Insights from Geometric Isomers in Therapeutic Nanoparticle Design
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Nanoparticle Design Evolution and Objectives
Nanoparticle design has undergone significant evolution since its inception in the field of therapeutic applications. The journey began with simple drug encapsulation techniques, primarily focusing on improving drug solubility and stability. As research progressed, scientists recognized the potential of nanoparticles for targeted drug delivery, leading to the development of surface-functionalized nanoparticles capable of recognizing specific cell types or tissues.
The advent of stimuli-responsive nanoparticles marked a crucial milestone in this evolution. These smart systems could release their payload in response to specific environmental cues such as pH, temperature, or enzymatic activity, enhancing therapeutic efficacy while minimizing side effects. Concurrently, the exploration of various nanoparticle compositions, including polymeric, lipid-based, and inorganic materials, expanded the toolkit for nanoparticle design.
Recent years have witnessed a shift towards more sophisticated nanoparticle architectures. Multi-functional nanoparticles capable of simultaneous imaging and drug delivery have emerged, paving the way for theranostic applications. The integration of artificial intelligence and machine learning in nanoparticle design has accelerated the optimization process, enabling rapid screening of countless design parameters.
The current focus on geometric isomers in therapeutic nanoparticle design represents a cutting-edge approach to fine-tuning nanoparticle properties. This strategy aims to exploit subtle structural differences to enhance drug loading capacity, cellular uptake, and overall therapeutic efficacy. By manipulating the spatial arrangement of atoms or functional groups within the nanoparticle structure, researchers seek to unlock new possibilities in drug delivery and targeting.
The primary objectives of this evolving field include improving drug efficacy, reducing side effects, and overcoming biological barriers. Researchers aim to develop nanoparticles with enhanced stability in biological environments, prolonged circulation times, and precise targeting capabilities. Additionally, there is a growing emphasis on creating biocompatible and biodegradable nanoparticles to address safety concerns and facilitate clinical translation.
Looking ahead, the field of therapeutic nanoparticle design is poised to tackle several ambitious goals. These include the development of nanoparticles capable of crossing the blood-brain barrier for treating neurological disorders, creating nanoparticle-based vaccines for infectious diseases and cancer immunotherapy, and engineering nanoparticles for gene therapy and CRISPR-based genome editing. The ultimate objective is to realize the full potential of nanomedicine in revolutionizing patient care and treatment outcomes.
The advent of stimuli-responsive nanoparticles marked a crucial milestone in this evolution. These smart systems could release their payload in response to specific environmental cues such as pH, temperature, or enzymatic activity, enhancing therapeutic efficacy while minimizing side effects. Concurrently, the exploration of various nanoparticle compositions, including polymeric, lipid-based, and inorganic materials, expanded the toolkit for nanoparticle design.
Recent years have witnessed a shift towards more sophisticated nanoparticle architectures. Multi-functional nanoparticles capable of simultaneous imaging and drug delivery have emerged, paving the way for theranostic applications. The integration of artificial intelligence and machine learning in nanoparticle design has accelerated the optimization process, enabling rapid screening of countless design parameters.
The current focus on geometric isomers in therapeutic nanoparticle design represents a cutting-edge approach to fine-tuning nanoparticle properties. This strategy aims to exploit subtle structural differences to enhance drug loading capacity, cellular uptake, and overall therapeutic efficacy. By manipulating the spatial arrangement of atoms or functional groups within the nanoparticle structure, researchers seek to unlock new possibilities in drug delivery and targeting.
The primary objectives of this evolving field include improving drug efficacy, reducing side effects, and overcoming biological barriers. Researchers aim to develop nanoparticles with enhanced stability in biological environments, prolonged circulation times, and precise targeting capabilities. Additionally, there is a growing emphasis on creating biocompatible and biodegradable nanoparticles to address safety concerns and facilitate clinical translation.
