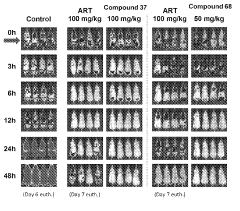
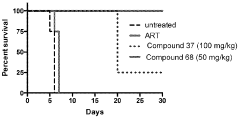
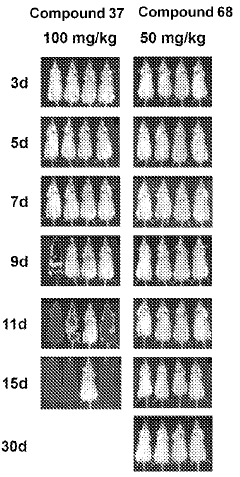
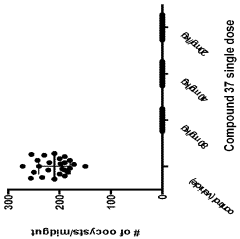
The Effect of Geometric Isomers on Solution Conductivity
AUG 1, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Geometric Isomers and Conductivity: Background and Objectives
Geometric isomers, a fascinating subset of stereoisomers, have long intrigued chemists and researchers due to their unique structural properties and their potential impact on various chemical and physical phenomena. The study of geometric isomers and their effect on solution conductivity represents a critical intersection of organic chemistry, physical chemistry, and materials science. This research area has gained significant attention in recent years, driven by the growing demand for advanced materials with tailored electrical properties in industries ranging from electronics to energy storage.
The evolution of this field can be traced back to the early 20th century when the concept of geometric isomerism was first established. However, it wasn't until the advent of modern analytical techniques and computational methods that researchers could delve deeper into the relationship between molecular structure and electrical conductivity. The past few decades have witnessed a surge in research activities aimed at understanding how subtle differences in molecular geometry can lead to significant variations in the conductivity of solutions.
As we look towards the future, the trajectory of this field appears to be heading towards more sophisticated applications and a deeper understanding of the underlying mechanisms. The primary objective of current research efforts is to elucidate the precise nature of the relationship between geometric isomerism and solution conductivity. This involves not only investigating the direct effects of molecular structure on ion mobility and charge transport but also exploring the secondary effects such as solvent interactions and ion-pairing phenomena.
Another crucial goal is to develop predictive models that can accurately forecast the conductivity of solutions based on the geometric isomers present. Such models would be invaluable in the design of new materials and the optimization of existing systems. Additionally, researchers aim to exploit the unique properties of geometric isomers to create novel conductive materials with tunable electrical characteristics.
The potential applications of this research are vast and diverse. In the field of energy storage, understanding the impact of geometric isomers on conductivity could lead to the development of more efficient electrolytes for batteries and supercapacitors. In the realm of organic electronics, this knowledge could pave the way for new conductive polymers with enhanced charge transport properties. Moreover, the insights gained from this research could have far-reaching implications in areas such as electrochemistry, sensor technology, and even biological systems where ion transport plays a crucial role.
The evolution of this field can be traced back to the early 20th century when the concept of geometric isomerism was first established. However, it wasn't until the advent of modern analytical techniques and computational methods that researchers could delve deeper into the relationship between molecular structure and electrical conductivity. The past few decades have witnessed a surge in research activities aimed at understanding how subtle differences in molecular geometry can lead to significant variations in the conductivity of solutions.
As we look towards the future, the trajectory of this field appears to be heading towards more sophisticated applications and a deeper understanding of the underlying mechanisms. The primary objective of current research efforts is to elucidate the precise nature of the relationship between geometric isomerism and solution conductivity. This involves not only investigating the direct effects of molecular structure on ion mobility and charge transport but also exploring the secondary effects such as solvent interactions and ion-pairing phenomena.
Another crucial goal is to develop predictive models that can accurately forecast the conductivity of solutions based on the geometric isomers present. Such models would be invaluable in the design of new materials and the optimization of existing systems. Additionally, researchers aim to exploit the unique properties of geometric isomers to create novel conductive materials with tunable electrical characteristics.
The potential applications of this research are vast and diverse. In the field of energy storage, understanding the impact of geometric isomers on conductivity could lead to the development of more efficient electrolytes for batteries and supercapacitors. In the realm of organic electronics, this knowledge could pave the way for new conductive polymers with enhanced charge transport properties. Moreover, the insights gained from this research could have far-reaching implications in areas such as electrochemistry, sensor technology, and even biological systems where ion transport plays a crucial role.
