function.
Hybrid quantum mechanics/molecular mechanics (QM/MM) methods combine the accuracy of QM calculations for the active site with the efficiency of MM simulations for the protein environment. This approach allows researchers to study how geometric isomers affect the electronic structure of the metal center while accounting for the influence of the surrounding protein matrix.
Machine learning (ML) techniques are increasingly being applied to analyze the vast amount of data generated by computational studies. ML algorithms can identify patterns and correlations between geometric isomers and metalloprotein properties, potentially uncovering novel structure-function relationships. Deep learning models, such as graph neural networks, are particularly promising for predicting how changes in geometric isomerism might affect protein function.
Docking and virtual screening methods are employed to investigate how geometric isomers influence ligand binding and substrate specificity in metalloprotein active sites. These techniques can rapidly evaluate large libraries of compounds, helping to identify potential inhibitors or substrates that may interact differently with various geometric isomers of the active site.
Continuum electrostatics calculations, such as Poisson-Boltzmann solvers, are used to study how geometric isomers affect the electrostatic properties of metalloprotein active sites. These methods can reveal changes in pKa values, protonation states, and redox potentials that may arise from different geometric configurations.
Quantum mechanical (QM) calculations are widely employed to study the electronic structure of metalloprotein active sites. Density Functional Theory (DFT) is particularly popular due to its balance between accuracy and computational efficiency. DFT calculations can reveal how different geometric isomers affect the electronic configuration of metal centers, ligand binding affinities, and catalytic mechanisms. Advanced QM methods, such as coupled cluster theory, are used for higher accuracy in smaller model systems.
Molecular dynamics (MD) simulations are essential for exploring the dynamic behavior of metalloproteins and their active sites. These simulations can reveal how geometric isomers influence protein flexibility, ligand binding, and conformational changes. Enhanced sampling techniques, like metadynamics or replica exchange, are often employed to overcome energy barriers and explore rare events that may be crucial for understanding isomer-dependent protein function.
Hybrid quantum mechanics/molecular mechanics (QM/MM) methods combine the accuracy of QM calculations for the active site with the efficiency of MM simulations for the protein environment. This approach allows researchers to study how geometric isomers affect the electronic structure of the metal center while accounting for the influence of the surrounding protein matrix.
Machine learning (ML) techniques are increasingly being applied to analyze the vast amount of data generated by computational studies. ML algorithms can identify patterns and correlations between geometric isomers and metalloprotein properties, potentially uncovering novel structure-function relationships. Deep learning models, such as graph neural networks, are particularly promising for predicting how changes in geometric isomerism might affect protein function.
Docking and virtual screening methods are employed to investigate how geometric isomers influence ligand binding and substrate specificity in metalloprotein active sites. These techniques can rapidly evaluate large libraries of compounds, helping to identify potential inhibitors or substrates that may interact differently with various geometric isomers of the active site.
Continuum electrostatics calculations, such as Poisson-Boltzmann solvers, are used to study how geometric isomers affect the electrostatic properties of metalloprotein active sites. These methods can reveal changes in pKa values, protonation states, and redox potentials that may arise from different geometric configurations.
Biocatalysis Potential
The potential of biocatalysis in the context of geometric isomers influencing metalloprotein active sites is significant and multifaceted. Metalloproteins, with their diverse active site configurations, offer a rich landscape for biocatalytic applications. The geometric isomerism of these active sites plays a crucial role in determining their catalytic properties and substrate specificity.
One of the key advantages of utilizing metalloproteins in biocatalysis is their ability to catalyze reactions under mild conditions, often with high selectivity and efficiency. The influence of geometric isomers on these active sites can be harnessed to fine-tune catalytic activities, potentially leading to the development of novel biocatalysts with enhanced performance characteristics.
The field of protein engineering has opened up new possibilities for manipulating the geometric isomerism of metalloprotein active sites. Through techniques such as directed evolution and rational design, researchers can modify the protein scaffold to alter the geometry of the metal-binding site. This approach allows for the creation of biocatalysts with tailored properties, optimized for specific industrial processes or environmental applications.
Furthermore, the study of geometric isomers in metalloprotein active sites has implications for understanding and mimicking natural enzymatic processes. By elucidating the relationship between active site geometry and catalytic function, scientists can develop biomimetic catalysts that replicate the efficiency and selectivity of natural enzymes. This knowledge can be applied to the design of artificial metalloenzymes with improved stability and broader substrate scope.
The potential applications of biocatalysis leveraging geometric isomerism in metalloprotein active sites span various industries. In the pharmaceutical sector, these biocatalysts could enable the synthesis of complex chiral molecules with high enantioselectivity. In environmental remediation, engineered metalloproteins could be employed for the degradation of persistent pollutants or the recovery of valuable metals from waste streams.
Moreover, the integration of computational methods with experimental approaches is accelerating the discovery and optimization of novel biocatalysts. Machine learning algorithms and molecular dynamics simulations can predict the effects of geometric isomerism on catalytic activity, guiding the rational design of improved metalloprotein catalysts. This synergy between computational and experimental techniques is poised to unlock new frontiers in biocatalysis, potentially revolutionizing chemical manufacturing processes and contributing to the development of sustainable technologies.
One of the key advantages of utilizing metalloproteins in biocatalysis is their ability to catalyze reactions under mild conditions, often with high selectivity and efficiency. The influence of geometric isomers on these active sites can be harnessed to fine-tune catalytic activities, potentially leading to the development of novel biocatalysts with enhanced performance characteristics.
The field of protein engineering has opened up new possibilities for manipulating the geometric isomerism of metalloprotein active sites. Through techniques such as directed evolution and rational design, researchers can modify the protein scaffold to alter the geometry of the metal-binding site. This approach allows for the creation of biocatalysts with tailored properties, optimized for specific industrial processes or environmental applications.
Furthermore, the study of geometric isomers in metalloprotein active sites has implications for understanding and mimicking natural enzymatic processes. By elucidating the relationship between active site geometry and catalytic function, scientists can develop biomimetic catalysts that replicate the efficiency and selectivity of natural enzymes. This knowledge can be applied to the design of artificial metalloenzymes with improved stability and broader substrate scope.
The potential applications of biocatalysis leveraging geometric isomerism in metalloprotein active sites span various industries. In the pharmaceutical sector, these biocatalysts could enable the synthesis of complex chiral molecules with high enantioselectivity. In environmental remediation, engineered metalloproteins could be employed for the degradation of persistent pollutants or the recovery of valuable metals from waste streams.
Moreover, the integration of computational methods with experimental approaches is accelerating the discovery and optimization of novel biocatalysts. Machine learning algorithms and molecular dynamics simulations can predict the effects of geometric isomerism on catalytic activity, guiding the rational design of improved metalloprotein catalysts. This synergy between computational and experimental techniques is poised to unlock new frontiers in biocatalysis, potentially revolutionizing chemical manufacturing processes and contributing to the development of sustainable technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!