Looking ahead, the field of therapeutic nanoparticle design is poised to tackle several ambitious goals. These include the development of nanoparticles capable of crossing the blood-brain barrier for treating neurological disorders, creating nanoparticle-based vaccines for infectious diseases and cancer immunotherapy, and engineering nanoparticles for gene therapy and CRISPR-based genome editing. The ultimate objective is to realize the full potential of nanomedicine in revolutionizing patient care and treatment outcomes.
Therapeutic Nanoparticle Market Analysis
The therapeutic nanoparticle market has been experiencing significant growth and is poised for further expansion in the coming years. This growth is driven by the increasing prevalence of chronic diseases, advancements in nanotechnology, and the rising demand for targeted drug delivery systems. The global therapeutic nanoparticle market was valued at approximately $50 billion in 2020 and is projected to reach over $100 billion by 2026, with a compound annual growth rate (CAGR) of around 12%.
One of the key factors contributing to market growth is the ability of therapeutic nanoparticles to enhance drug efficacy and reduce side effects. This is particularly important in cancer treatment, where nanoparticles can selectively target tumor cells, minimizing damage to healthy tissues. As a result, oncology applications currently dominate the therapeutic nanoparticle market, accounting for over 40% of the total market share.
The market is also witnessing increased demand in other therapeutic areas, including cardiovascular diseases, neurodegenerative disorders, and infectious diseases. This diversification of applications is expected to further drive market growth in the coming years.
Geographically, North America holds the largest market share, followed by Europe and Asia-Pacific. The United States, in particular, is a major contributor to market growth due to its advanced healthcare infrastructure and significant investments in research and development. However, emerging economies in Asia-Pacific, such as China and India, are expected to exhibit the highest growth rates in the coming years, driven by increasing healthcare expenditure and growing awareness of advanced treatment options.
The therapeutic nanoparticle market is characterized by intense competition and rapid technological advancements. Key players in the market include Merck & Co., Pfizer, Novartis, and Johnson & Johnson, among others. These companies are investing heavily in research and development to develop innovative nanoparticle-based therapies and gain a competitive edge in the market.
Recent trends in the market include the development of multifunctional nanoparticles that combine therapeutic and diagnostic capabilities, known as theranostics. This approach allows for simultaneous treatment and monitoring of disease progression, potentially revolutionizing personalized medicine. Additionally, there is growing interest in the use of biodegradable and biocompatible materials for nanoparticle synthesis, addressing concerns about long-term toxicity and environmental impact.
One of the key factors contributing to market growth is the ability of therapeutic nanoparticles to enhance drug efficacy and reduce side effects. This is particularly important in cancer treatment, where nanoparticles can selectively target tumor cells, minimizing damage to healthy tissues. As a result, oncology applications currently dominate the therapeutic nanoparticle market, accounting for over 40% of the total market share.
The market is also witnessing increased demand in other therapeutic areas, including cardiovascular diseases, neurodegenerative disorders, and infectious diseases. This diversification of applications is expected to further drive market growth in the coming years.
Geographically, North America holds the largest market share, followed by Europe and Asia-Pacific. The United States, in particular, is a major contributor to market growth due to its advanced healthcare infrastructure and significant investments in research and development. However, emerging economies in Asia-Pacific, such as China and India, are expected to exhibit the highest growth rates in the coming years, driven by increasing healthcare expenditure and growing awareness of advanced treatment options.
The therapeutic nanoparticle market is characterized by intense competition and rapid technological advancements. Key players in the market include Merck & Co., Pfizer, Novartis, and Johnson & Johnson, among others. These companies are investing heavily in research and development to develop innovative nanoparticle-based therapies and gain a competitive edge in the market.
Recent trends in the market include the development of multifunctional nanoparticles that combine therapeutic and diagnostic capabilities, known as theranostics. This approach allows for simultaneous treatment and monitoring of disease progression, potentially revolutionizing personalized medicine. Additionally, there is growing interest in the use of biodegradable and biocompatible materials for nanoparticle synthesis, addressing concerns about long-term toxicity and environmental impact.