Market Analysis for Isomer-Based Conductive Solutions
The market for isomer-based conductive solutions is experiencing significant growth, driven by increasing demand across various industries. The unique properties of geometric isomers, particularly their ability to influence solution conductivity, have opened up new possibilities in electronics, energy storage, and materials science.
In the electronics sector, isomer-based conductive solutions are finding applications in flexible and printed electronics. The market for these advanced materials is projected to expand rapidly as manufacturers seek more efficient and adaptable components for next-generation devices. The automotive industry is also showing keen interest, particularly in the development of improved battery technologies and sensor systems.
The energy storage market presents another substantial opportunity for isomer-based conductive solutions. With the global push towards renewable energy and electric vehicles, there is a growing need for advanced electrolytes and electrode materials. Isomers that can enhance conductivity while maintaining stability are highly sought after in this sector.
In the field of materials science, researchers are exploring the potential of isomer-based conductive solutions for smart materials and coatings. These applications could revolutionize industries ranging from aerospace to consumer electronics, offering improved performance and new functionalities.
The healthcare and biomedical sectors are emerging as promising markets for isomer-based conductive solutions. Applications in biosensors, drug delivery systems, and tissue engineering are being actively researched, with potential for significant market growth in the coming years.
Geographically, North America and Europe currently lead in research and development of isomer-based conductive solutions, but Asia-Pacific is expected to see the fastest market growth. This is largely due to the region's expanding electronics manufacturing base and increasing investments in advanced materials research.
Market analysts predict that the global market for isomer-based conductive solutions will grow at a compound annual growth rate (CAGR) of over 8% in the next five years. This growth is expected to be fueled by advancements in synthesis techniques, increasing awareness of the benefits of isomer-based materials, and the continuous drive for innovation in high-tech industries.
However, challenges remain in scaling up production and reducing costs, which could impact market penetration in price-sensitive applications. Additionally, regulatory considerations, particularly in healthcare applications, may influence the pace of market adoption in certain sectors.
In the electronics sector, isomer-based conductive solutions are finding applications in flexible and printed electronics. The market for these advanced materials is projected to expand rapidly as manufacturers seek more efficient and adaptable components for next-generation devices. The automotive industry is also showing keen interest, particularly in the development of improved battery technologies and sensor systems.
The energy storage market presents another substantial opportunity for isomer-based conductive solutions. With the global push towards renewable energy and electric vehicles, there is a growing need for advanced electrolytes and electrode materials. Isomers that can enhance conductivity while maintaining stability are highly sought after in this sector.
In the field of materials science, researchers are exploring the potential of isomer-based conductive solutions for smart materials and coatings. These applications could revolutionize industries ranging from aerospace to consumer electronics, offering improved performance and new functionalities.
The healthcare and biomedical sectors are emerging as promising markets for isomer-based conductive solutions. Applications in biosensors, drug delivery systems, and tissue engineering are being actively researched, with potential for significant market growth in the coming years.
Geographically, North America and Europe currently lead in research and development of isomer-based conductive solutions, but Asia-Pacific is expected to see the fastest market growth. This is largely due to the region's expanding electronics manufacturing base and increasing investments in advanced materials research.
Market analysts predict that the global market for isomer-based conductive solutions will grow at a compound annual growth rate (CAGR) of over 8% in the next five years. This growth is expected to be fueled by advancements in synthesis techniques, increasing awareness of the benefits of isomer-based materials, and the continuous drive for innovation in high-tech industries.
However, challenges remain in scaling up production and reducing costs, which could impact market penetration in price-sensitive applications. Additionally, regulatory considerations, particularly in healthcare applications, may influence the pace of market adoption in certain sectors.
Current Challenges in Isomer-Conductivity Research
The field of isomer-conductivity research faces several significant challenges that hinder progress and limit our understanding of the relationship between geometric isomers and solution conductivity. One of the primary obstacles is the complexity of isolating and studying individual isomers in solution. Geometric isomers often exist in equilibrium, making it difficult to measure the conductivity effects of a single isomer without interference from its counterparts.