Geometric Isomers in Nanoparticle Design: Current Status
The field of geometric isomers in therapeutic nanoparticle design has seen significant advancements in recent years. Researchers have recognized the importance of molecular geometry in influencing the behavior and efficacy of nanoparticles for drug delivery and other therapeutic applications. Currently, the focus is on understanding and exploiting the unique properties of geometric isomers to enhance nanoparticle performance.
One of the key areas of progress is in the development of precise synthesis methods for creating nanoparticles with specific geometric configurations. Scientists have made strides in controlling the spatial arrangement of atoms and molecules within nanoparticles, allowing for the creation of isomeric structures with tailored properties. This level of control has opened up new possibilities for optimizing drug encapsulation, release kinetics, and targeting efficiency.
The current status of the field also reflects a growing emphasis on the relationship between geometric isomerism and nanoparticle-cell interactions. Researchers have observed that subtle changes in the geometric configuration of nanoparticles can significantly impact their cellular uptake, biodistribution, and therapeutic efficacy. This has led to the development of isomer-specific nanoparticle designs that can enhance drug delivery to specific tissues or cell types.
Another important aspect of the current state of research is the investigation of how geometric isomers affect the stability and degradation of nanoparticles in biological environments. Studies have shown that different isomeric forms can exhibit varying degrees of resistance to enzymatic breakdown and clearance from the body, potentially leading to improved pharmacokinetic profiles for therapeutic nanoparticles.
The field is also witnessing increased efforts in computational modeling and simulation of geometric isomers in nanoparticle systems. These in silico approaches are enabling researchers to predict the behavior of different isomeric configurations and guide experimental design, accelerating the development of more effective nanoparticle-based therapies.
Furthermore, there is growing interest in leveraging geometric isomerism for the development of stimuli-responsive nanoparticles. Researchers are exploring how external stimuli such as light, pH, or temperature can induce conformational changes in geometric isomers, potentially allowing for controlled release of therapeutic payloads or activation of specific functionalities.
One of the key areas of progress is in the development of precise synthesis methods for creating nanoparticles with specific geometric configurations. Scientists have made strides in controlling the spatial arrangement of atoms and molecules within nanoparticles, allowing for the creation of isomeric structures with tailored properties. This level of control has opened up new possibilities for optimizing drug encapsulation, release kinetics, and targeting efficiency.
The current status of the field also reflects a growing emphasis on the relationship between geometric isomerism and nanoparticle-cell interactions. Researchers have observed that subtle changes in the geometric configuration of nanoparticles can significantly impact their cellular uptake, biodistribution, and therapeutic efficacy. This has led to the development of isomer-specific nanoparticle designs that can enhance drug delivery to specific tissues or cell types.
Another important aspect of the current state of research is the investigation of how geometric isomers affect the stability and degradation of nanoparticles in biological environments. Studies have shown that different isomeric forms can exhibit varying degrees of resistance to enzymatic breakdown and clearance from the body, potentially leading to improved pharmacokinetic profiles for therapeutic nanoparticles.
The field is also witnessing increased efforts in computational modeling and simulation of geometric isomers in nanoparticle systems. These in silico approaches are enabling researchers to predict the behavior of different isomeric configurations and guide experimental design, accelerating the development of more effective nanoparticle-based therapies.
Furthermore, there is growing interest in leveraging geometric isomerism for the development of stimuli-responsive nanoparticles. Researchers are exploring how external stimuli such as light, pH, or temperature can induce conformational changes in geometric isomers, potentially allowing for controlled release of therapeutic payloads or activation of specific functionalities.
Geometric Isomer-based Nanoparticle Solutions
01 Geometric isomer design in nanoparticle synthesis
The design of geometric isomers plays a crucial role in nanoparticle synthesis. By controlling the spatial arrangement of atoms or molecules, researchers can create nanoparticles with specific shapes and properties. This approach allows for the tailoring of nanoparticles for various applications, including drug delivery, catalysis, and optical devices.- Geometric isomer design in nanoparticle synthesis: The design of geometric isomers plays a crucial role in nanoparticle synthesis. By controlling the spatial arrangement of atoms or molecules, researchers can create nanoparticles with specific shapes and properties. This approach allows for the tailoring of nanoparticles for various applications, including drug delivery, catalysis, and sensing.
- Computational modeling of geometric isomers in nanoparticles: Advanced computational techniques are employed to model and predict the behavior of geometric isomers in nanoparticle structures. These models help researchers understand the relationship between isomer configuration and nanoparticle properties, enabling more efficient design and optimization of nanoparticle-based systems.
- Characterization methods for geometric isomers in nanoparticles: Various analytical techniques are used to characterize geometric isomers in nanoparticles. These methods include spectroscopic techniques, microscopy, and diffraction-based approaches. Accurate characterization is essential for understanding the structure-property relationships of nanoparticles containing geometric isomers.
- Applications of geometric isomer-based nanoparticles: Nanoparticles designed with specific geometric isomers find applications in various fields. These include targeted drug delivery systems, enhanced catalytic materials, and advanced sensing devices. The unique properties arising from the geometric arrangement of isomers in nanoparticles contribute to their effectiveness in these applications.
- Visualization and representation of geometric isomers in nanoparticles: Specialized visualization techniques and software tools are developed to represent and analyze geometric isomers in nanoparticles. These tools aid in the design process, allowing researchers to manipulate and study complex isomeric structures at the nanoscale level, facilitating better understanding and optimization of nanoparticle designs.
02 Computational modeling of geometric isomers in nanoparticles
Advanced computational techniques are employed to model and predict the behavior of geometric isomers in nanoparticle structures. These models help researchers understand the relationship between isomer configuration and nanoparticle properties, enabling more efficient design and optimization of nanoparticle-based systems.Expand Specific Solutions03 Characterization methods for geometric isomers in nanoparticles
Various analytical techniques are used to characterize geometric isomers in nanoparticles. These methods include spectroscopic techniques, electron microscopy, and X-ray diffraction, which provide detailed information about the spatial arrangement and composition of nanoparticle structures. Such characterization is essential for validating design concepts and ensuring the quality of synthesized nanoparticles.Expand Specific Solutions04 Applications of geometric isomer-based nanoparticles
Nanoparticles designed with specific geometric isomers find applications in various fields. These include targeted drug delivery systems, where the isomer configuration affects the nanoparticle's interaction with biological systems, and in the development of novel catalysts, where the spatial arrangement of atoms can enhance catalytic activity.Expand Specific Solutions05 Visualization and representation of geometric isomers in nanoparticles
Advanced visualization techniques are employed to represent and analyze geometric isomers in nanoparticles. These include 3D modeling software and virtual reality systems that allow researchers to interact with and manipulate nanoparticle structures, facilitating a deeper understanding of isomer configurations and their effects on nanoparticle properties.Expand Specific Solutions
Key Players in Therapeutic Nanoparticle Industry
The field of therapeutic nanoparticle design utilizing geometric isomers is in its early developmental stages, with significant potential for growth. The market size is expanding as researchers explore novel applications in drug delivery and targeted therapies. While the technology is still evolving, several key players are driving innovation. Academic institutions like Case Western Reserve University, Washington University in St. Louis, and Yale University are conducting foundational research. Pharmaceutical giants such as Pfizer, AbbVie, and Agilent Technologies are investing in translating this research into commercial applications. The competitive landscape is characterized by collaborations between academia and industry, with a focus on developing patentable technologies and overcoming regulatory hurdles for clinical implementation.
Pfizer Inc.