Another challenge lies in the development of precise measurement techniques capable of detecting subtle differences in conductivity caused by isomeric variations. Current methods may lack the sensitivity required to accurately quantify these small but potentially significant changes, leading to inconsistent or inconclusive results across different studies.
The influence of environmental factors on isomer-conductivity relationships presents an additional hurdle. Temperature, pressure, and solvent properties can all affect both the isomeric equilibrium and the overall conductivity of the solution. Controlling these variables to ensure reproducible results across different experimental setups remains a significant challenge for researchers in the field.
Furthermore, the lack of comprehensive theoretical models to explain the observed conductivity differences between geometric isomers hampers progress. While some theories have been proposed, they often fail to account for all observed phenomena, leaving gaps in our understanding of the underlying mechanisms.
Researchers also face difficulties in synthesizing and purifying sufficient quantities of specific geometric isomers for conductivity studies. This limitation often results in a scarcity of data for certain isomeric pairs, making it challenging to draw broad conclusions about the relationship between molecular structure and conductivity.
The interdisciplinary nature of isomer-conductivity research presents its own set of challenges. Effective collaboration between chemists, physicists, and materials scientists is crucial for advancing the field, but differences in terminology, methodologies, and research priorities can impede progress.
Lastly, the application of findings from isomer-conductivity studies to real-world scenarios remains a significant challenge. Translating laboratory results into practical applications, such as the design of more efficient electrolytes or conductive materials, requires overcoming numerous technical and engineering obstacles.
Another challenge lies in the development of precise measurement techniques capable of detecting subtle differences in conductivity caused by isomeric variations. Current methods may lack the sensitivity required to accurately quantify these small but potentially significant changes, leading to inconsistent or inconclusive results across different studies.
The influence of environmental factors on isomer-conductivity relationships presents an additional hurdle. Temperature, pressure, and solvent properties can all affect both the isomeric equilibrium and the overall conductivity of the solution. Controlling these variables to ensure reproducible results across different experimental setups remains a significant challenge for researchers in the field.
Furthermore, the lack of comprehensive theoretical models to explain the observed conductivity differences between geometric isomers hampers progress. While some theories have been proposed, they often fail to account for all observed phenomena, leaving gaps in our understanding of the underlying mechanisms.
Researchers also face difficulties in synthesizing and purifying sufficient quantities of specific geometric isomers for conductivity studies. This limitation often results in a scarcity of data for certain isomeric pairs, making it challenging to draw broad conclusions about the relationship between molecular structure and conductivity.
The interdisciplinary nature of isomer-conductivity research presents its own set of challenges. Effective collaboration between chemists, physicists, and materials scientists is crucial for advancing the field, but differences in terminology, methodologies, and research priorities can impede progress.
Lastly, the application of findings from isomer-conductivity studies to real-world scenarios remains a significant challenge. Translating laboratory results into practical applications, such as the design of more efficient electrolytes or conductive materials, requires overcoming numerous technical and engineering obstacles.
Existing Methods for Measuring Isomer-Induced Conductivity
01 Conductivity differences in geometric isomers
Geometric isomers can exhibit different electrical conductivity properties due to variations in their molecular structure. These differences can be attributed to factors such as bond angles, molecular symmetry, and electron distribution, which affect the overall electronic properties of the compounds.- Conductivity differences in geometric isomers: Geometric isomers can exhibit different electrical conductivity properties due to variations in their molecular structure. These differences can be attributed to factors such as bond angles, molecular symmetry, and electron distribution, which affect the overall electronic properties of the compounds.
- Measurement techniques for isomer conductivity: Various methods and devices have been developed to measure and compare the conductivity of geometric isomers. These techniques may involve specialized sensors, electrochemical cells, or spectroscopic methods to accurately determine the electrical properties of different isomeric forms.
- Applications of isomer conductivity in materials science: The conductivity differences between geometric isomers have found applications in materials science, particularly in the development of novel electronic materials and devices. By exploiting these properties, researchers can design materials with tailored electrical characteristics for specific applications.
- Computational modeling of isomer conductivity: Advanced computational methods have been employed to model and predict the conductivity of geometric isomers. These simulations help researchers understand the underlying mechanisms of conductivity differences and guide the design of new materials with desired electrical properties.