Technical Solution: Pfizer has developed a novel approach to therapeutic nanoparticle design utilizing geometric isomers. Their technology involves creating nanoparticles with specific geometric configurations to enhance drug delivery and efficacy. The company employs advanced computational modeling to predict and optimize the three-dimensional structure of nanoparticles, allowing for precise control over their shape and surface properties[1]. This approach enables the creation of nanoparticles with improved stability, targeted delivery, and controlled release profiles. Pfizer's method incorporates the use of biodegradable polymers and lipids to form nanoparticles with distinct geometric isomers, each tailored for specific therapeutic applications[3]. The company has also invested in developing scalable manufacturing processes to ensure consistent production of these geometrically-optimized nanoparticles for clinical use[5].
Strengths: Advanced computational modeling capabilities, precise control over nanoparticle geometry, potential for improved drug efficacy and reduced side effects. Weaknesses: Complex manufacturing process, potential regulatory challenges due to novel approach.
AbbVie, Inc.
Technical Solution: AbbVie has focused on leveraging geometric isomers in nanoparticle design to enhance the delivery of small molecule drugs and biologics. Their approach involves creating nanoparticles with specific geometric configurations that can improve drug solubility, stability, and cellular uptake. AbbVie's technology platform utilizes a combination of lipid-based and polymer-based nanoparticles, each designed with precise geometric isomers to optimize drug encapsulation and release kinetics[2]. The company has developed a proprietary method for controlling the self-assembly of nanoparticles, allowing for the creation of complex geometric structures with multiple compartments for drug loading[4]. This multi-compartment approach enables the co-delivery of multiple therapeutic agents with different physicochemical properties, potentially enhancing treatment efficacy for complex diseases[6].
Strengths: Versatile platform for both small molecules and biologics, potential for combination therapies. Weaknesses: Complexity in manufacturing and quality control, potential for increased production costs.
Breakthrough Isomer Technologies for Nanoparticles
nanoparticles
PatentActiveUS20180177728A1
Innovation
- Development of thermosensitive lipid nanoparticles (LNPs) comprising phospholipids, lysolipids, hydrophilic polymers, and structural lipids with MRI and NIRF labels, which are administered with high-frequency ultrasound to enhance drug delivery and monitoring, allowing for image-guided focused hyperthermia.
Therapeutic nanoparticles comprising a therapeutic agent and methods of making and using same
PatentActiveUS20150258102A1
Innovation
- Incorporation of a substantially hydrophobic acid to stabilize the basic therapeutic agent in the nanoparticle formulation.
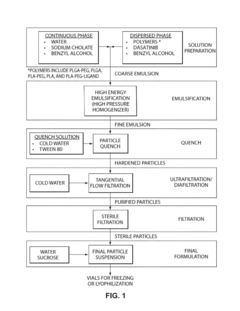
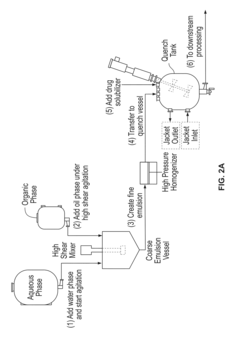
- Use of diblock copolymers (PLA-PEG or PLGA-PEG) to create a nanoparticle structure with controlled drug release properties.
- Precise control of PEG content (10-30 wt%) in the nanoparticle to balance drug loading, stability, and circulation time.
Regulatory Framework for Nanomedicine
The regulatory framework for nanomedicine is a complex and evolving landscape that aims to ensure the safety and efficacy of nanoparticle-based therapeutic interventions. As the field of nanomedicine continues to advance, regulatory agencies worldwide are adapting their approaches to address the unique challenges posed by nanoscale materials in medical applications.
In the United States, the Food and Drug Administration (FDA) has taken a lead role in developing guidelines for the regulation of nanomedicines. The FDA's approach is product-specific, considering the intended use and potential risks associated with each nanomedicine application. The agency has established the Nanotechnology Task Force to coordinate regulatory efforts across different centers within the FDA.