- Influence of environmental factors on isomer conductivity: Environmental factors such as temperature, pressure, and solvent effects can significantly impact the conductivity of geometric isomers. Understanding these influences is crucial for accurately characterizing and utilizing the electrical properties of isomeric compounds in various applications.
02 Measurement techniques for isomer conductivity
Various techniques and instruments are used to measure and compare the conductivity of geometric isomers. These may include specialized electrodes, impedance spectroscopy, or other electrical characterization methods that can detect subtle differences in conductivity between isomeric forms.Expand Specific Solutions03 Applications of isomer conductivity in materials science
The conductivity differences between geometric isomers are exploited in materials science for developing novel electronic materials, sensors, and devices. By selectively using specific isomers or isomer mixtures, researchers can fine-tune the electrical properties of materials for various applications.Expand Specific Solutions04 Computational modeling of isomer conductivity
Advanced computational methods are employed to predict and model the conductivity of geometric isomers. These simulations help researchers understand the relationship between molecular structure and electrical properties, guiding the design of new materials with desired conductivity characteristics.Expand Specific Solutions05 Influence of environmental factors on isomer conductivity
Environmental factors such as temperature, pressure, and solvent effects can significantly impact the conductivity of geometric isomers. Understanding these influences is crucial for accurately characterizing and utilizing the electrical properties of isomeric compounds in various applications and conditions.Expand Specific Solutions
Key Players in Geometric Isomer Research
The research into "The Effect of Geometric Isomers on Solution Conductivity" is in a developing stage, with potential for significant growth. The market size is expanding as industries recognize the importance of understanding isomeric effects on conductivity. While the technology is not yet fully mature, several key players are advancing the field. Universities like The Regents of the University of California and Northwestern University are leading academic research, while companies such as BASF Corp. and Sumitomo Chemical Co., Ltd. are likely focusing on industrial applications. The involvement of diverse organizations, from academic institutions to large chemical corporations, indicates a competitive landscape with opportunities for both fundamental research and practical innovations.
The Regents of the University of California
Technical Solution: The University of California has conducted extensive research on the effect of geometric isomers on solution conductivity. Their approach involves studying various organic compounds with different geometric isomers and their impact on ionic conductivity in solutions. They have developed a novel method to synthesize and characterize geometric isomers of conductive polymers, which has shown significant differences in conductivity based on the spatial arrangement of atoms[1]. Their research also includes the use of advanced spectroscopic techniques to analyze the molecular structure and interactions of isomers in solution, providing insights into how geometric configuration affects charge transport mechanisms[2]. Additionally, they have explored the application of these findings in developing more efficient electrolytes for energy storage devices[3].
Strengths: Comprehensive research approach, advanced characterization techniques, and practical applications in energy storage. Weaknesses: Potential limitations in scaling up synthesis methods for industrial applications.
BASF Corp.
Technical Solution: BASF Corp. has developed innovative approaches to study the effect of geometric isomers on solution conductivity, particularly in the context of electrolyte solutions for batteries and other electrochemical applications. Their research focuses on synthesizing and characterizing novel organic electrolytes with different geometric isomers, aiming to optimize ionic conductivity and electrochemical stability[1]. BASF has employed advanced computational modeling techniques to predict the behavior of geometric isomers in solution, allowing for more efficient screening of potential electrolyte candidates[2]. They have also investigated the impact of geometric isomerism on the solvation structure and dynamics of ions in solution, which directly influences conductivity[3]. Furthermore, BASF has developed proprietary additives that can modulate the geometric configuration of electrolyte molecules to enhance conductivity in specific applications[4].
Strengths: Strong integration of computational and experimental approaches, focus on practical applications in energy storage. Weaknesses: Potential intellectual property restrictions limiting broader scientific collaboration.
Breakthrough Studies on Isomer-Conductivity Relationships
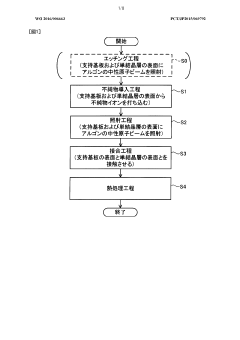
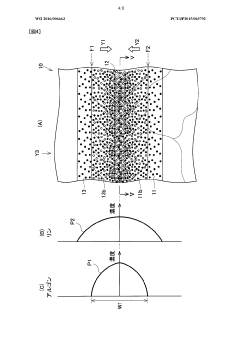
Semiconductor substrate and semiconductor substrate production method
PatentWO2016006663A1
Innovation
- A method involving surface irradiation of semiconductor layers with inert impurities, followed by bonding and heat treatment to narrow the concentration profile of these impurities, thereby reducing the distance they exist in the current path and suppressing non-ohmic conductive characteristics, includes steps like impurity introduction, irradiation, bonding, and heat treatment to achieve improved electrical characteristics.
Compounds and methods for the treatment of malaria
PatentInactiveIN202118043692A
Innovation
- Development of specific compounds, such as those represented by Formula I and listed in Table 1, which offer new structural features and functional groups to target malaria parasites effectively, including those resistant to existing drugs.
Environmental Impact of Isomer-Based Conductive Solutions
The environmental impact of isomer-based conductive solutions is a critical consideration in the development and application of these technologies. Geometric isomers, which have the same molecular formula but different spatial arrangements of atoms, can significantly influence the conductivity of solutions. This difference in conductivity may lead to varying environmental consequences when these solutions are used in industrial processes or released into ecosystems.
One of the primary environmental concerns is the potential for bioaccumulation of isomeric compounds in aquatic organisms. Certain geometric isomers may be more readily absorbed by fish, algae, and other aquatic life, leading to higher concentrations in the food chain. This bioaccumulation can result in long-term ecological effects, potentially disrupting ecosystem balance and biodiversity.
The persistence of isomers in the environment is another crucial factor. Some geometric isomers may be more resistant to natural degradation processes, leading to prolonged presence in soil and water systems. This persistence can contribute to chronic exposure for various organisms and may alter soil and water chemistry over time.
Water treatment facilities face challenges in effectively removing isomer-based conductive solutions from wastewater. The structural differences between geometric isomers can affect their solubility and reactivity, potentially requiring specialized treatment methods to ensure complete removal. Ineffective treatment could result in the release of these compounds into natural water bodies, impacting aquatic ecosystems and potentially contaminating drinking water sources.
The production and disposal of isomer-based conductive solutions also raise concerns about energy consumption and greenhouse gas emissions. Manufacturing processes for specific isomers may require more energy or produce more waste than others, contributing to the overall carbon footprint of the technology. Additionally, the disposal of products containing these solutions must be carefully managed to prevent environmental contamination.
Soil contamination is another potential consequence of using isomer-based conductive solutions. Leaching from improperly disposed products or accidental spills can introduce these compounds into soil ecosystems. This contamination may affect soil microorganisms, plant growth, and ultimately, the health of terrestrial ecosystems.
To mitigate these environmental impacts, research into green chemistry approaches for synthesizing and utilizing geometric isomers is essential. Developing biodegradable alternatives or designing closed-loop systems for the use and recycling of these solutions could significantly reduce their environmental footprint. Furthermore, comprehensive life cycle assessments of different isomeric compounds can help identify the most environmentally friendly options for specific applications.
One of the primary environmental concerns is the potential for bioaccumulation of isomeric compounds in aquatic organisms. Certain geometric isomers may be more readily absorbed by fish, algae, and other aquatic life, leading to higher concentrations in the food chain. This bioaccumulation can result in long-term ecological effects, potentially disrupting ecosystem balance and biodiversity.
The persistence of isomers in the environment is another crucial factor. Some geometric isomers may be more resistant to natural degradation processes, leading to prolonged presence in soil and water systems. This persistence can contribute to chronic exposure for various organisms and may alter soil and water chemistry over time.
Water treatment facilities face challenges in effectively removing isomer-based conductive solutions from wastewater. The structural differences between geometric isomers can affect their solubility and reactivity, potentially requiring specialized treatment methods to ensure complete removal. Ineffective treatment could result in the release of these compounds into natural water bodies, impacting aquatic ecosystems and potentially contaminating drinking water sources.
The production and disposal of isomer-based conductive solutions also raise concerns about energy consumption and greenhouse gas emissions. Manufacturing processes for specific isomers may require more energy or produce more waste than others, contributing to the overall carbon footprint of the technology. Additionally, the disposal of products containing these solutions must be carefully managed to prevent environmental contamination.