The European Medicines Agency (EMA) has also been proactive in addressing the regulatory needs of nanomedicines. The EMA has published several reflection papers and guidelines specific to nanomedicines, including those related to block copolymer micelle products and intravenous liposomal products. These documents provide guidance on quality, safety, and efficacy considerations for nanomedicine development.
Internationally, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) plays a crucial role in harmonizing regulatory standards across different regions. While there are no specific ICH guidelines for nanomedicines yet, existing guidelines on quality, safety, and efficacy are being applied and adapted to nanomedicine products.
One of the key challenges in regulating nanomedicines is the characterization of nanoparticles. Regulatory agencies require comprehensive physicochemical characterization data, including size distribution, surface properties, and stability. The FDA and EMA have emphasized the importance of developing standardized methods for nanoparticle characterization to ensure consistency in regulatory submissions.
Safety assessment of nanomedicines presents unique challenges due to their small size and potential for altered biodistribution compared to conventional drugs. Regulatory frameworks are evolving to address these concerns, with increased focus on long-term safety studies and potential accumulation of nanoparticles in organs.
The regulatory landscape for nanomedicines is also influenced by environmental and ethical considerations. Agencies are developing guidelines for assessing the environmental impact of nanomaterials and addressing potential ethical issues related to their use in medicine.
As the field of nanomedicine continues to evolve, regulatory frameworks are expected to become more refined and specific. Collaboration between regulatory agencies, researchers, and industry stakeholders will be crucial in developing appropriate guidelines that balance innovation with patient safety.
In the United States, the Food and Drug Administration (FDA) has taken a lead role in developing guidelines for the regulation of nanomedicines. The FDA's approach is product-specific, considering the intended use and potential risks associated with each nanomedicine application. The agency has established the Nanotechnology Task Force to coordinate regulatory efforts across different centers within the FDA.
The European Medicines Agency (EMA) has also been proactive in addressing the regulatory needs of nanomedicines. The EMA has published several reflection papers and guidelines specific to nanomedicines, including those related to block copolymer micelle products and intravenous liposomal products. These documents provide guidance on quality, safety, and efficacy considerations for nanomedicine development.
Internationally, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) plays a crucial role in harmonizing regulatory standards across different regions. While there are no specific ICH guidelines for nanomedicines yet, existing guidelines on quality, safety, and efficacy are being applied and adapted to nanomedicine products.
One of the key challenges in regulating nanomedicines is the characterization of nanoparticles. Regulatory agencies require comprehensive physicochemical characterization data, including size distribution, surface properties, and stability. The FDA and EMA have emphasized the importance of developing standardized methods for nanoparticle characterization to ensure consistency in regulatory submissions.
Safety assessment of nanomedicines presents unique challenges due to their small size and potential for altered biodistribution compared to conventional drugs. Regulatory frameworks are evolving to address these concerns, with increased focus on long-term safety studies and potential accumulation of nanoparticles in organs.
The regulatory landscape for nanomedicines is also influenced by environmental and ethical considerations. Agencies are developing guidelines for assessing the environmental impact of nanomaterials and addressing potential ethical issues related to their use in medicine.
As the field of nanomedicine continues to evolve, regulatory frameworks are expected to become more refined and specific. Collaboration between regulatory agencies, researchers, and industry stakeholders will be crucial in developing appropriate guidelines that balance innovation with patient safety.
Nanoparticle Safety and Toxicology Considerations
The safety and toxicology considerations of nanoparticles are crucial aspects in therapeutic nanoparticle design, particularly when exploring the potential of geometric isomers. As nanoparticles interact with biological systems in complex ways, their safety profiles can be significantly influenced by their geometric properties.