Soil contamination is another potential consequence of using isomer-based conductive solutions. Leaching from improperly disposed products or accidental spills can introduce these compounds into soil ecosystems. This contamination may affect soil microorganisms, plant growth, and ultimately, the health of terrestrial ecosystems.
To mitigate these environmental impacts, research into green chemistry approaches for synthesizing and utilizing geometric isomers is essential. Developing biodegradable alternatives or designing closed-loop systems for the use and recycling of these solutions could significantly reduce their environmental footprint. Furthermore, comprehensive life cycle assessments of different isomeric compounds can help identify the most environmentally friendly options for specific applications.
Applications in Electronic and Energy Storage Industries
The impact of geometric isomers on solution conductivity has significant applications in the electronic and energy storage industries. In the field of electronics, this phenomenon is particularly relevant to the development of advanced electrolytes for supercapacitors and batteries. Geometric isomers can influence the ionic mobility and charge transport mechanisms within electrolyte solutions, directly affecting the performance of energy storage devices.
In supercapacitor technology, the conductivity of electrolytes plays a crucial role in determining the device's power density and overall efficiency. By leveraging the effects of geometric isomers, researchers can fine-tune electrolyte compositions to optimize ionic conductivity. This optimization can lead to supercapacitors with enhanced charge-discharge rates and improved energy storage capabilities, making them more suitable for high-power applications in consumer electronics and electric vehicles.
The battery industry also stands to benefit from a deeper understanding of how geometric isomers affect solution conductivity. Lithium-ion batteries, which dominate the portable electronics and electric vehicle markets, rely heavily on efficient ion transport through electrolyte solutions. By manipulating the geometric configuration of electrolyte components, battery manufacturers can potentially increase the ionic conductivity of their systems, leading to faster charging times and improved overall battery performance.
In the realm of flexible electronics, the influence of geometric isomers on solution conductivity becomes particularly relevant. As the demand for bendable and stretchable electronic devices grows, so does the need for electrolytes that can maintain their conductivity under various deformation conditions. Geometric isomers offer a potential avenue for developing electrolyte formulations that retain their conductive properties even when subjected to mechanical stress, thus enabling the creation of more robust and versatile flexible electronic components.
The energy storage industry is also exploring the use of geometric isomers in the development of solid-state electrolytes. These advanced materials promise safer and more energy-dense batteries by eliminating the need for liquid electrolytes. The arrangement of molecules in solid-state electrolytes, influenced by their geometric isomerism, can significantly impact ion mobility and, consequently, the overall conductivity of the material. This research direction holds the potential to revolutionize energy storage technologies, offering improved safety and higher energy densities for a wide range of applications, from portable devices to grid-scale energy storage systems.
In supercapacitor technology, the conductivity of electrolytes plays a crucial role in determining the device's power density and overall efficiency. By leveraging the effects of geometric isomers, researchers can fine-tune electrolyte compositions to optimize ionic conductivity. This optimization can lead to supercapacitors with enhanced charge-discharge rates and improved energy storage capabilities, making them more suitable for high-power applications in consumer electronics and electric vehicles.
The battery industry also stands to benefit from a deeper understanding of how geometric isomers affect solution conductivity. Lithium-ion batteries, which dominate the portable electronics and electric vehicle markets, rely heavily on efficient ion transport through electrolyte solutions. By manipulating the geometric configuration of electrolyte components, battery manufacturers can potentially increase the ionic conductivity of their systems, leading to faster charging times and improved overall battery performance.
In the realm of flexible electronics, the influence of geometric isomers on solution conductivity becomes particularly relevant. As the demand for bendable and stretchable electronic devices grows, so does the need for electrolytes that can maintain their conductivity under various deformation conditions. Geometric isomers offer a potential avenue for developing electrolyte formulations that retain their conductive properties even when subjected to mechanical stress, thus enabling the creation of more robust and versatile flexible electronic components.
The energy storage industry is also exploring the use of geometric isomers in the development of solid-state electrolytes. These advanced materials promise safer and more energy-dense batteries by eliminating the need for liquid electrolytes. The arrangement of molecules in solid-state electrolytes, influenced by their geometric isomerism, can significantly impact ion mobility and, consequently, the overall conductivity of the material. This research direction holds the potential to revolutionize energy storage technologies, offering improved safety and higher energy densities for a wide range of applications, from portable devices to grid-scale energy storage systems.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!