One of the primary concerns in nanoparticle safety is their ability to penetrate cellular membranes and accumulate in various organs. The geometric isomerism of nanoparticles can affect their cellular uptake and biodistribution, potentially leading to unexpected toxicological outcomes. For instance, rod-shaped nanoparticles may exhibit different tissue penetration patterns compared to their spherical counterparts, necessitating thorough evaluation of each geometric variant.
The surface chemistry of nanoparticles, which can be altered by geometric isomerism, plays a vital role in their toxicological profile. Changes in surface area and curvature can affect protein adsorption, leading to the formation of protein coronas that may influence nanoparticle-cell interactions. This, in turn, can impact the immune response and potential inflammatory reactions triggered by the nanoparticles.
Oxidative stress induction is another critical factor in nanoparticle toxicity. The geometric configuration of nanoparticles can influence their catalytic properties, potentially leading to varying degrees of reactive oxygen species (ROS) generation. Understanding these structure-activity relationships is essential for predicting and mitigating potential oxidative damage to cells and tissues.
The clearance mechanisms of nanoparticles from the body are also affected by their geometric properties. Differences in shape and size can impact the rate of renal filtration, hepatic metabolism, and immune system recognition. Consequently, the persistence of nanoparticles in the body may vary among geometric isomers, necessitating long-term toxicity studies for each variant.
In the context of therapeutic nanoparticle design, it is crucial to consider the potential for genotoxicity and carcinogenicity. The interaction of geometrically diverse nanoparticles with genetic material may differ, potentially leading to varying degrees of DNA damage or mutagenic effects. Comprehensive genotoxicity assessments must be conducted to ensure the safety of each geometric isomer.
To address these safety and toxicology considerations, researchers must employ a multifaceted approach. This includes in vitro studies to assess cellular uptake, cytotoxicity, and genotoxicity, as well as in vivo studies to evaluate biodistribution, organ-specific toxicity, and long-term effects. Advanced imaging techniques and computational modeling can provide valuable insights into the behavior of geometric isomers in biological systems.
One of the primary concerns in nanoparticle safety is their ability to penetrate cellular membranes and accumulate in various organs. The geometric isomerism of nanoparticles can affect their cellular uptake and biodistribution, potentially leading to unexpected toxicological outcomes. For instance, rod-shaped nanoparticles may exhibit different tissue penetration patterns compared to their spherical counterparts, necessitating thorough evaluation of each geometric variant.
The surface chemistry of nanoparticles, which can be altered by geometric isomerism, plays a vital role in their toxicological profile. Changes in surface area and curvature can affect protein adsorption, leading to the formation of protein coronas that may influence nanoparticle-cell interactions. This, in turn, can impact the immune response and potential inflammatory reactions triggered by the nanoparticles.
Oxidative stress induction is another critical factor in nanoparticle toxicity. The geometric configuration of nanoparticles can influence their catalytic properties, potentially leading to varying degrees of reactive oxygen species (ROS) generation. Understanding these structure-activity relationships is essential for predicting and mitigating potential oxidative damage to cells and tissues.
The clearance mechanisms of nanoparticles from the body are also affected by their geometric properties. Differences in shape and size can impact the rate of renal filtration, hepatic metabolism, and immune system recognition. Consequently, the persistence of nanoparticles in the body may vary among geometric isomers, necessitating long-term toxicity studies for each variant.
In the context of therapeutic nanoparticle design, it is crucial to consider the potential for genotoxicity and carcinogenicity. The interaction of geometrically diverse nanoparticles with genetic material may differ, potentially leading to varying degrees of DNA damage or mutagenic effects. Comprehensive genotoxicity assessments must be conducted to ensure the safety of each geometric isomer.
To address these safety and toxicology considerations, researchers must employ a multifaceted approach. This includes in vitro studies to assess cellular uptake, cytotoxicity, and genotoxicity, as well as in vivo studies to evaluate biodistribution, organ-specific toxicity, and long-term effects. Advanced imaging techniques and computational modeling can provide valuable insights into the behavior of geometric isomers in biological systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!